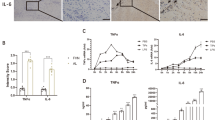
Abstract
Aseptic implant loosening is a devastating long-term complication of total joint arthroplasty. It is mainly initiated by the interaction of wear debris and macrophages. However, how does the chronic inflammation persist and how to stop it is poorly understood. Sphingosine kinases (SPHKs) are an essential feature of immunosuppressive M2 polarisation in macrophages and a promoter for chronic inflammation. In this study, RAW 264.7 macrophages were exposed to stimulation with titanium particles (0.1 mg/ml), and the subsequent expression of SPHKs and pro-inflammatory cytokines was evaluated. The effect of inhibitors of SPHKs (FTY720, PF543, and ABC294640) on titanium particle-challenged macrophages was analysed. As for results, the amount of sphingosine kinase (SPHK)-1 and SPHK-2 in RAW264.7 macrophages increased in the presence of titanium particles in a time-dependent manner. Two inhibitors of SPHKs (FTY720 and ABC294640) suppressed titanium particle-induced tumour necrosis factor (TNF)-α and interleukin (IL)-6 production in RAW264.7 macrophages. These findings suggest that persistent stimulation with titanium particles may lead to a consistent release of TNF-α and IL-6 via SPHK-2 activity, which may lead to aseptic implant loosening. Appropriate regulation of SPHK-2 may serve as a potential new strategy in the treatment of aseptic implant loosening.





Similar content being viewed by others
References
Pivec, R., A.J. Johnson, S.C. Mears, and M.A. Mont. 2012. Hip arthroplasty. Lancet 380 (9855): 1768–1777. https://doi.org/10.1016/S0140-6736(12)60607-2.
Thiele, K., C. Perka, G. Matziolis, H.O. Mayr, M. Sostheim, and R. Hube. 2015. Current failure mechanisms after knee arthroplasty have changed: polyethylene wear is less common in revision surgery. The Journal of Bone and Joint Surgery. American Volume 97 (9): 715–720. https://doi.org/10.2106/JBJS.M.01534.
Yang, F., W. Wu, L. Cao, Y. Huang, Z. Zhu, T. Tang, and K. Dai. 2011. Pathways of macrophage apoptosis within the interface membrane in aseptic loosening of prostheses. Biomaterials 32 (35): 9159–9167. https://doi.org/10.1016/j.biomaterials.2011.08.039.
Ingham, E., and J. Fisher. 2005. The role of macrophages in osteolysis of total joint replacement. Biomaterials 26 (11): 1271–1286. https://doi.org/10.1016/j.biomaterials.2004.04.035.
Takagi, M., Y. Tamaki, H. Hasegawa, Y. Takakubo, L. Konttinen, V.M. Tiainen, R. Lappalainen, Y.T. Konttinen, and J. Salo. 2007. Toll-like receptors in the interface membrane around loosening total hip replacement implants. Journal of Biomedical Materials Research. Part A 81 (4): 1017–1026. https://doi.org/10.1002/jbm.a.31235.
Islam, A.S., M.A. Beidelschies, A. Huml, and E.M. Greenfield. 2011. Titanium particles activate toll-like receptor 4 independently of lipid rafts in RAW264.7 murine macrophages. Journal of Orthopaedic Research 29 (2): 211–217. https://doi.org/10.1002/jor.21199.
Pajarinen, J., V.P. Kouri, E. Jamsen, T.F. Li, J. Mandelin, and Y.T. Konttinen. 2013. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomaterialia 9 (11): 9229–9240. https://doi.org/10.1016/j.actbio.2013.06.027.
Luo, G., Z. Li, Y. Wang, H. Wang, Z. Zhang, W. Chen, Y. Zhang, Y. Xiao, C. Li, Y. Guo, and P. Sheng. 2016. Resveratrol protects against titanium particle-induced aseptic loosening through reduction of oxidative stress and inactivation of NF-kappaB. Inflammation 39 (2): 775–785. https://doi.org/10.1007/s10753-016-0306-6.
Chen, W., Z. Li, Y. Guo, Y. Zhou, Z. Zhang, Y. Zhang, G. Luo, et al. 2015. Wear particles promote reactive oxygen species-mediated inflammation via the nicotinamide adenine dinucleotide phosphate oxidase pathway in macrophages surrounding loosened implants. Cellular Physiology and Biochemistry 35 (5): 1857–1867. https://doi.org/10.1159/000373996.
Baumann, B., J. Seufert, F. Jakob, U. Noth, O. Rolf, J. Eulert, and C.P. Rader. 2005. Activation of NF-kappaB signalling and TNFalpha-expression in THP-1 macrophages by TiAlV- and polyethylene-wear particles. Journal of Orthopaedic Research 23 (6): 1241–1248. https://doi.org/10.1016/j.orthres.2005.02.017.1100230602.
Hallab, N.J., and J.J. Jacobs. 2009. Biologic effects of implant debris. Bulletin of the NYU Hospital for Joint Diseases 67 (2): 182–188.
Zhang, Y., S. Yu, J. Xiao, C. Hou, Z. Li, Z. Zhang, Q. Zhai, M. Lehto, Y.T. Konttinen, and P. Sheng. 2013. Wear particles promote endotoxin tolerance in macrophages by inducing interleukin-1 receptor-associated kinase-M expression. Journal of Biomedical Materials Research. Part A 101 (3): 733–739. https://doi.org/10.1002/jbm.a.34375.
Zhang, Y., C. Hou, S. Yu, J. Xiao, Z. Zhang, Q. Zhai, J. Chen, Z. Li, X. Zhang, M. Lehto, Y.T. Konttinen, and P. Sheng. 2012. IRAK-M in macrophages around septically and aseptically loosened hip implants. Journal of Biomedical Materials Research. Part A 100 (1): 261–268. https://doi.org/10.1002/jbm.a.33258.
Chen, W., Z. Li, Y. Guo, Y. Zhou, Y. Zhang, G. Luo, X. Yang, C. Li, W. Liao, and P. Sheng. 2015. Wear particles impair antimicrobial activity via suppression of reactive oxygen species generation and ERK1/2 phosphorylation in activated macrophages. Inflammation 38 (3): 1289–1296. https://doi.org/10.1007/s10753-014-0099-4.
Antonios, J.K., Z. Yao, C. Li, A.J. Rao, and S.B. Goodman. 2013. Macrophage polarization in response to wear particles in vitro. Cellular & Molecular Immunology 10 (6): 471–482. https://doi.org/10.1038/cmi.2013.39.
Biswas, S.K., and E. Lopez-Collazo. 2009. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends in Immunology 30 (10): 475–487. https://doi.org/10.1016/j.it.2009.07.009.
Grin'kina, N.M., E.E. Karnabi, D. Damania, S. Wadgaonkar, I.A. Muslimov, and R. Wadgaonkar. 2012. Sphingosine kinase 1 deficiency exacerbates LPS-induced neuroinflammation. PLoS One 7 (5): e36475. https://doi.org/10.1371/journal.pone.0036475.
Lei, Y.C., L.L. Yang, W. Li, and P. Luo. 2015. Sphingosine kinase 1 dependent protein kinase C-delta activation plays an important role in acute liver failure in mice. World Journal of Gastroenterology 21 (48): 13438–13446. https://doi.org/10.3748/wjg.v21.i48.13438.
Lai, W.Q., A.W. Irwan, H.H. Goh, H.S. Howe, D.T. Yu, R. Valle-Onate, I.B. McInnes, A.J. Melendez, and B.P. Leung. 2008. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. Journal of Immunology 181 (11): 8010–8017.
Baker, D.A., J. Barth, R. Chang, L.M. Obeid, and G.S. Gilkeson. 2010. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. Journal of Immunology 185 (4): 2570–2579. https://doi.org/10.4049/jimmunol.1000644.
Liang, J., M. Nagahashi, E.Y. Kim, K.B. Harikumar, A. Yamada, W.C. Huang, N.C. Hait, et al. 2013. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23 (1): 107–120. https://doi.org/10.1016/j.ccr.2012.11.013.
Crespo, I., B. San-Miguel, J. L. Mauriz, J. J. Ortiz de Urbina, M. Almar, M. J. Tunon, and J. Gonzalez-Gallego. 2017. Protective effect of protocatechuic acid on TNBS-induced colitis in mice is associated with modulation of the SphK/S1P signaling pathway. Nutrients 9 (3): E288. https://doi.org/10.3390/nu9030288.
Wu, W., R.D. Mosteller, and D. Broek. 2004. Sphingosine kinase protects lipopolysaccharide-activated macrophages from apoptosis. Molecular and Cellular Biology 24 (17): 7359–7369. https://doi.org/10.1128/MCB.24.17.7359-7369.2004.
Edwards, J.P., X. Zhang, K.A. Frauwirth, and D.M. Mosser. 2006. Biochemical and functional characterization of three activated macrophage populations. Journal of Leukocyte Biology 80 (6): 1298–1307. https://doi.org/10.1189/jlb.0406249.
Xiong, Y., H.J. Lee, B. Mariko, Y.C. Lu, A.J. Dannenberg, A.S. Haka, F.R. Maxfield, E. Camerer, R.L. Proia, and T. Hla. 2016. Sphingosine kinases are not required for inflammatory responses in macrophages. The Journal of Biological Chemistry 291 (21): 11465. https://doi.org/10.1074/jbc.A113.483750.
Alvarez, S.E., K.B. Harikumar, N.C. Hait, J. Allegood, G.M. Strub, E.Y. Kim, M. Maceyka, H. Jiang, C. Luo, T. Kordula, S. Milstien, and S. Spiegel. 2010. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465 (7301): 1084–1088. https://doi.org/10.1038/nature09128.
Hisano, Y., T. Nishi, and A. Kawahara. 2012. The functional roles of S1P in immunity. Journal of Biochemistry 152 (4): 305–311. https://doi.org/10.1093/jb/mvs090.
Maceyka, M., K.B. Harikumar, S. Milstien, and S. Spiegel. 2012. Sphingosine-1-phosphate signaling and its role in disease. Trends in Cell Biology 22 (1): 50–60. https://doi.org/10.1016/j.tcb.2011.09.003.
Theiss, A.L. 2013. Sphingosine-1-phosphate: driver of NFkappaB and STAT3 persistent activation in chronic intestinal inflammation and colitis-associated cancer. JAKSTAT 2 (3): e24150. https://doi.org/10.4161/jkst.24150.
Puneet, P., C.T. Yap, L. Wong, Y. Lam, D.R. Koh, S. Moochhala, J. Pfeilschifter, A. Huwiler, and A.J. Melendez. 2010. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science 328 (5983): 1290–1294. https://doi.org/10.1126/science.1188635.
Pchejetski, D., J. Nunes, K. Coughlan, H. Lall, S.M. Pitson, J. Waxman, and V.V. Sumbayev. 2011. The involvement of sphingosine kinase 1 in LPS-induced Toll-like receptor 4-mediated accumulation of HIF-1alpha protein, activation of ASK1 and production of the pro-inflammatory cytokine IL-6. Immunology and Cell Biology 89 (2): 268–274. https://doi.org/10.1038/icb.2010.91.
Lei, Y.C., C.L. Lu, L. Chen, K. Ge, L.L. Yang, W. Li, and Y.H. Wu. 2016. C5a/C5aR pathway is essential for up-regulating SphK1 expression through p38-MAPK activation in acute liver failure. World Journal of Gastroenterology 22 (46): 10148–10157. https://doi.org/10.3748/wjg.v22.i46.10148.
Maceyka, M., H. Sankala, N.C. Hait, H. Le Stunff, H. Liu, R. Toman, C. Collier, et al. 2005. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. The Journal of Biological Chemistry 280 (44): 37118–37129. https://doi.org/10.1074/jbc.M502207200.
Lee, H.G., A. Hsu, H. Goto, S. Nizami, J.H. Lee, E.R. Cadet, P. Tang, R. Shaji, C. Chandhanayinyong, S.H. Kweon, D.S. Oh, H. Tawfeek, and F.Y. Lee. 2013. Aggravation of inflammatory response by costimulation with titanium particles and mechanical perturbations in osteoblast- and macrophage-like cells. American Journal of Physiology. Cell Physiology 304 (5): C431–C439. https://doi.org/10.1152/ajpcell.00202.2012.
Yu, H., B.A. Herbert, M. Valerio, L. Yarborough, L.C. Hsu, and K.M. Argraves. 2015. FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans. Lipids in Health and Disease 14: 66. https://doi.org/10.1186/s12944-015-0057-7.
Pyne, N.J., M. McNaughton, S. Boomkamp, N. MacRitchie, C. Evangelisti, A.M. Martelli, H.R. Jiang, S. Ubhi, and S. Pyne. 2016. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Advances in Biological Regulation 60: 151–159. https://doi.org/10.1016/j.jbior.2015.09.001.
French, K.J., Y. Zhuang, L.W. Maines, P. Gao, W. Wang, V. Beljanski, J.J. Upson, C.L. Green, S.N. Keller, and C.D. Smith. 2010. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. The Journal of Pharmacology and Experimental Therapeutics 333 (1): 129–139. https://doi.org/10.1124/jpet.109.163444.
Fox, E.S., P. Thomas, and S.A. Broitman. 1990. Hepatic mechanisms for clearance and detoxification of bacterial endotoxins. The Journal of Nutritional Biochemistry 1 (12): 620–628.
Wooley, P.H., and E.M. Schwarz. 2004. Aseptic loosening. Gene Therapy 11 (4): 402–407. https://doi.org/10.1038/sj.gt.3302202.
Nine, M.J., D. Choudhury, A.C. Hee, R. Mootanah, and N.A.A. Osman. 2014. Wear debris characterization and corresponding biological response: artificial hip and knee joints. Materials (Basel) 7 (2): 980–1016. https://doi.org/10.3390/ma7020980.
Acknowledgments
We thank Xiujian Lan and Xuanhong Zhang (Department of Medical Science Experimentation Center, Sun Yat-sen University) for technical support.
Funding
This study was funded by the National Natural Science Foundation of China (grant No. 81672149, No. 81171710) and the Natural Science Foundation of Guangdong Province (No. 2015A030311004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This study did not involve human participants and animals.
Rights and permissions
About this article
Cite this article
Yang, G., Gu, M., Chen, W. et al. SPHK-2 Promotes the Particle-Induced Inflammation of RAW264.7 by Maintaining Consistent Expression of TNF-α and IL-6. Inflammation 41, 1498–1507 (2018). https://doi.org/10.1007/s10753-018-0795-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0795-6