Abstract
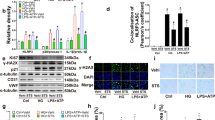
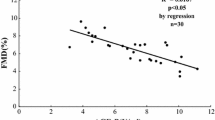
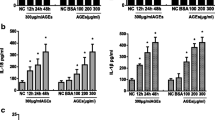
Advanced glycation end products (AGEs) have been confirmed to induce dysfunction in endothelial progenitor cells (EPCs) and play key roles in pathogenesis of diabetes-related vascular complications. The major function of sirtuin 3 (SIRT3) is to orchestrate oxidative metabolism and control reactive oxygen species (ROS) homeostasis, which are more closely related to EPCs’ dysfunction. Our study therefore was designed to explore the role of SIRT3 on AGEs-induced EPCs dysfunction of. EPCs isolated from healthy adults were stimulated with AGEs and the expression of SIRT3 was assessed. Then, EPCs transfected with ad-SIRT3 or siRNA-SIRT3 were cultured with or without AGEs. EPCs function, including proliferation, migration; expression of manganese superoxide dismutase (MnSOD), ROS production, and interleukin-8 (IL-8); and vascular endothelial growth factor (VEGF) production were measured. In some experiments, EPCs were pre-cultured with anti-receptor for advanced glycation end products (RAGE) antibody or anti-neutralizing antibody, and then proliferation, migration, expression of MnSOD, ROS production, and IL-8 and VEGF production were measured. Our results showed that SIRT3 expressed in EPCs and AGEs decreased SIRT3 expression. SIRT3 knockdown with siRNA-SIRT3 promoted dysfunction in EPCs whereas SIRT3 activation with ad-SIRT3 strengthened anti-oxidant capacity and protected AGE-impaired dysfunction. Moreover, RAGE may involve in AGEs-decreased SIRT3 expression in EPCs. These data suggested an important role of SIRT3 in regulating EPCs bioactivity.









Similar content being viewed by others
REFERENCES
Urbich, C., and S. Dimmeler. 2004. Endothelial progenitor cells: characterization and role in vascular biology. Circulation Research 95: 343–353.
Rabelink, T.J., H.C. de Boer, E.J. de Koning, and A.J. van Zonneveld. 2004. Endothelial progenitor cells: more than an inflammatory response. Arteriosclerosis, Thrombosis, and Vascular Biology (Dallas, TX) 24: 834–838.
Groleau, J., S. Dussault, P. Haddad, J. Turgeon, C. Menard, J.S. Chan, and A. Rivard. 2010. Essential role of copper- zinc superoxide dismutase for ischemia-induced neovascularization via modulation of bone marrow-derived endothelial progenitor cells. Arteriosclerosis, Thrombosis, and Vascular Biology (Dallas, TX) 30: 2173–2181.
Heller, G.V. 2005. Evaluation of the patient with diabetes mellitus and suspected coronary artery disease. The American Journal of Medicine 118: 9S–14S.
Waltenberger, J. 2001. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovascular Research 49: 554–560.
Palombo, C., M. Kozakova, C. Morizzo, L. Gnesi, M.C. Barsotti, P. Spontoni, F. Massart, P. Salvi, A. Balbarini, G. Saggese, et al. 2011. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovascular Diabetology 10: 88.
Tepper, O.M., R.D. Galiano, J.M. Capla, et al. 2002. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106:2781–6
Vasa, M., S. Fichtlscherer, A. Aicher, et al. 2001. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circulation Research 89: E1–E7.
Jandeleit-Dahm, K., A. Watson, and A. Soro-Paavonen. 2008. The AGEs/RAGEs axis in diabetes-accelerated atherosclerosis. Clinical and Experimental Pharmacology and Physiology 35: 329–334.
Moriyama, T., M. Kemi, C. Okumura, K. Yoshihara, and T. Horie. 2010. Involvement of advanced glycation end-products, pentosidine and N(epsilon)-(carboxymethyl)lysine, in doxorubicin-induced cardiomyopathy in rats. Toxicology 268: 89–97.
Madonna, R., and R. de Caterina. 2011. Cellular and molecular mechanisms of vascular injury in diabetes—part II: cellular mechanisms and therapeutic targets. Vascular Pharmacology 54: 75–79.
Zhou, Y.J., H.W. Yang, X.G. Wang, and H. Zhang. 2009. Hepatocyte growth factor prevents advanced glycation end products-induced injury and oxidative stress through a PI3K/Akt-dependent pathway in human endothelial cells. Life Sciences 85: 670–677.
Chen, J., M. Song, S. Yu, P. Gao, Y. Yu, H. Wang, and L. Huang. 2010. Advanced glycation endproducts alter functions and promote apoptosis in endothelial progenitor cells through receptor for advanced glycation endproducts mediate overpression of cell oxidant stress. Molecular and Cellular Biochemistry 335: 137–146.
Li, H., X. Zhang, X. Guan, X. Cui, Y. Wang, H. Chu, and M. Cheng. 2012. Advanced glycation end products impair the migration, adhesion and secretion potentials of late endothelial progenitor cells. Cardiovascular Diabetology 11: 46.
Finkel, T., C.X. Deng, and R. Mostoslavsky. 2009. Recent progress in the biology and physiology of sirtuin. Nature 460: 587–591.
Schwer, B., and E. Verdin. 2008. Conserved metabolic regulatory functions of sirtuins. Cell Metabolism 7: 104–112.
Lombard, D.B., F.W. Alt, H.L. Cheng, J. Bunkenborg, R.S. Streeper, R. Mostoslavsky, J. Kim, G. Yancopoulos, D. Valenzuela, A. Murphy, Y. Yang, Y. Chen, M.D. Hirschey, R.T. Bronson, M. Haigis, L.P. Guarente, R.V. Farese, S. Weissman, E. Verdin, and B. Schwer. 2007. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Molecular and Cellular Biology 27: 8807–8814.
Tseng, A.H., S.S. Shieh, and D.L. Wang. 2013. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radical Biology and Medicine 63: 222–234.
Sundaresan, N.R., M. Gupta, G. Kim, S.B. Rajamohan, A. Isbatan, and M.P. Gupta. 2009. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. Journal of Clinical Investigation 119: 2758–2771.
Hirschey, M.D., T. Shimazu, E. Jing, C.A. Grueter, A.M. Collins, B. Aouizerat, A. Stancakova, E. Goetzman, M.M. Lam, B. Schwer, R.D. Stevens, M.J. Muehlbauer, S. Kakar, N.M. Bass, J. Kuusisto, M. Laakso, F.W. Alt, C.B. Newgard, R.V. Farese Jr., C.R. Kahn, and E. Verdin. 2011. Sirt3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Molecular Cell 44: 177–90.
Zeng, H., X. He, X. Hou, L. Li, and J.X. Chen. 2014. Apelin gene therapy increases myocardial vascular density and ameliorates diabetic cardiomyopathy via upregulation of sirtuin 3. American Journal of Physiology. Heart and Circulatory Physiology 306: 585–597.
Cheng, C.C., S.J. Chang, Y.N. Chueh, T.S. Huang, P.H. Huang, S.M. Cheng, T.N. Tsai, J. Chen, and H.W. Wang. 2013. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genomics 14: 182.
Hur, J., C.H. Yoon, H.S. Kim, J.H. Choi, H.J. Kang, K.K. Hwang, B.H. Oh, M.M. Lee, and Y.B. Park. 2004. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology (Dallas, TX) 24: 288–293.
Luo, X., Z. Yang, S. Zheng, Y. Cao, and Y. Wu. 2014. Sirt3 activation attenuated oxidized low-density lipoprotein-induced human umbilical vein endothelial cells’ apoptosis. Cell Biology International 24: 288–293.
Mortuza, R., S. Chen, B. Feng, S. Sen, and S. Chakrabarti. 2013. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PloS One 8, e54514.
Shi, T., G.Q. Fan, and S.D. Xiao. 2010. SIRT3 reduces lipid accumulation in human hepatic cells via AMPK activation. Journal of Digestive Diseases 11: 55–62.
Chen, Y., J. Zhang, Y. Lin, Q. Lei, K.L. Guan, S. Zhao, and Y. Xiong. 2011. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Reports 12: 534–541.
Qiu, X., K. Brown, M.D. Hirschey, E. Verdin, and D. Chen. 2010. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metabolism 12: 662–667.
Ji, K.T., L. Qian, J.L. Nan, Y.J. Xue, S.Q. Zhang, G.Q. Wang, R.P. Yin, Y.J. Zhu, L.P. Wang, J. Ma, L.M. Liao, and J.F. Tang. 2015. Ox-LDL induces dysfunction of endothelial progenitor cells via activation of NF-kappaB. BioMed Research International 2015: 175291.
Someya, S., W. Yu, W.C. Hallows, J. Xu, J.M. Vann, C. Leeuwenburgh, M. Tanokura, J.M. Denu, and T.A. Prolla. 2010. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812.
Iwaguro, H., J. Yamaguchi, C. Kalka, et al. 2002. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 105: 732–738.
Kocher, A.A., M.D. Schuster, N. Bonaros, K. Lietz, G. Xiang, T.P. Martens, P.A. Kurlansky, H. Sondermeijer, P. Witkowski, A. Boyle, S. Homma, S.F. Wang, and S. Itescu. 2006. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. Journal of Molecular and Cellular Cardiology 40: 455–464.
Sun, C., C. Liang, Y. Ren, Y. Zhen, Z. He, H. Wang, H. Tan, X. Pan, and Z. Wu. 2009. Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Research in Cardiology 104: 42–49.
Scheubel, R.J., S. Kahrstedt, H. Weber, J. Holtz, I. Friedrich, J. Borgermann, R.E. Silber, and A. Simm. 2006. Depression of progenitor cell function by advanced glycation endproducts (AGEs): potential relevance for impaired angiogenesis in advanced age and diabetes. Experimental Gerontology 41: 540–548.
Kawamura, Y., Y. Uchijima, N. Horike, K. Tonami, K. Nishiyama, T. Amano, T. Asano, Y. Kurihara, and H. Kurihara. 2010. SIRT3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. Journal of Clinical Investigation 120: 2817–2828.
Someya, S., W. Yu, W.C. Hallows, J. Xu, J.M. Vann, C. Leeuwenburgh, M. Tanokura, J.M. Denu, and T.A. Prolla. 2010. SIRT3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chang, M., Zhang, B., Tian, Y. et al. AGEs Decreased SIRT3 Expression and SIRT3 Activation Protected AGEs-Induced EPCs’ Dysfunction and Strengthened Anti-oxidant Capacity. Inflammation 40, 473–485 (2017). https://doi.org/10.1007/s10753-016-0493-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0493-1