Abstract
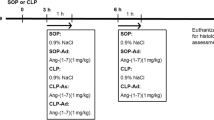
Sepsis is a life-threatening condition with high mortality rates that is caused by dysregulation of the host response to infection. We previously showed that treatment with the cannabinoid CB1 receptor antagonist rimonabant reduced mortality rates in animals with sepsis that was induced by cecal ligation and puncture (CLP). This improvement in the survival rate appeared to be related to an increase in arginine vasopressin (AVP) levels 12 h after CLP. The present study investigated the effects of rimonabant on organ dysfunction, hematologic parameters, and vascular reactivity in male Wistar rats with sepsis induced by CLP. Intraperitoneal treatment with rimonabant (10 mg/kg, 4 h after CLP) abolished the increase in the plasma levels of lactate, lactate dehydrogenase, glucose, and creatinine kinase MB without altering hematological parameters (i.e., leukopenia and a reduction of platelet counts). CLP increased plasma levels of nitrate/nitrite (NOx) and induced vasoconstriction in the tail artery. The treatment of CLP rats with rimonabant did not alter NOx production but reduced the vasoconstriction. Rimonabant also attenuated the hyperreactivity to AVP induced by CLP without affecting hyporesponsiveness to phenylephrine in aortic rings. These results suggest that rimonabant reduces organ dysfunction during sepsis, and this effect may be related to AVP signaling in blood vessels. This effect may have contributed to the higher survival rate in rimonabant-treated septic animals.





Similar content being viewed by others
References
Wiersinga, W.J., S.J. Leopold, D.R. Cranendonk, and T. van der Poll. 2014. Host innate immune responses to sepsis. Virulence 5 (1): 36–44.
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, G.R. Bernard, J.D. Chiche, C.M. Coopersmith, R.S. Hotchkiss, M.M. Levy, J.C. Marshall, G.S. Martin, S.M. Opal, G.D. Rubenfeld, T. van der Poll, J.L. Vincent, and D.C. Angus. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315 (8): 801–810.
Bakker, J., M.W. Nijsten, and T.C. Jansen. 2013. Clinical use of lactate monitoring in critically ill patients. Annals of Intensive Care 3 (1): 12.
Duman, A., A. Akoz, M. Kapci, M. Ture, S. Orun, K. Karaman, and K.A. Turkdogan. 2016. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: predictive role of lactate dehydrogenase. The American Journal of Emergency Medicine 34 (11): 2167–2171.
Zhang, L., et al. 2015. Poly (ADP-ribose) synthetase inhibitor has a heart protective effect in a rat model of experimental sepsis. International Journal of Clinical and Experimental Pathology 8 (9): 9824–9835.
Lafreniere, J.D., and C. Lehmann. 2017. Parameters of the endocannabinoid system as novel biomarkers in sepsis and septic shock. Metabolites 7 (4): pii: E55.
Maccarrone, M., I. Bab, T. Bíró, G.A. Cabral, S.K. Dey, V. di Marzo, J.C. Konje, G. Kunos, R. Mechoulam, P. Pacher, K.A. Sharkey, and A. Zimmer. 2015. Endocannabinoid signaling at the periphery: 50 years after THC. Trends in Pharmacological Sciences 36 (5): 277–296.
Sarker, K.P., S. Obara, M. Nakata, I. Kitajima, and I. Maruyama. 2000. Anandamide induces apoptosis of PC-12 cells: involvement of superoxide and caspase-3. FEBS Letters 472 (1): 39–44.
Villanueva, A., S.M. Yilmaz, W.R. Millington, R.A. Cutrera, D.G. Stouffer, L.H. Parsons, J.F. Cheer, and C. Feleder. 2009. Central cannabinoid 1 receptor antagonist administration prevents endotoxic hypotension affecting norepinephrine release in the preoptic anterior hypothalamic area. Shock 32 (6): 614–620.
Pacher, P., and G. Kunos. 2013. Modulating the endocannabinoid system in human health and disease--successes and failures. The FEBS Journal 280 (9): 1918–1943.
Zampronio, A.R., J.B. Kuzmiski, C.M. Florence, S.J. Mulligan, and Q.J. Pittman. 2010. Opposing actions of endothelin-1 on glutamatergic transmission onto vasopressin and oxytocin neurons in the supraoptic nucleus. The Journal of Neuroscience 30 (50): 16855–16863.
Leite-Avalca, M.C., et al. 2016. Involvement of central endothelin ETA and cannabinoid CB1 receptors and arginine vasopressin release in sepsis induced by cecal ligation and puncture in rats. Shock 46 (3): 290–296.
Rattmann, Y.D., S.M. Malquevicz-Paiva, M. Iacomini, and L.M.C. Cordeiro. 2013. Galactofuranose-rich polysaccharides from Trebouxia sp. induce inflammation and exacerbate lethality by sepsis in mice. Phytochemistry 94: 206–210.
Fernandes, D., et al. 2006. Nitric oxide-dependent reduction in soluble guanylate cyclase functionality accounts for early lipopolysaccharide-induced changes in vascular reactivity. Molecular Pharmacology 69 (3): 983–990.
Gordon, C.J. 1990. Thermal biology of the laboratory rat. Physiology & Behavior 47 (5): 963–991.
Karnatovskaia, L.V., and E. Festic. 2012. Sepsis: a review for the neurohospitalist. Neurohospitalist 2 (4): 144–153.
Brooks, H.F., C.K. Osabutey, R.F. Moss, P.L.R. Andrews, and D.C. Davies. 2007. Caecal ligation and puncture in the rat mimics the pathophysiological changes in human sepsis and causes multi-organ dysfunction. Metabolic Brain Disease 22 (3–4): 353–373.
Yang, W.L., and G. Ma. 2016. Combined administration of human ghrelin and human growth hormone attenuates organ injury and improves survival in aged septic rats. Molecular Medicine 22: 1.
Zhou, L., M. Gao, Z. Xiao, J. Zhang, X. Li, and A. Wang. 2015. Protective effect of astaxanthin against multiple organ injury in a rat model of sepsis. The Journal of Surgical Research 195 (2): 559–567.
Maitra, S.R., M.M. Wojnar, and C.H. Lang. 2000. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycemic phases of sepsis. Shock 13 (5): 379–385.
Sharma, A.C., S.J. Motew, S. Farias, K.J. Alden, H.B. Bosmann, W.R. Law, and J.L. Ferguson. 1997. Sepsis alters myocardial and plasma concentrations of endothelin and nitric oxide in rats. Journal of Molecular and Cellular Cardiology 29 (5): 1469–1477.
Osuchowski, M.F., J. Connett, K. Welch, J. Granger, and D.G. Remick. 2009. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Critical Care Medicine 37 (5): 1567–1573.
Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420 (6917): 885–891.
Katchan, V., P. David, and Y. Shoenfeld. 2016. Cannabinoids and autoimmune diseases: A systematic review. Autoimmunity Reviews 15 (6): 513–528.
Ho, J.T., M.J. Chapman, S. O’Connor, S. Lam, J. Edwards, G. Ludbrook, J.G. Lewis, and D.J. Torpy. 2010. Characteristics of plasma NOx levels in severe sepsis: high interindividual variability and correlation with illness severity, but lack of correlation with cortisol levels. Clinical Endocrinology 73 (3): 413–420.
Vincent, J.L. 1998. Cardiovascular alterations in septic shock. The Journal of Antimicrobial Chemotherapy 41 (Suppl A): 9–15.
Lorente, J.A., et al. 1993. L-arginine pathway in the sepsis syndrome. Critical Care Medicine 21 (9): 1287–1295.
Bernardelli, A.K., et al. 2016. Vasoplegia in sepsis depends on the vascular system, vasopressor, and time-point: a comparative evaluation in vessels from rats subjected to the cecal ligation puncture model. Canadian Journal of Physiology and Pharmacology 94 (11): 1227–1236.
OBrien, J.M., Jr., et al. 2007. Sepsis. The American Journal of Medicine 120 (12): 1012–1022.
Bennett, T., R.P. Mahajan, J.E. March, P.A. Kemp, and S.M. Gardiner. 2004. Regional and temporal changes in cardiovascular responses to norepinephrine and vasopressin during continuous infusion of lipopolysaccharide in conscious rats. British Journal of Anaesthesia 93 (3): 400–407.
Barrett, L.K., N.N. Orie, V. Taylor, R.P. Stidwill, L.H. Clapp, and M. Singer. 2007. Differential effects of vasopressin and norepinephrine on vascular reactivity in a long-term rodent model of sepsis. Critical Care Medicine 35 (10): 2337–2343.
Kienbaum, P., C. Prante, N. Lehmann, A. Sander, A. Jalowy, and J. Peters. 2008. Alterations in forearm vascular reactivity in patients with septic shock. Anaesthesia 63 (2): 121–128.
Acknowledgments
The authors thank MSc. Tatiane M.B.B. Telles for the help with the analysis in the Municipal Laboratory of Curitiba.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant # 473194/2012-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were approved by the institution’s Ethical Committee for Animal Use and were in accordance with Brazilian and EU Directive 2010/63/EU Guidelines for Animal Care. All efforts were made to minimize the number of animals used and their suffering.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leite-Avalca, M.C.G., Staats, F.T., Verona, D. et al. Cannabinoid CB1 Receptor Antagonist Rimonabant Decreases Levels of Markers of Organ Dysfunction and Alters Vascular Reactivity in Aortic Vessels in Late Sepsis in Rats. Inflammation 42, 618–627 (2019). https://doi.org/10.1007/s10753-018-0919-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0919-z