Abstract
Cyanobacterium Microcoleus anatoxicus, isolated from a coastal stream in northern California, produces both anatoxin-a (ATX) and dihydroanatoxin-a (dhATX), responsible for dog deaths, but its environmental preferences are unknown. We tested the effect of environmentally relevant stressors, e.g., salinity enrichment and nitrogen (N) depletion, on mat formation and toxicity of M. anatoxicus during the stationary growth phase in culture. Microcoleus anatoxicus showed broad salinity tolerance and the potential to enter estuaries and produce toxins in mesohaline conditions. Maximum growth was observed in oligohaline waters with salinity of 4.6 ppt. Moderate salinity stress (up to 7.8 ppt) did not affect dhATX production significantly. In contrast, higher salinity above 9.3 ppt had a detrimental effect on cell growth and significantly suppressed dhATX production. Formation of a common polysaccharide sheath covering multiple filaments was characteristic with increased salinity and may provide protection against osmotic stress. Microcoleus anatoxicus grown for 40 days in N-depleted medium formed mats with significantly elevated dhATX and increased ATX concentrations. Phycobilisome degradation was a possible acclimation response to N-limitation, as indicated by distinctly keritomized and pale cells in these cultures. In both experiments, most of the anatoxins were extracellular, probably due to toxin leaking during the stationary growth phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proliferations of benthic cyanobacterial mats in running waters, both toxic and non-toxic, are an emerging environmental issue globally which harm ecosystem health and interfere with recreational water use. Benthic cyanobacterial mats may produce potent neurotoxins, such as anatoxin-a and its congeners, which have been responsible for domestic animal mortalities (Testai, 2021). Filamentous mat-forming cyanobacteria in the genus Microcoleus are recognized as a major source of anatoxins in streams and rivers worldwide. However, the diversity and ecology of its toxigenic species in flowing freshwaters are poorly known. Molecular analyses of anatoxin-containing cyanobacterial mats from environmental samples showed that several different genotypes of Microcoleus (both toxigenic and non-toxigenic) contribute to the spatial variation of anatoxins in rivers in New Zealand (Heath et al., 2010, 2011), northern California (Bouma-Gregson et al., 2019, 2021) and Canada (Valadez-Cano et al., 2023a). A metagenomic study of field mats collected from the Eel and Russian Rivers in northern California (Bouma-Gregson et al., 2021) identified two common non-toxic Microcoleus species (sp. 1 and sp. 3) in both rivers and one rare toxic species in Eel River only (Microcoleus sp. 2) which contained the anatoxin-a biosynthesis gene cluster and shared 99.9% 16S rRNA sequence similarity to recently described Microcoleus anatoxicus Stancheva & Conklin, isolated from the Russian River (Conklin et al., 2020). The toxic Microcoleus species in both rivers in northern California were associated with dog-poisonings.
Microcoleus anatoxicus produces anatoxin-a (ATX) and dihydroanatoxin-a (dhATX) and has broad ecotoxicity for aquatic macroinvertebrates. Crude extracts containing ATX and dhATX from M. anatoxicus caused significant mortality in two benthic organisms (midges and amphipods) and the planktonic crustacean Ceriodaphnia dubia Richard (Anderson et al., 2018). Microcoleus anatoxicus produces a higher concentration of dhATX than ATX. Previously, dhATX was considered as a degradation product of ATX with lower toxicity, but recent work has shown dhATX is 3–4 times more acutely toxic for mice by oral administration (feeding and gavage) than ATX (Puddick et al., 2021). Furthermore, the complete ATX gene cassette recovered in M. anatoxicus contains anaK (Conklin et al., 2020), the gene product responsible for biosynthesis of dhATX (Méjean et al., 2009, 2014, 2016). This indicates that dhATX was internally synthesized, not just derived following cell lysis (Heath et al., 2014). The acute toxicity of M. anatoxicus may not be completely understood as other studies have shown that crude extracts of planktonic cyanobacteria containing a mixture of microcystin and anatoxin-a were more toxic to the sediment-dwelling midge Chironomus than purified cyanotoxins (Wood et al., 2020).
Mitigation of benthic cyanobacterial proliferations of Microcoleus and its detrimental effects in stream ecosystems requires an understanding of environmental tolerances and adaptations of toxic and non-toxic species. Our current knowledge is limited to studies of composite benthic mats with mixed Microcoleus species (e.g., Heath et al., 2010, 2011; Bouma-Gregson et al., 2019, 2021; Valades-Cano et al., 2023a), analyses of Microcoleus genomes (e.g., Tee et al., 2021; Valades-Cano et al., 2023b) and lab tests of effect of iron, copper, nitrogen and phosphorus on unialgal Microcoleus strains (Harland et al., 2013; Heath et al., 2014, 2016). A comparative genomic study by Tee et al. (2021), which included M. anatoxicus, elucidated genetic mechanisms differentiating non-toxic and toxic strains of Microcoleus. There was substantial genome streamlining in toxic strains and a potential dependency on external sources for thiamine and sucrose. Tee et al. (2021) suggested that toxic strains may be less tolerant to salt stress and nutrient fluctuations, possibly due to expendable loss of the genes for sucrose synthesis, and differences in nitrogen and carbon uptake and storage. Lab experiments with toxic Microcoleus strains can test the actual tolerances to variable stressors and their effect on filament growth and toxin production. Currently, there are no experimental data on the effect of salinity on anatoxin-producing filamentous cyanobacterial species. The influence of temperature, light irradiance, culture duration, culture medium composition, and nitrogen source on ATX-producing cyanobacteria have been studied in N2-fixing Anabaena and Aphanizomenon (see for review Neilan et al., 2013; Cirés et al., 2017), but not in Microcoleus.
Microcoleus anatoxicus is known to inhabit the coastal Russian and Eel Rivers in northern California, where it flourishes in benthic algal communities dominated by N2-fixing algae indicative of N-limiting conditions (Stancheva et al., 2013). Geographic distribution and environmental preferences of this important toxic cyanobacterium are poorly known, but microscopic analysis of hundreds of samples from streams across California collected by the Surface Water Ambient Monitoring Program (SWAMP) suggested potential wide-spread distribution M. anatoxicus in streams with variable environmental conditions, particularly in northern California (Stancheva, unpublished data). Given the ecological plasticity of cyanobacteria in general, and their tolerance to extreme environmental conditions, we hypothesize that toxic Microcoleus species could be substantially more widely distributed under a larger range of environmental conditions than previously reported. Therefore, we were interested in testing the potential of M. anatoxicus to inhabit water bodies with elevated salinity, because the increasing salinization of freshwater ecosystems in arid areas with a Mediterranean climate caused by droughts, or sea level rise are likely to affect the distribution of benthic cyanobacteria. To answer this question, we used two of the original strains of M. anatoxicus (e.g., strains PTRS1 and PTRS2) described in Conklin et al. (2020) maintained in our lab. The aims of this study were to investigate: 1) the salinity tolerance of M. anatoxicus and its ability to grow and produce toxins under increased salinity as an indication of its potential to enter coastal and tidal ecosystems in the land–ocean interface along the Pacific Ocean and 2) its toxin-production under prolonged nitrogen depletion as indication of its ability to grow and produce toxins in N-limited natural habitats.
Materials and methods
Cyanobacterial cultures
In this study, we tested the original M. anatoxicus strains isolated from the Russian River in northern California in 2015 (Conklin et al., 2020). We maintain three strains of M. anatoxicus (e.g., the reference strain PTRS2 and strains PTRS1 and PTRS3 described in Conklin et al., 2020) since their isolation in 2015 at 21 °C, light irradiance of 100 µmoles/ m2/ s and a 12:12-h light:dark cycle. The strains are unialgal, monoclonal, and non-axenic and originally produced both ATX and dhATX (see Table 1 in Conklin et al., 2020). We kept the cyanobacterial strains constantly growing by transferring filaments every 60 days into fresh BG11 medium with pH 7.17 (Millipore Sigma, St. Louis, MO). We used M. anatoxicus strain PTRS2 which produced both ATX and dhATX and strain PTRS1 which produced detectable dhATX only at the time of the experiments.
Experimental design
Salinity enrichment experiment
Microcoleus anatoxicus strain PTRS1 was cultured for 30 days in six different liquid media representing combinations of freshwater BG11 medium (control) and sea salt solutions with variable salinity, ion content, and nutrient concentrations. For each experimental treatment, four 125-ml Pyrex® glass Erlenmeyer flasks were inoculated with a small clump of Microcoleus filaments (6 mg ± 0.5 mg) in 45 ml culture medium. Cyanobacteria were grown at 21 °C, with a light irradiance of 100 µmoles/m2/s and a 12:12-h light:dark cycle. To minimize variation in growth due to different light intensities reaching each culture, the flask positions were randomized every day. The following media were included in this study:
BG-11 freshwater media: BG11 medium, a commonly used growth medium for freshwater cyanobacteria with exceptionally high nitrate and phosphate levels (Anderson, 2005), was used as a control.
Sodium chloride medium (NaCl): BG11 enriched with 0.25 g/L NaCl was used to test the effect of salinity stress caused by NaCl alone.
Artificial sea salts media: Four artificial media treatments prepared by the addition of those salts found in seawater to test the salinity stress separately from the effect of the nutrient availability. A solution of five sea salts was prepared in deionized water at concentrations approximating their natural occurrence in marine waters (NaCl 26.88 g/L, MgCl2 4.09 g/L, CaCl2 1.11 g/L, MgSO4 1.08 g/L, K2SO4 0.85 g/L (Emerson and Hedges 2008). This solution was mixed it with BG11 in the following proportions:
SS10 medium – BG11 with addition of 10% sea salts (NaCl 2.688 g/L, MgCl2 0.409 g/L, CaCl2 0.111 g/L, MgSO4 0.108 g/L, K2SO4 0.085 g/L).
SS20 medium – BG11 with addition of 20% sea salts (NaCl 5.376 g/L, MgCl2 0.818 g/L, CaCl2 0.222 g/L, MgSO4 0.216 g/L, K2SO4 0.17 g/L).
SS25 medium – BG11 with addition of 25% sea salts (NaCl 6.72 g/L, MgCl2 1.0225 g/L, CaCl2 0.2775 g/L, MgSO4 0.27 g/L, K2SO4 0.2125 g/L).
SS30 medium – BG11 with addition of 30% sea salts (NaCl 8.064 g/L, MgCl2 1.227 g/L, CaCl2 0.333 g/L, MgSO4 0.324 g/L, K2SO4 0.85 g/L).
Nutrients (NO3-N, NH4-N, PO4-P), chloride, and sulfate concentrations in these experimental media were determined by ion-chromatography at the University of Utah. The detection limit for N was 0.05 mg/L. We used an EXTECH EC400: ExStik® Conductivity/TDS/Salinity meter to measure pH, conductivity, salinity and total dissolved solids (TDS).
Three flasks per treatment were used for toxin measurements and RNA extraction (work in progress), and one flask was used for microscopic observations of the filaments. The sample from each flask for toxin measurement and RNA extractions was split to three equal portions containing approximately 15 ml (± 0.01 ml) of culturing medium and filaments. Filaments were gently removed from the walls of the culture flask, and mats were fragmented into visually similar small-in-size clumps using sterile, disposable loops. Then, the flask was gently agitated to evenly disperse the filaments in the culturing medium, and 15 ml of the sample content (e.g., liquid with floating filaments) was transferred to a plastic tube using a sterile serological pipette. The samples were shipped overnight on ice to the State University of New York (SUNY-ESF) in Syracuse for toxin and dry weight measurement. Dry weight was obtained by lyophilization as first step of the toxin analysis and used as a proxy of cell density. The experiment was conducted in June 2021 and M. anatoxicus strain PTRS1 produced only dhATX at that time.
Nitrogen depletion experiment
To compare the growth and anatoxins production of M. anatoxicus strain PTRS2 in optimal nutrient conditions and N-depleted conditions, M. anatoxicus was cultured for 40 days in standard BG11 and BG11 medium with no added nitrogen (BG11-N from UTEX Culture Collection of Algae, Austin, TX). For each experimental treatment, six 125 ml Pyrex® glass Erlenmeyer flasks were inoculated with a small clump of Microcoleus filaments (8 mg ± 0.5 mg) in 75 ml culture medium. Both treatments were inoculated with filaments grown for 30 days in BG11 until reach stationary phase. For the N-depleted treatment, mats were removed with sterile forceps and rinsed with BG11-N prior to inoculation to reduce the carryover, but were not N-starved in advance of the experiment. Flask positions were randomized every day to minimize variation in growth due to different light intensities in the incubators.
Three flasks per treatment were used for toxin measurements and microscopic observations of the filaments, and three flasks were used for dry weight measurements. The sample from each flask for toxin measurement and microscopic observations was split to three equal portions. Filaments were gently removed from the walls of the culture flask, and mats were fragmented into visually similar small-in-size clumps using sterile, disposable loops. Then, the flask was gently agitated to evenly disperse the filaments in the culturing medium, and 50 ml (± 0.01 ml) of the sample content (e.g., liquid with floating filaments) was transferred to a plastic tube using a sterile serological pipette. The tubes with culture material were shipped overnight on ice to the State University of New York (SUNY-ESF) in Syracuse for toxin measurement. The remaining 25 ml of Microcoleus filaments in culture liquid were used for microscopic observations.
The second set of triplicates were used to measure the dry weight. The entire sample was filtered on polycarbonate membrane filters, 47 mm in diameter, 0.4 µm pore size (Millipore Sigma, Merck KGaA, Darmstadt, Germany) and dried in desiccator for 72 h. The wet weight and dry weight of the sample and dry weight of the filter were measured using an analytical balance (ME54, Mettler-Toledo, Columbus, OH). Dry weight was again used as a proxy of cell density. The experiment was conducted in June 2019 and M. anatoxicus strain PTRS2 produced both ATX and dhATX at that time.
Anatoxin-a variants analysis
Upon receipt at SUNY-ESF, the samples were filtered onto tared Whatman 934 AH glass fiber filters (nominal pore size 1.5 µm), dried by lyophilization, and reweighed. The filtrate was collected and analyzed for extracellular toxin content; the filaments retained on the filter were analyzed for intracellular toxins. The dry weight mass retained by the filters was measured and used as a proxy of cell density in salinity enrichment experiments.
Filters were extracted in 5 ml of 50% acidified methanol containing 1% acetic acid using a Branson 450W probe sonicator equipped with a microtip for 1 min on ice. After sonication, the extract was clarified by centrifugation at 14,000×g, and the filtrate passed through a 0.2 µm pore nylon filter prior to LC–MS (/MS) analysis. Prior studies using cyanobacterial cultures have shown that this protocol released greater than 90% of microcystins, anatoxins, and PSP toxins from the cyanobacteria cells (Boyer 2007). The filtrate was analyzed directly without sonication after passage through a 0.2 µm nylon filter. All samples were analyzed for microcystins by LC–MS (21 congeners, Boyer 2020), cylindrospermopsins (EPA method 545 modified to include deoxy and epi-cylindrospermopsin and confirmation ions) and six anatoxins (anatoxin-a, homoanatoxin-a and their respective α and β dihydro epimers) (EPA method 545 modified include the additional congeners and confirmation ions, Smith et al., 2020). Only the α and β dihydroanatoxin-a was found in these samples and is reported here. Dihydroanatoxin-a was quantified against a secondary anatoxin-a standard, which in turn was quantified against an anatoxin-a certified reference standard obtained from NRC Canada. Chemical synthesis of dihydroanatoxin-a confirmed that the response factor for this toxin was similar to anatoxin-a and not a significant source of error. Spike experiments confirmed that ion suppression in these samples was < 10% and thus final concentrations were not corrected for matrix effects. Method detection limits were calculated individually for all samples using the instrument detection limits at the time the same was run, the extract volume and the weight or volume of the sample extracted. Method detection limits for microcystins averaged < 6 µg/g for intracellular toxins or 5 µg/L for extracellular toxins. Method detection limits for anatoxins (including the dihydro derivatives) were several orders of magnitude less than the measured concentrations and were generally less than 0.2 µg/g or 0.1 µg/L. Method detection limits for cylindrospermopsin were slightly higher and averaged less than 1 µg/g or < 0.7 µg/l, respectively.
Microscopic observations of Microcoleus morphology
Morphological observations of filaments from each treatment were conducted from fresh material on the date of collection with an Olympus microscope BX41 with an attached Olympus SC30 digital camera (Olympus Imaging America). Taxonomically important morphological features, also indicative of the physiological state, including cell color, granulation, keritomization, filament disintegration, extracellular mucilage, and motility were characterized. For each sample, we counted 300 random cells and classified them in the following qualitative categories indicative of cellular stress: 1) Cells with storage granules along the cross-walls or scattered throughout the cell—this is taxonomically important but variable feature related to phosphorus, sulfur, or light starvation (Komárek & Anagnostidis, 2005); 2) Keritomized cells (e.g., “vacuolized” cells with “net-like” structures caused by possibly widened thylakoids)—indication of light, salinity, and other stress (Komárek & Anagnostidis, 2005); 3) Cells with discolored thylakoids (reddish, yellowish to colorless)—indication of phycobilisome breakdown as result of nitrogen starvation (Herrero et al., 2001); and 4) Cell within short fragments (fragments with less than 20 cells)—indication for filament disintegration and hormogonia formation.
Statistical data analysis
Data were checked to meet assumptions of normality using Shapiro–Wilk test and homogeneity using the Bartlett test prior to running all statistical analyses. All statistical analyses were calculated using R: (R Core Team, 2022). Graphs were generated using the package ‘ggplot2’ (Wickham, 2023). The differences between dry weight accrual and total dhATX production across treatments in the salinity enrichment experiments were analyzed using a one-way analysis of variance (ANOVA) for each metric. Where significant differences were detected, additional post hoc Tukey’s Honest Significant Difference (HSD) tests where run to identify which treatments varied significantly from each other. One data point from SS25 media was omitted, due to low sample yield, and the average of the duplicates was used. To analyze whether dry weight accrual, total ATX, and total dhATX were different between the control BG11 and BG11 (-N) treatments in the nitrogen starvation experiments, two sample t tests assuming equal variance were run for each metric.
Results
Salinity enrichment experiment
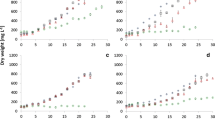
Microcoleus anatoxicus grew continuously for 30 days in culture under a wide gradient of salinity (1.1–9.3 ppt), chlorides (41.5–4400. mg/l) and sulfates (29.2–333.4 mg/l) concentrations (Table 1). The culturing medium with highest salinity (11.1 ppt), chlorides (5230 mg/l) and sulfate (345.9 mg/l) concentrations did not support the growth of Microcoleus, and 76% of the cells were colorless without visible intracellular content. Nutrient concentrations in the culture media were within a narrow range of nitrate (237.3–258.5 mg/l) and phosphate (4.84–5.39 mg/L) concentrations, which minimized the influence of nutrient variability as compared to salinity.
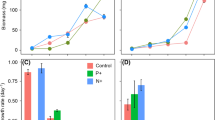
The highest salinity of 11.1 ppt significantly reduced growth (ANOVA, F5,12 = 10.59, P = 0.00045) and dhATX production (ANOVA, F5,12 = 6.023, P = 0.00516) in M. anatoxicus (Fig. 1 and Table 2 for Tukey HSD post hoc test data). Slight increase in salinity up to 4.6 ppt (both in NaCl and SS10) stimulated the growth of M. anatoxicus compared to the BG11 control medium, although not statistically significantly (Tukey-HSD post hoc test, P > 0.05, Table 2). In contrast, salinity above 4.6 ppt progressively inhibited cyanobacterial growth, where cells nearly cease to grow and produce dhATX at a salinity of 11.1 ppt (Fig. 1a, b). The highest dhATX concentration was recorded in medium with salinity 4.6 ppt (SS10 – 2268 µg/g dry weight). However, increase in salinity from 1.1 to 7.8 ppt did not significantly affect the average dhATX production, which was within a narrow range of 2067–2278 µg/g (Fig. 1b). The dhATX concentrations in salinity 11.1 ppt dropped more than twice the average of the lower salinity treatments and were significantly lower than all other treatments (Table 2, Fig. 1b). The extracellular dhATX quota was between 76 and 100% of the total measured dhATX (Fig. 1c). The highest intracellular dhATX concentrations were recorded at salinity 7.8 ppt (13–23%), followed by 4.6 ppt (7–17%) and 9.3 ppt (1.2–16.7%), while in the rest of the media the intracellular dhATX quota was below 1%.
Microcoleus anatoxicus strain PTRS1 dry weight (a), dhATX concentrations (b), and percentage of extracellular dhATX (c) under different salinity enrichment treatments. Error bars denote upper limits of 95% confidence interval. See Table 2 for statistically significant differences between treatments and electronic supplementary material for the raw data
Effect of salinity stress on Microcoleus anatoxicus morphology
Microcoleus filaments in all salinity conditions were consolidated into mats and embedded in extensive colorless mucilage after 30 days of culturing (Fig. 2). Microcoleus anatoxicus grown in BG11 control medium had trichomes enclosed in an individual colorless mucilaginous sheath; cells were olive-green with small storage granules scattered (87% of the cells) or along the cross-walls (13% of the cells) (Fig. 2a). The addition of NaCl caused a proportional increase in granulation along the cross-walls, but the filaments were otherwise similar to the BG11 control (Fig. 2b). Exposure of Microcoleus to sea salts led to intense accumulation of extracellular polysaccharide layers and formation of common sheaths surrounding multiple trichomes (Fig. 2d, g), rarely observed in other culturing conditions. Furthermore, additional mucilaginous accumulations on top of the calyptra were observed in all sea salt treatments (Fig. 2b, c, e, f). Microcoleus cells in salinity treatments from 4.6 to 9.3 ppt were slightly constricted (Fig. 2b, f), with metabolites stored in large granules along both sides of the cross-walls, and 28 to 36% cells had keritomized content, reddish or pale color (Fig. 2c). Some filaments showed bluish marks which might be a sign of cell wall damage (Fig. 2c, e). The highest salinity concentration of 11.1 ppt had strongly inhibiting effect on cellular growth, as 76% of the cells were colorless and structureless, and there were many empty individual and common mucilaginous sheaths remaining after cell death (Fig. 2g). The rarely observed colorful filaments in salinity 11.1 ppt were embedded in thick common mucilage and had constricted, yellowish-red colored cells, without granules, or seldom few large scattered granules (Fig. 2g). Microcoleus filaments cultured in all SS media produced distinctive odor from volatile compounds, in contrast to the BG11 control. The disintegration of the filaments was comparable in all treatments with elevated salinity with 28 to 39% of the cells contained within short hormogonia (Fig. 2d).
Filament morphology of Microcoleus anatoxicus strain PTRS1 in salinity enrichment experiment: (A) BG11 (control), (B) NaCl, (C) SS10, (D, E) SS20, (F) SS25, (G) SS30. Note multiple filaments in common sheath (D, G); Symbols indicate colorless polysaccharide sheath (triangles), apical calyptra with additional mucilaginous accumulations (stars); storage granules along both sides of the cross-walls or dispersed (black arrows); cells with keritomized content (white arrows). Black boxes border areas with damaged cell walls with phycobilin marks. Images obtained by differential interference contrast light microscopy. Scale bar is 10 µm
Nitrogen depletion experiment
Microcoleus anatoxicus grew continuously in both control BG11 and N-free BG11 medium for 40 days. The lack of nitrogen source reduced the dry weight (Fig. 3a), but not significantly (t = 2.30, df = 4, P > 0.05). The ATX production in the N-depleted Microcoleus cultures increased (Fig. 3b) but was also not significantly different from the control medium (t = − 0.78, df = 4, P > 0.05), while dhATX production was significantly higher under N-depleted conditions (− 3.45, df = 4, P = 0.03, Fig. 3c). The extracellular dhATX fraction was 68–77% of the total measured dhATX in BG11 and 90–95% in BG11-N (Fig. 3d). ATX was detected in the extracellular fraction only, but not in the cells, except for one BG11-N replicate where intracellular ATX was 3.8% of the total (Fig. 3d).
Microcoleus anatoxicus strain PTRS2 dry weight (a), ATX (b), dhATX (c), and percentage of extracellular toxins concentrations (d) in control BG11 medium and under N-depleted conditions in BG11-N medium. Error bars denote upper limits of 95% confidence interval. Legend: *Significant at P < 0.05; Diagonal lines patterns indicate ATX and cross lines pattern—dhATX (b–d)
Effect of nitrogen depletion on Microcoleus anatoxicus morphology
Microcoleus anatoxicus strain PTRS2 grown in the BG11 control had cells with pale olive-green color and scattered granules in 92% of the cells (Fig. 4a). Cellular and filament morphology was very similar to strain PTRS1 grown in the BG11 control for the salinity experiment (see Fig. 2a for comparison). In contrast, nearly 100% of the cells in the N-depleted treatment were keritomized, pale, without visible granules (Fig. 4b, c), enclosed in extensive diffuse colorless mucilage. In addition, filaments were disintegrated to hormogonia in the BG11-N treatment (67% of the cells, Fig. 4c).
Filament morphology of Microcoleus anatoxicus strain PTRS2 in nitrogen depletion experiments: (A) BG11 (control), (B and C) BG11-N – note filaments are disintegrated to hormogonia and cell are with keritomized content. Symbols indicate storage granules along both sides of the cross-walls or dispersed (black arrows) and cells with keritomized content (white arrows). Scale bar is 10 µm
Discussion
Salinity enrichment experiment
We tested the effect of two environmentally relevant stressors (salinity and N-limitation) on mat formation and toxicity of M. anatoxicus during the stationary growth phase in culture. To our knowledge, the effect of salinity on anatoxin producing freshwater cyanobacteria has not been reported, in contrast to its effect on cyanobacteria producing microcystins, nodularin, and paralytic shellfish toxins (Cirés et al., 2017 and reference therein).
Microcoleus anatoxicus tolerated a wide range of salinity and chloride concentrations corresponding to oligohaline and mesohaline conditions commonly found in estuaries (Montagna et al., 2013), suggesting that Microcoleus would grow in these environments which may in turn have management implications for its monitoring and mitigation. In contrast, Microcoleus potentially transported out of the estuaries into the coastal waters would be unlikely to survive as salinity above 9.3 ppt had damaging effect on cell physiology and significantly inhibited growth and toxin production. Microcoleus anatoxicus growth was remarkedly tolerant to oligohaline conditions considering that it was isolated from the freshwater Russian River (0.1–0.16 ppt). Microcoleus grew well at salinities between 1 and 7 ppt, and then, its growth (dry weight) and toxin formation declined at salinities above this level. High salinity is a chemical stressor that can alter the osmotic balance of the cells. Both the salinity and other osmotic stressors can change the water potential, inducing water loss and raising ionic concentration of the cell, which can decrease cellular growth and metabolism (Yadav et al., 2022). Most studies on the effect of salinity on freshwater benthic algae and cyanobacteria have focused on inland saline lakes, soil crusts and other extreme habitats (Wehr & Sheath, 2015). Additions of NaCl to soil biocrusts showed that Microcoleus vaginatus Gomont could survive 1% salinity (~ 10 ppt), though both salt and drought stresses inhibited microbial biomass and metabolism (Wu et al., 2022).
We observed a similar response with M. anatoxicus in that the cultures grew well at salinity levels below 7.8 ppt as evidenced by the final dry weight, even though there were obvious morphological changes in cells, such as constricted cell walls, abundant accumulation of large storage granules along cross-walls, and intense production of extracellular polysaccharide mucilage with multiple trichomes within a common sheath (Fig. 2d, g). It is tempting to speculate that these large storage granules are important in the storage of carbon as glycogen (Ortega-Martínez et al., 2023) and that the extracellular mucilage layers serve as protective mechanisms against damaging osmotic stress by creating a more favorable chemical environment beneath of the sheath than the ambient conditions. This study does not provide information about the chemical composition of the granules, and another hypothesis is that moderate salinity stress may promote luxury phosphorus uptake and storage of polyphosphate granules which can be used for subsequent growth when phosphorus concentrations are low (Rier et al., 2016). Though it was not quantified, all sea salts treatments, but not in NaCl by itself, stimulated production of earthy/musty odor compounds. Tee et al. (2021) detected geosmin and 2-methylisoborneol genes in both toxic and non-toxic Microcoleus strains, and our results suggest that salinity stress may initiate their expression in M. anatoxicus.
Moderate salinity stress up to 7.8 ppt did not change dhATX production significantly. Higher salinity (9.3–11.1 ppt) significantly suppressed dhATX production, but that could be due to detrimental effect of salinity on cell growth and the corresponding cellular collapse. Tonk et al. (2007) observed very similar responses in lab experiments with Microcystis, where microcystin production was more or less unaffected by salinity up to 10 ppt, and salinities above this concentration caused a collapse in cell growth. Our results indicate that M. anatoxicus has a high salinity tolerance for a strictly freshwater species and the observed physiological responses to salinity stress deserve further transcriptomic exploration. According to Tee et al. (2021), one proposed explanation for lower tolerance to salt stress of toxic Microcoleus species is expendable gene loss, more specifically, lack of genes for sucrose synthesis. This was not measured in these experiments. However, M. anatoxicus might have other mechanisms to cope with salt stress, including the formation of protective extracellular polysaccharide mucilaginous layers which function as mechanical and chemical barrier. Our study showed that low-levels of salinity may actually promote M. anatoxicus growth relative to control media. This should be further investigated using smaller increments of change in the salinity concentrations at these low levels. However, we have no evidence to support that there was a corresponding increase in toxin production with increasing salinity stress at these low levels.
Nitrogen depletion experiment
Previous nutrient lab experiments with Microcoleus autumnalis (Gomont) Strunecky, Komárek & J. R. Johansen strain CAWBG557 isolated from a stream in New Zealand, which produces all four anatoxin congeners, showed that low nitrogen levels reduced the cell density and increased the production of dhATX only (Heath et al., 2014). Rapala et al. (1993) found that growth in nitrogen-free media elevated anatoxin-a concentration in N2-fixing Anabaena and Aphanizomenon. Our N-depleted experiment with M. anatoxicus showed a similar trend. Microcoleus anatoxicus, although non-diazotrophic, was resilient to N-starvation and grew and produced both ATX and dhATX for the duration of the 40-day experiments. Nitrogen-depleted medium reduced its dry weight and increased the ATX production, although both changes were insignificant at the P = 0.05 level, while the effect on dhATX production was strong, with significant threefold increase. Heath et al. (2014) speculated that the increase in cellular anatoxins under nutrient stress may be linked to increased levels of free intracellular amino acid proline, which is precursor to ATX and homoanatoxin-a (Méjean et al., 2009). It is interesting to note that the N-rich microcystins decrease under nitrogen stress due to the presence of a global nitrogen regulatory element as part of their biosynthetic operon (Hellwegeret al., 2022); however, a similar regulation has not be reported for anatoxin biosynthesis.
Cell morphology of N-starved M. anatoxicus showed complete lack of storage granules (sign for depleted stored metabolites), pale coloration, and keritomized protoplast (e.g., “vacuolized” or “net-like”) caused by possibly widened thylakoids and damaged photosynthetic pigments. Keritomization of this type is indicative for generalized cell stress, including salts, light regime, or age, and is not specific to salinity stress (Komárek & Anagnostidis, 2005). The observed morphological changes under N-depletion could be indicative of the use of phycobilins as nitrogen source for cell metabolism leading to destruction of the phycobilisomes and reduced cell growth. Chlorosis or the recycling of pigment nitrogen in response to N-starvation is well studied in non-diazotrophic cyanobacteria (Allen & Smith 1969; Herrero et al., 2001; Sauer et al., 2001). During this process, N-rich pigments, predominantly phycocyanin contained within the phycobilisomes, are degraded to provide nitrogen needed for the cellular amino acid pools and the excess carbon is accumulated as carbon reserves, particularly glycogen (Gründel et al., 2012; Krauspe et al., 2022). A similar approach may be used by Microcoleus to cope with N-limitation. Pigment breakdown may not be the only approach used by Microcoleus in response to N-limitation. Nitrogen-rich environments have been found to promote the growth of toxic Microcoleus strains, as they contain an additional nitrate (NitT/TauT) transport system when compared to their non-toxic counterparts (Tee et al., 2021). It was suggested that toxic Microcoleus transport and store additional nitrogen under nutrient-rich conditions to utilize in N-depleted environments. Our cultures easily survived for 40 days in N-depleted media following their transfer from N-sufficient conditions. Tee et al. (2020, 2021) proposed that cyanophycin may be stored and catabolized as a nitrogen and/or carbon source and non-toxic strains harbor additional gene clusters, therefore, bolstering their ability to persist in low-nutrient environments. While our study presented evidence that a toxic-strain of M. anatoxicus can persist in and produce toxins in a N-depleted environment, the mechanism promoting their survival is unknown and requires further investigation.
Morphological observations of the filaments indicated there were physiological stress responses, but future transcriptomic studies are needed to reveal the genetic mechanisms for stress acclimation. The formation of intense extracellular polysaccharide sheaths in M. anatoxicus, especially at the mat formation stage, made RNA extraction and representative cell density counts challenging in the current experiments. Successful methods for RNA extraction and biomass estimate, such as pigment analysis, applied to shorter experiment duration will mitigate the difficulties caused by extracellular sheath accumulations as demonstrated by Kelly et al. (2019). Hence, the culture conditions used here should be viewed as pilot data for further studies of both environmental stressors at more steady discrete increments analyzed along the entire growth cycle.
Our lab experiments consistently showed that the extracellular toxin concentrations are much higher than the intracellular toxin concentrations in M. anatoxicus under all types of growth conditions. The current hypothesis is that the extracellular fraction of anatoxin congeners is due mainly to cell lysis (Testai, 2021; Wood et al., 2018); thus, it was a concern that the transport of the cell mats to Syracuse for toxin analysis could disrupt these Microcoleus mats and lead to toxin release from the cells prior to analysis. However, culturing conditions could influence the release of anatoxins to the extracellular fraction as well. The development of additional mucilaginous layers around the M. anatoxicus filaments under moderate salinity stress could stabilize the cells and increase the retention of anatoxin in the intracellular fraction. Additional experiments with M. anatoxicus from streams in California (unpublished data) showed that the strains from the Russian River studied here consistently produce more extracellular toxins, in contrast to strains from other rivers characterized by prevalence of intracellular toxin fraction. Similarly, Wood et al. (2018) documented dominance of extracellular (dissolved) anatoxins in two streams in New Zealand with M. autumnalis mats, and intracellular toxins within the mats only in one stream. Some cyanotoxins, for instance, cylindrospermopsins are believed to be actively exported outside the cells, due to the presence of a putative transporter (cyrK) in the gene cluster (Mihali et al., 2008). In Microcoleus we detected cell damage and visible cyanophycin leakage in the stationary phase of growth (Fig. 2 c, e) which may explain the high proportion of extracellular toxins.
Conclusion
We presented here the response of M. anatoxicus to an environmentally relevant salinity gradient and nitrogen-deplete conditions. Our results showed that M. anatoxicus has the potential to grow and produce toxins under mesohaline conditions (up to 9.3 ppt) with maximum growth in oligohaline waters with salinity up to 4.6 ppt. Microcoleus anatoxicus had a broad salinity tolerance, and moderate salinity stress did not increase dhATX production. In contrast, salinity above 9.3 ppt had detrimental effect on cell growth and significantly suppressed its dhATX production. Formations of a common sheath incorporating multiple filaments were observed at higher salinity and may serve as possible protection against increased osmotic stress. Microcoleus anatoxicus can grow for 40 days in N-depleted medium and form mats with significantly elevated dhATX concentration. Phycobilisome degradation was suggested as a possible acclimation response to N-starvation, as indicated by distinctly keritomized and pale cells observed in this culture medium. Our study showed the potential of controlled lab experiments with unialgal monoclonal Microcoleus strains to reveal the autecology of toxigenic Microcoleus species and inform environmentally relevant management decisions.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Allen, M. M. & A. J. Smith, 1969. Nitrogen chlorosis in blue-green algae. Archiv für Mikrobiologie 69: 114–120.
Anderson, B., J. Voorhees, B. Phillips, R. Fadness, R. Stancheva, J. Nichols, D. Orr & S. A. Wood, 2018. Extracts from benthic anatoxin-producing Phormidium are toxic to three macroinvertebrate taxa at environmentally relevant concentrations. Environmental Toxicology and Chemistry 37: 2851–2859.
Bouma-Gregson, K., M. R. Olm, A. J. Probst, K. Anantharaman, M. E. Power & J. F. Banfield, 2019. Impacts of microbial assemblage and environmental conditions on the distribution of anatoxin-a producing cyanobacteria within a river network. ISME 13: 1618–1634.
Bouma-Gregson, K., A. Crits-Christov, M. R. Olm, M. E. Power & J. F. Banfield, 2021. Microcoleus (Cyanobacteria) form watershed-wide populations without strong gradients in population structure. Molecular Ecology 31(1): 86–103. https://doi.org/10.1111/mec.16208.
Boyer, G. L., 2007. The occurrence of cyanobacterial toxins in New York lakes: Lessons from the MERHAB-Lower Great Lakes program. Lake and Reservoir Management 23: 153–160. https://doi.org/10.1080/07438140709353918.
Boyer, G. L., 2020. LCMS-SOP Determination of microcystins in water samples by high performance liquid chromatography (HPLC) with single quadrupole mass spectrometry (MS). protocols.io. https://doi.org/10.17504/protocols.io.bck2iuye
Cirés, S., M. C. Casero & A. Quesada, 2017. Toxicity at the edge of life: A review on cyanobacterial toxins from extreme environments. Marine Drugs 15(7): 233. https://doi.org/10.3390/md15070233.
Conklin, K. Y., R. Stancheva, T. Otten, R. Fadness, G. Boyer, B. Read, X. Zhang & R. G. Sheath, 2020. Molecular and morphological characterization of a novel dihydroanatoxin-a producing Microcoleus species (cyanobacteria) from the Russian River, California, USA. Harmful Algae 93: 101767.
Emerson, S. & J. Hedges, 2008. Geochemical mass balance: Dissolved chemical inflow and outflow from the ocean, Chemical Oceanography and the Marine Carbon Cycle. Cambridge University, Cambridge.
Gründel, M., R. Scheunemann, W. Lockau & Y. Zilliges, 2012. Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 158(12): 3032–3043. https://doi.org/10.1099/mic.0.062950-0.
Harland, F. M. J., S. A. Wood, E. Moltchanova, W. M. Williamson & S. Gaw, 2013. Phormidium autumnale Growth and Anatoxin-a Production under Iron and Copper Stress. Toxins 5(12): 2504–2521. https://doi.org/10.3390/toxins5122504.
Heath, M., S. A. Wood & K. G. Ryan, 2010. Polyphasic assessment of fresh-water benthic mat-forming cyanobacteria isolated from New Zealand. FEMS Microbiology Ecology 73(1): 95–109. https://doi.org/10.1111/j.1574-6941.2010.00867.x.
Heath, M., S. A. Wood & K. G. Ryan, 2011. Spatial and temporal variability in Phormidium mats and associated anatoxin-a and homoanatoxin-a in two New Zealand rivers. Aquatic Microbiology Ecology 64: 69–79. https://doi.org/10.3354/ame01516.
Heath, M. W., S. A. Wood, R. F. Barbieri, R. G. Young & K. G. Ryan, 2014. Effects of nitrogen and phosphorus on anatoxin-a, homoanatoxin-a, dihydroanatoxin-a and dihydrohomoanatoxin-a production by Phormidium autumnale. Toxicon 92: 179–185. https://doi.org/10.1016/j.toxicon.2014.10.014.
Heath, M., S. A. Wood, R. G. Young & K. G. Ryan, 2016. The role of nitrogen and phosphorus in regulating Phormidium sp. (cyanobacteria) growth and anatoxin production. FEMS Microbiology Ecology 92(3): fiw021. https://doi.org/10.1093/femsec/fiw021.
Hellweger, F. L., R. M. Martin, F. Eigemann, D. J. Smith, G. J. Dick & S. W. Wilhelm, 2022. Models predict planned phosphorus load reduction will make Lake Erie more toxic. Science 376(6596): 1001–1005. https://doi.org/10.1126/science.abm6791.
Herrero, A., A. M. Muro-Pastor & E. Flores, 2001. Nitrogen control in cyanobacteria. Journal of Bacteriology 183(2): 411–425. https://doi.org/10.1128/JB.183.2.411-425.2001.
Kelly, L. T., K. G. Ryan & S. A. Wood, 2019. Differential strain response in alkaline phosphatase activity to available phosphorus in Microcoleus autumnalis. Harmful Algae 89: 101664.
Komárek, J. & K. Anagnostidis, 2005. Cyanoprokaryota: Oscillatoriales. In Büdel, B., G. Gärtner, L. Krienitz & M. Schagerl (eds), Süsswasserflora von Mitteleuropa 19/2. Elsevier, München.
Krauspe, V., S. Timm, M. Hagemann & W. R. Hess, 2022. Phycobilisome breakdown effector NblD is required to maintain the cellular amino acid composition during nitrogen starvation. Journal of Bacteriology 204(1): e00158. https://doi.org/10.1128/jb.00158-21.
Méjean, A., S. Mann, T. Maldiney, G. Vassiliadis, O. Lequin & O. Ploux, 2009. Evidence that biosynthesis of the neurotoxic alkaloids anatoxin-a and homoanatoxin-a in the cyanobacterium Oscillatoria PCC 6506 occurs on a modular polyketide synthase initiated by L-proline. Journal of the American Chemical Society 131(22): 7512–7313. https://doi.org/10.1021/ja9024353.
Méjean, A., G. Paci, V. Gautier & O. Ploux, 2014. Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 91: 15–22. https://doi.org/10.1016/j.toxicon.2014.07.016.
Méjean, A., K. Dalle, G. Paci, S. Bouchonnet, S. Mann, V. Pichon & O. Ploux, 2016. Dihydroanatoxin-a is biosynthesized from Proline in Cylindrospermum stagnale PCC 7417: isotopic incorporation experiments and mass spectrometry analysis. Journal of Natural Products 79(7): 1775–1782. https://doi.org/10.1021/acs.jnatprod.6b00189.
Mihali, T. K., R. Kellmann, J. Muenchhoff, K. D. Barrow & B. A. Neilan, 2008. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Applied and Environmental Microbiology 74: 716–722.
Montagna, P. A., T. A. Palmer & J. B. Pollack, 2013. Hydrological Changes and Estuarine Dynamics, Springer, New York:, 94.
Neilan, B. A., L. A. Pearson, J. Muenchhoff, M. C. Moffitt & E. Dittmann, 2013. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environmental Microbiology 15(5): 1239–1253. https://doi.org/10.1111/j.1462-2920.2012.02729.x.
Ortega-Martínez, P., M. Roldán, S. Díaz-Troya & F. J. Florencio, 2023. Stress response requires an efficient connection between glycogen and central carbon metabolism by phosphoglucomutases in cyanobacteria. Journal of Experimental Botany 74(5): 1532–1550. https://doi.org/10.1093/jxb/erac474.
Puddick, J., R. van Ginkel, C. D. Page, J. S. Murray, H. E. Greenhough, J. Bowater, A. I. Selwood, S. A. Wood, M. R. Prinsep, P. Truman, R. Munday & S. C. Finch, 2021. Acute toxicity of dihydroanatoxin-a from Microcoleus autumnalis in comparison to anatoxin-a. Chemosphere 263: 127937. https://doi.org/10.1016/j.chemosphere.2020.127937.
R core team. 2022. R: A language and environment for statistical computing [online]. Vienna: R Foundation for Statistical Computing [viewed 4 December 2023]. Available from: https://www.R-project.org/
Rapala, J., K. Sivonen, R. Luukainen & S. I. Niemala, 1993. Anatoxin-a concentration in Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxic Anabaena strains in a laboratory study. Journal of Applied Phycology 5: 581–591. https://doi.org/10.1007/BF02184637.
Rier, S. T., K. C. Kinek, S. E. Hay & S. N. Francoeur, 2016. Polyphosphate plays a vital role in the phosphorus dynamics of stream periphyton. Freshwater Science 35: 490–502.
Sauer, J., U. Schreiber, R. Schmid, U. Völker & K. Forchhammer, 2001. Nitrogen starvation-induced chlorosis in Synechococcus PCC 7942. Low-level photosynthesis as a mechanism of long-term survival. Plant Physiol 126(1): 233–243.
Smith, Z. J., D. W. Conroe, K. L. Schulz & G. L. Boyer, 2020. Limnological differences in a two-basin lake help to explain the occurrence of anatoxin-a, paralytic shellfish poisoning toxins, and microcystins. Toxins 12(9): 559. https://doi.org/10.3390/toxins12090559.
Stancheva, R., R. G. Sheath, B. A. Read, K. D. McArthur, C. Schroepfer, J. P. Kociolek & A. E. Fetscher, 2013. Nitrogen-fixing cyanobacteria (free-living and diatom endosymbionts): their use in southern California stream bioassessment. Hydrobiologia 720: 111–127.
Tee, H. S., D. Waite, L. Payne, M. Middleditch, S. Wood & K. M. Handley, 2020. Tools for successful proliferation: diverse strategies of nutrient acquisition by a benthic cyanobacterium. The ISME Journal 14: 2164–2178. https://doi.org/10.1038/s41396-020-0676-5.
Tee, H. S., S. A. Wood, K. Bouma-Gregson, G. Lear & K. M. Handley, 2021. Genome streamlining, plasticity, and metabolic versatility distinguish co-occurring toxic and nontoxic cyanobacterial strains of Microcoleus. mBio 12: e02235. https://doi.org/10.1128/mBio.02235-21.
Testai, E., 2021. Anatoxin-a and analogues. In Chorus, I. & M. Welker (eds), Toxic Cyanobacteria in Water 2nd ed. CRC Press, Boca Raton.
Tonk, L., K. Bosch, P. M. Visser & J. Huisman, 2007. Salt tolerance of the harmful cyanobacterium Microcystis aeruginosa. Aquatic Microbial Ecology 46(2): 117–123. https://doi.org/10.3354/ame046117.
Valadez-Cano, C., A. Reyes-Prieto & J. Lawrence, 2023a. Novel virulent and temperate cyanophages predicted to infect Microcoleus associated with anatoxin-producing benthic mats. Environmental Microbiology. https://doi.org/10.1111/1462-2920.16527.
Valadez-Cano, C., A. Reyes-Prieto, D. G. Beach, C. Rafuse, P. McCarron & J. Lawrence, 2023b. Genomic characterization of coexisting anatoxin-producing and non-toxigenic Microcoleus subspecies in benthic mats from the Wolastoq, New Brunswick Canada. Harmful Algae 124: 102405. https://doi.org/10.1016/j.hal.2023.102405.
Wehr, J. D. & R. Sheath, 2015. Habitats of freshwater algae. Freshwater Algae of North America, . Academic Press, San Diego:, 13–74.
Wickham, H., W. Chang, L. Henry, T. L. Pedersen, K. Takahashi, C. Wilke, K. Woo, H.D. Yutani. 2023. Dunnington. ggplot2: Create elegant data visualizations using the grammar of graphics. In: R package version 4.3.1.
Wood, S. A., L. Biessy & J. Puddick, 2018. Anatoxins are consistently released into the water of streams with Microcoleus autumnalis-dominated (cyanobacteria) proliferations. Harmful Algae 80: 88–95.
Wood, S. A., L. T. Kelly, K. Bouma-Gregson, J. F. Humbert, H. D. Laughinghouse, J. Lazorchak, T. McAllister, A. McQueen, K. Pokrzywinski, J. Puddick, C. Quiblier, L. A. Reitz, K. Ryan, Y. Vadeboncoeur, A. Zastepa & T. Davis, 2020. Toxic benthic freshwater cyanobacterial proliferations: challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshwater Biology 65(10): 1824–1842. https://doi.org/10.1111/fwb.13532.
Wu, L., M. E. Farías, R. M. Torres, L. Xia, S. Song, A. A. Saber & S. Lan, 2022. Salinity affects microbial composition and function in artificially induced biocrusts: implications for cyanobacterial inoculation in saline soils. Soil Biology and Biochemistry 170: 108691. https://doi.org/10.1016/j.soilbio.2022.108691.
Yadav, P., R. P. Singh, S. Rana, D. Joshi, D. Kumar, N. Bhardwaj, R. K. Gupta & A. Kumar, 2022. Mechanisms of stress tolerance in cyanobacteria under extreme conditions. Stresses 2(4): 531–549. https://doi.org/10.3390/stresses2040036.
Acknowledgements
This work was funded by NSF-REU: 1852189 to PI Betsy Read and student mentor Rosalina Stancheva. Additional support was provided by NSF-URoL:EN: 2222322 to Ramesh Goel (PI) and Rosalina Stancheva (Co-PI), NIEHS (1P01ES028939-01) and NSF (OCE- 1840715) through the Great Lakes Center for Fresh Waters and Human Health at Bowling Green State University to Gregory Boyer for toxin measurements. We are thankful to the CSUSM NSF REU students Brianna Vegas, Allison Sullivan, David Castro, and CSUSM lab member Kim Conklin for the help with the lab experiments, and to Anne McElwee and Sydney Hall (SUNY-ESF) for assistance with the toxin extractions. The authors thank two anonymous reviewers and the Associate Editor in Chief of Hydrobiologia Sidinei M. Thomaz who helped us to improve the presentation of this study.
Author information
Authors and Affiliations
Contributions
RS contributed toward conceptualization, experimental design, student mentoring, data analysis, and article writing. SB contributed toward data analysis and article writing. GLB, BW, RG contributed toward toxin and chemistry analyses, and article writing. SH and NVK conducted experimental work. BR contributed toward student mentoring.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Yandong Pan, Steven T. Rier & Kalina Manoylov / Advances in Freshwater Algal Ecology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stancheva, R., Brown, S., Boyer, G.L. et al. Effect of salinity stress and nitrogen depletion on growth, morphology and toxin production of freshwater cyanobacterium Microcoleus anatoxicus Stancheva & Conklin. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05586-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05586-3