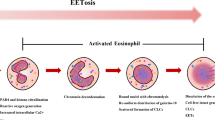
Abstract
It is becoming increasingly clear that nanoparticles (NPs) possess many potential applications in both clinical medicine and research. Potential utilization of NPs in nanomedicine for the treatment of respiratory diseases where eosinophils exert pathogenic roles is gaining increasing attention. Even though several NPs were found to possess pro-inflammatory activities in in vivo models based on an increased number of eosinophils in rodent airways, it is not clear how NPs could directly activate eosinophils themselves and how they can alter their biology. In this review, we discuss the most recent data in this new area of research demonstrating that NPs could now be added as new eosinophils modulators. Indeed, activation of eosinophils with NPs could lead to modulation of spontaneous apoptosis, caspase activation, and cytoskeleton breakdown when apoptosis is induced; cytokine production, de novo protein synthesis, cellular adhesion onto a cell substratum, and cell signalling events such as activation of the phosphoinositide 3-kinase/Akt pathway and actin re-localization are involved in NP-induced adhesion. Therefore, future development of therapeutic strategies with NPs aiming at targeting diseases where eosinophils are involved should now consider the capacity of NPs to modulate human eosinophil biology.





Similar content being viewed by others
References
Giembycz, M.A., and M.A. Lindsay. 1999. Pharmacology of the eosinophil. Pharmacological Reviews 51 (2): 213–340.
Park, Y.M., and B.S. Bochner. 2010. Eosinophil survival and apoptosis in health and disease. Allergy, Asthma & Immunology Research 2 (2): 87–101.
Ilmarinen, P., E. Moilanen, and H. Kankaanranta. 2014. Regulation of spontaneous eosinophil apoptosis-a neglected area of importance. Journal of Cell Death 7: 1–9.
Shamri, R., J.J. Xenakis, and L.A. Spencer. 2011. Eosinophils in innate immunity: an evolving story. Cell and Tissue Research 343 (1): 57–83.
Johansson, M.W. 2014. Activation states of blood eosinophils in asthma. Clinical and Experimental Allergy 44 (4): 482–498.
Blanchard, C., and M.E. Rothenberg. 2009. Biology of the eosinophil. Advances in Immunology 101: 81–121.
Walsh, G.M. 2013. Eosinophil apoptosis and clearance in asthma. Journal of Cell Death 6: 17–25.
Shen, Z.J., and J.S. Malter. 2015. Determinants of eosinophil survival and apoptotic cell death. Apoptosis 20 (2): 224–234.
Braun, R.K., M. Franchini, F. Erard, S. Rihs, I.J. De Vries, K. Blaser, T.T. Hansel, and C. Walker. 1993. Human peripheral blood eosinophils produce and release interleukin-8 on stimulation with calcium ionophore. European Journal of Immunology 23 (4): 956–960.
Stone, K.D., C. Prussin, and D.D. Metcalfe. 2010. IgE, mast cells, basophils, and eosinophils. The Journal of Allergy and Clinical Immunology 125 (2 Suppl 2): S73–S80.
Rosenberg, H.F., S. Phipps, and P.S. Foster. 2007. Eosinophil trafficking in allergy and asthma. The Journal of Allergy and Clinical Immunology 119 (6): 1303–1310 quiz 1311-1302.
Stern, M., J. Savill, and C. Haslett. 1996. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. The American Journal of Pathology 149 (3): 911–921.
Robb, C.T., K.H. Regan, D.A. Dorward, and A.G. Rossi. 2016. Key mechanisms governing resolution of lung inflammation. Seminars in Immunopathology 38 (4): 425–448.
Gaipl, U.S., A. Sheriff, S. Franz, L.E. Munoz, R.E. Voll, J.R. Kalden, and M. Herrmann. 2006. Inefficient clearance of dying cells and autoreactivity. Current Topics in Microbiology and Immunology 305: 161–176.
Ilmarinen, P., and H. Kankaanranta. 2014. Eosinophil apoptosis as a therapeutic target in allergic asthma. Basic & Clinical Pharmacology & Toxicology 114 (1): 109–117.
Tai, P.C., L. Sun, and C.J. Spry. 1991. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clinical and Experimental Immunology 85 (2): 312–316.
Reis, A.C., A.L. Alessandri, R.M. Athayde, D.A. Perez, J.P. Vago, T.V. Avila, T.P. Ferreira, et al. 2015. Induction of eosinophil apoptosis by hydrogen peroxide promotes the resolution of allergic inflammation. Cell Death & Disease 6: e1632.
Lavastre, V., S. Chiasson, H. Cavalli, and D. Girard. 2005. Viscum album agglutinin-I induces apoptosis and degradation of cytoskeletal proteins via caspases in human leukaemia eosinophil AML14.3D10 cells: differences with purified human eosinophils. British Journal of Haematology 130 (4): 527–535.
Druilhe, A., S. Letuve, and M. Pretolani. 2003. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis 8 (5): 481–495.
Barnes, P.J. 1996. Mechanisms of action of glucocorticoids in asthma. American Journal of Respiratory and Critical Care Medicine 154 (2 Pt 2): S26–S27.
Almeida, J.P., E.R. Figueroa, and R.A. Drezek. 2014. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine 10 (3): 503–514.
Chandolu, V., and C.R. Dass. 2013. Treatment of lung cancer using nanoparticle drug delivery systems. Current Drug Discovery Technologies 10 (2): 170–176.
Szelenyi, I. 2012. Nanomedicine: evolutionary and revolutionary developments in the treatment of certain inflammatory diseases. Inflammation Research 61 (1): 1–9.
Lu, X., T. Zhu, C. Chen, and Y. Liu. 2014. Right or left: the role of nanoparticles in pulmonary diseases. International Journal of Molecular Sciences 15 (10): 17577–17600.
Onoue, S., S. Yamada, and H.K. Chan. 2014. Nanodrugs: pharmacokinetics and safety. International Journal of Nanomedicine 9: 1025–1037.
Al-Qubaisi, M.S., A. Rasedee, M.H. Flaifel, S.H. Ahmad, S. Hussein-Al-Ali, M.Z. Hussein, E.E. Eid, et al. 2013. Cytotoxicity of nickel zinc ferrite nanoparticles on cancer cells of epithelial origin. International Journal of Nanomedicine 8: 2497–2508.
Castiglioni, S., A. Cazzaniga, C. Perrotta, and J.A. Maier. 2015. Silver nanoparticles-induced cytotoxicity requires ERK activation in human bladder carcinoma cells. Toxicology Letters 237 (3): 237–243.
Cheng, G., W. Guo, L. Han, E. Chen, L. Kong, L. Wang, W. Ai, N. Song, H. Li, and H. Chen. 2013. Cerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathways. Toxicology In Vitro 27 (3): 1082–1088.
Choi, S.Y., S. Jeong, S.H. Jang, J. Park, J.H. Park, K.S. Ock, S.Y. Lee, and S.W. Joo. 2012. In vitro toxicity of serum protein-adsorbed citrate-reduced gold nanoparticles in human lung adenocarcinoma cells. Toxicology In Vitro 26 (2): 229–237.
Zhao, W., X. Lu, Y. Yuan, C. Liu, B. Yang, H. Hong, G. Wang, and F. Zeng. 2011. Effect of size and processing method on the cytotoxicity of realgar nanoparticles in cancer cell lines. International Journal of Nanomedicine 6: 1569–1577.
Yin, H., P.S. Casey, M.J. McCall, and M. Fenech. 2015. Size-dependent cytotoxicity and genotoxicity of ZnO particles to human lymphoblastoid (WIL2-NS) cells. Environmental and Molecular Mutagenesis 56 (9): 767–776.
Magrez, A., S. Kasas, V. Salicio, N. Pasquier, J.W. Seo, M. Celio, S. Catsicas, B. Schwaller, and L. Forro. 2006. Cellular toxicity of carbon-based nanomaterials. Nano Letters 6 (6): 1121–1125.
Babin, K., F. Antoine, D.M. Goncalves, and D. Girard. 2013. TiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicology Letters 221 (1): 57–63.
Babin, K., D.M. Goncalves, and D. Girard. 2015. Nanoparticles enhance the ability of human neutrophils to exert phagocytosis by a Syk-dependent mechanism. Biochimica et Biophysica Acta 1850 (11): 2276–2282.
Goncalves, D.M., S. Chiasson, and D. Girard. 2010. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicology In Vitro 24 (3): 1002–1008.
Goncalves, D.M., and D. Girard. 2014. Zinc oxide nanoparticles delay human neutrophil apoptosis by a de novo protein synthesis-dependent and reactive oxygen species-independent mechanism. Toxicology In Vitro 28 (5): 926–931.
Liz, R., J.C. Simard, L.B. Leonardi, and D. Girard. 2015. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. International Immunopharmacology 28 (1): 616–625.
Noel, C., J.C. Simard, and D. Girard. 2016. Gold nanoparticles induce apoptosis, endoplasmic reticulum stress events and cleavage of cytoskeletal proteins in human neutrophils. Toxicology In Vitro 31: 12–22.
Poirier, M., J.C. Simard, F. Antoine, and D. Girard. 2014. Interaction between silver nanoparticles of 20 nm (AgNP20) and human neutrophils: induction of apoptosis and inhibition of de novo protein synthesis by AgNP20 aggregates. Journal of Applied Toxicology 34 (4): 404–412.
Poirier, M., J. C. Simard, and D. Girard. 2015. Silver nanoparticles of 70 nm and 20 nm affect differently the biology of human neutrophils. Journal of Immunotoxicology 1–11.
Baumann, M.A., and C.C. Paul. 1998. The AML14 and AML14.3D10 cell lines: a long-overdue model for the study of eosinophils and more. Stem Cells 16 (1): 16–24.
Karlsson, A.K., K. Walles, H. Bladh, S. Connolly, M. Skrinjar, and A. Rosendahl. 2011. Small molecule antagonists of CCR8 inhibit eosinophil and T cell migration. Biochemical and Biophysical Research Communications 407 (4): 764–771.
Ryu, S.I., W.K. Kim, H.J. Cho, P.Y. Lee, H. Jung, T.S. Yoon, J.H. Moon, et al. 2007. Phosphoproteomic analysis of AML14.3D10 cell line as a model system of eosinophilia. Journal of Biochemistry and Molecular Biology 40 (5): 765–772.
Dent, G., S.C. Loweth, A.M. Hasan, and F.M. Leslie. 2014. Synergic production of neutrophil chemotactic activity by colonic epithelial cells and eosinophils. Immunobiology 219 (10): 793–797.
Mahajan, L., P. Gautam, E. Dodagatta-Marri, T. Madan, and U. Kishore. 2014. Surfactant protein SP-D modulates activity of immune cells: proteomic profiling of its interaction with eosinophilic cells. Expert Review of Proteomics 11 (3): 355–369.
Vallieres, F., J.C. Simard, C. Noel, M. Murphy-Marion, V. Lavastre, and D. Girard. 2016. Activation of human AML14.3D10 eosinophils by nanoparticles: modulatory activity on apoptosis and cytokine production. Journal of Immunotoxicology 13 (6): 817–826.
Kim, M.H., J.H. Seo, H.M. Kim, and H.J. Jeong. 2014. Zinc oxide nanoparticles, a novel candidate for the treatment of allergic inflammatory diseases. European Journal of Pharmacology 738: 31–39.
Huang, K.L., Y.H. Lee, H.I. Chen, H.S. Liao, B.L. Chiang, and T.J. Cheng. 2015. Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. Journal of Hazardous Materials 297: 304–312.
Silva, L.R., and D. Girard. 2016. Human eosinophils are direct targets to nanoparticles: zinc oxide nanoparticles (ZnO) delay apoptosis and increase the production of the pro-inflammatory cytokines IL-1beta and IL-8. Toxicology Letters 259: 11–20.
Murphy-Marion, M., and D. Girard. 2017. Titanium dioxide nanoparticles induce human eosinophil adhesion onto endothelial EA.hy926 cells via activation of phosphoinositide 3-kinase/Akt cell signalling pathway. Immunobiology.
Chhay, P., M. Murphy-Marion, Y. Samson, and D. Girard. 2018. Activation of human eosinophils with palladium nanoparticles (Pd NPs): importance of the actin cytoskeleton in Pd NPs-induced cellular adhesion. Environmental Toxicology and Pharmacology 57: 95–103.
Simard, J.C., F. Vallieres, R. de Liz, V. Lavastre, and D. Girard. 2015. Silver nanoparticles induce degradation of the endoplasmic reticulum stress sensor activating transcription factor-6 leading to activation of the NLRP-3 inflammasome. The Journal of Biological Chemistry 290 (9): 5926–5939.
Acknowledgments
The study was partially supported by grants from the Institut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST) and Natural Sciences and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vanharen, M., Girard, D. Activation of Human Eosinophils with Nanoparticles: a New Area of Research. Inflammation 43, 8–16 (2020). https://doi.org/10.1007/s10753-019-01064-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01064-4