Abstract
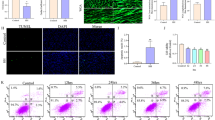
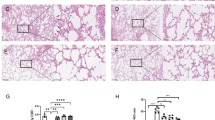
Acute lung injury (ALI) is a critical complication of the severe acute pancreatitis (SAP), characterized by increased pulmonary permeability with high mortality. Pulmonary microvascular endothelial cells (PMVECs) injury and apoptosis play a key role in ALI. Previous studies indicated that store-operated calcium entry (SOCE) could regulate a variety of cellular processes. The present study was to investigate the effects of SOCE inhibition on ALI induced by SAP in Sprague-Dawley rats, and PMVECs injury induced by lipopolysaccharide (LPS). Rat model of SAP-associated ALI were established by the retrograde infusion of sodium deoxycholate. Serum levels of amylase, TNF-α, and IL-6, histological changes, water content of the lung, oxygenation index, and ultrastructural changes of PMVECs were examined in ALI rats with or without store-operated Ca2+ channels (SOCs) pharmacological inhibitor (2-aminoethoxydiphenyl borate, 2-APB) pretreatment. For in vitro studies, PMVECs were transiently transfected with or without small interfering RNA (siRNA) against calcium release-activated calcium channel protein1 (Orai1) and stromal interaction molecule1 (STIM1), the two main molecular constituents of SOCs, then exposed to LPS. The viability of PMVECs was determined. The expression of STIM1, Orai1, Bax, and caspase3, both in lung tissue and in PMVECs, were assessed by quantitative real-time PCR and western blot. Administration of sodium deoxycholate upregulated the expression of SOCs proteins in lung tissue. Similarly, the SOCs proteins were increased in PMVECs induced by LPS. 2-APB reduced the serum levels of amylase, TNF-α, and IL-6, and attenuated lung water content and histological findings. In addition, the decreased oxygenation index and ultrastructural damage in PMVECs associated with SAP were ameliorated after administration of 2-APB. Knockdown of STIM1 and Orai1 inhibited LPS-induced PMVECs death. Furthermore, blockade of SOCE significantly suppressed Orai1, STIM1, Bax, and caspase3 expression both in vivo and in vitro. These results suggest that SOCE may play a critical role in SAP-associated ALI and the protective effects of inhibition of SOCs could be mediated, at least partially, by restraining mitochondrial associated apoptosis of PMVECs.








Similar content being viewed by others
References
Tenner, S., G. Sica, M. Hughes, E. Noordhoek, S. Feng, M. Zinner, et al. 1997. Relationship of necrosis to organ failure in severe acute pancreatitis. Gastroenterology 113: 899–903.
Buter, A., C.W. Imrie, C.R. Carter, S. Evans, and C.J. McKay. 2002. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. British Journal of Surgery 89: 298–302.
Shields, C.J., D.C. Winter, and H.P. Redmond. 2002. Lung injury in acute pancreatitis: mechanisms, prevention, and therapy. Current Opinion in Critical Care 8: 158–163.
Yang, Y., Q. Li, Z. Deng, Z. Zhang, J. Xu, G. Qian, et al. 2011. Protection from lipopolysaccharide-induced pulmonary microvascular endothelial cell injury by activation of hedgehog signaling pathway. Molecular Biology Reports 38: 3615–3622.
Pan, Z., X. Zhao, and M. Brotto. 2012. Fluorescence-based measurement of store-operated calcium entry in live cells: from cultured cancer cell to skeletal muscle fiber. Journal of visualized experiments: JoVE 60: 1–6.
Motiani, R.K., M.C. Hyzinski-Garcia, X. Zhang, M.M. Henkel, I.F. Abdullaev, Y.H. Kuo, et al. 2013. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Archiv: European journal of physiology 465: 1249–1260.
Lodola, F., U. Laforenza, E. Bonetti, D. Lim, S. Dragoni, C. Bottino, et al. 2012. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PloS One 7: e42541.
Moccia, F., F. Tanzi, and L. Munaron. 2014. Endothelial remodelling and intracellular calcium machinery. Current Molecular Medicine 14: 457–480.
Gandhirajan, R.K., S. Meng, H.C. Chandramoorthy, K. Mallilankaraman, S. Mancarella, H. Gao, et al. 2013. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. The Journal of clinical investigation 123: 887–902.
Varga-Szabo, D., A. Braun, and B. Nieswandt. 2011. STIM and Orai in platelet function. Cell Calcium 50: 270–278.
Zhang, J.W., G.X. Zhang, H.L. Chen, G.L. Liu, L. Owusu, Y.X. Wang, et al. 2015. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World Journal of Gastroenterology 21: 3537–3546.
Li, Y., H. Jiang, C.C. Ruan, J.C. Zhong, D.L. Zhu, W.Q. Niu, et al. 2013. Effect of TRPM7 Inhibitor on Pressure Overload Induced Cardiac Hypertrophy. Molecular Cardiology of China 501–504.
Sari, E., H. Aksit, H.A. Erken, A. Yay, D. Aksit, A.S. Amasyali, et al. 2015. Protective effect of 2-APB on testicular ischemia-reperfusion injury in rats. Journal of Urology 193: 1036–1041.
Parrau, D., G. Ebensperger, E.A. Herrera, F. Moraga, R.A. Riquelme, C.E. Ulloa, et al. 2013. Store-operated channels in the pulmonary circulation of high- and low-altitude neonatal lambs. American Journal of Physiology - Lung Cellular and Molecular Physiology 304: L540–548.
Rongione, A.J., A.M. Kusske, K. Kwan, S.W. Ashley, H.A. Reber, and D.W. McFadden. 1997. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology 112: 960–967.
Mayer, J., V.J. Laine, B. Rau, H.G. Hotz, T. Foitzik, T.J. Nevalainen, et al. 1999. Systemic lymphocyte activation modulates the severity of diet-induced acute pancreatitis in mice. Pancreas 19: 62–68.
Osman, M.O., J.U. Kristensen, N.O. Jacobsen, S.B. Lausten, B. Deleuran, M. Deleuran, et al. 1998. A monoclonal anti-interleukin 8 antibody (WS-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits. Gut 43: 232–239.
Meng, G., J. Zhao, H.M. Wang, R.G. Ding, X.C. Zhang, C.Q. Huang, et al. 2010. Cell injuries of the blood-air barrier in acute lung injury caused by perfluoroisobutylene exposure. Journal of Occupational Health 52: 48–57.
Steer, M.L. 2001. Relationship between pancreatitis and lung diseases. Respiration Physiology 128: 13–16.
Ikei, S., M. Ogawa, and Y. Yamaguchi. 1998. Blood concentrations of polymorphonuclear leucocyte elastase and interleukin-6 are indicators for the occurrence of multiple organ failures at the early stage of acute pancreatitis. Journal of Gastroenterology and Hepatology 13: 1274–1283.
Aoun, E., J. Chen, D. Reighard, F.C. Gleeson, D.C. Whitcomb, and G.I. Papachristou. 2009. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al.] 9: 777–785.
Malleo, G., E. Mazzon, A.K. Siriwardena, and S. Cuzzocrea. 2007. TNF-alpha as a therapeutic target in acute pancreatitis—lessons from experimental models. The Scientific World Journal 7: 431–448.
De Campos, T., J. Deree, and R. Coimbra. 2007. From acute pancreatitis to end-organ injury: mechanisms of acute lung injury. Surgical Infections 8: 107–120.
Maniatis, N.A., A. Kotanidou, J.D. Catravas, and S.E. Orfanos. 2008. Endothelial pathomechanisms in acute lung injury. Vascular Pharmacology 49: 119–133.
Yanyan, C., Q. Guoxian, G. Yang, and W. Leting. 2008. Mechanism of hypoxia-induced factor 1alpha expression in endothelial cells of the human umbilical vein and its induction of apoptosis. Molecular Biology Reports 35: 285–290.
Mizuta, M., H. Nakajima, N. Mizuta, Y. Kitamura, Y. Nakajima, S. Hashimoto, et al. 2008. Fas ligand released by activated monocytes causes apoptosis of lung epithelial cells in human acute lung injury model in vitro. Biological and Pharmaceutical Bulletin 31: 386–390.
Lucas, R., A.D. Verin, S.M. Black, and J.D. Catravas. 2009. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochemical Pharmacology 77: 1763–1772.
Rudkowski, J.C., E. Barreiro, R. Harfouche, P. Goldberg, O. Kishta, P. D’Orleans-Juste, et al. 2004. Roles of iNOS and nNOS in sepsis-induced pulmonary apoptosis. American Journal of Physiology Lung Cellular and Molecular Physiology 286: L793–800.
Salido, G.M., S.O. Sage, and J.A. Rosado. 2009. Biochemical and functional properties of the store-operated Ca2+ channels. Cellular Signalling 21: 457–461.
Zhang, S.L., Y. Yu, J. Roos, J.A. Kozak, T.J. Deerinck, M.H. Ellisman, et al. 2005. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905.
Prakriya, M., S. Feske, Y. Gwack, S. Srikanth, A. Rao, and P.G. Hogan. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233.
Rao, W., L. Zhang, N. Su, K. Wang, H. Hui, L. Wang, et al. 2013. Blockade of SOCE protects HT22 cells from hydrogen peroxide-induced apoptosis. Biochemical and Biophysical Research Communications 441: 351–356.
Li, N., P. Lin, C. Cai, Z. Pan, N. Weisleder, and J. Ma. 2009. The amino-terminal peptide of Bax perturbs intracellular Ca2+ homeostasis to enhance apoptosis in prostate cancer cells. American journal of physiology Cell physiology 296: C267–272.
Zhong, L.R., X. Chen, and K.M. Wei. 2013. Radix tetrastigma hemsleyani flavone induces apoptosis in human lung carcinoma a549 cells by modulating the MAPK pathway. Asian Pacific journal of cancer prevention: APJCP 14: 5983–5987.
Marzo, I., C. Brenner, N. Zamzami, J.M. Jurgensmeier, S.A. Susin, H.L. Vieira, et al. 1998. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science (New York, NY) 281: 2027–2031.
Liu, Z.B., Y.F. Hou, G.H. Di, J. Wu, Z.Z. Shen, and Z.M. Shao. 2009. PA-MSHA inhibits proliferation and induces apoptosis through the up-regulation and activation of caspases in the human breast cancer cell lines. Journal of Cellular Biochemistry 108: 195–206.
Heimlich, G., A.D. McKinnon, K. Bernardo, D. Brdiczka, J.C. Reed, R. Kain, et al. 2004. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. The Biochemical journal 378: 247–255.
Li, N., L. Zheng, P. Lin, D. Danielpour, Z. Pan, and J. Ma. 2008. Overexpression of Bax induces down-regulation of store-operated calcium entry in prostate cancer cells. Journal of Cellular Physiology 216: 172–179.
Wang, Y., H. Chen, H. Li, J. Zhang, and Y. Gao. 2013. Effect of angiopoietin-like protein 4 on rat pulmonary microvascular endothelial cells exposed to LPS. International Journal of Molecular Medicine 32: 568–576.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by National Natural Science Foundation of China (81173452, 81573751), and Basic Research Fund of Key Laboratory of Liaoning provincial education department (LZ2014042).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Committee for Research and Animal Ethics of Dalian Medical University.
Rights and permissions
About this article
Cite this article
Wang, G., Zhang, J., Xu, C. et al. Inhibition of SOCs Attenuates Acute Lung Injury Induced by Severe Acute Pancreatitis in Rats and PMVECs Injury Induced by Lipopolysaccharide. Inflammation 39, 1049–1058 (2016). https://doi.org/10.1007/s10753-016-0335-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0335-1