Abstract
The sea walnut, Mnemiopsis leidyi, has invaded and expanded throughout the whole Mediterranean Sea basin. Large blooms were recorded also in the Venice Lagoon (Italy), an ecosystem rich with biodiversity which supports multiple services, including artisanal fishery production. To investigate M. leidyi impacts on lagoon artisanal fisheries, we combined fishers’ local ecological knowledge, fishery landing time series analysis, and field sampling. Firstly, we interviewed artisanal fishers to date the blooms of M. leidyi. Secondly, we analyzed long-term fishery landings records to detect whether changes in landings quantity and composition were related to the ctenophore invasion. Thirdly, we sampled catches of the lagoon fyke nets. This interdisciplinary approach overcame the weaknesses of single methodologies and allowed us to reconstruct the temporal phases of M. leidyi invasion in the Venice Lagoon. Moreover, our results indicate that the lagoon landings significantly declined with the blooms, paralleled by the increase of water temperature. Finally, we showed that the mechanical obstruction of the nets, caused by the massive ctenophore blooms, strongly impacts fishing activities. Our results are a first step in assessing the short and long-term impacts of this invasive species on lagoon ecosystems, including its socioeconomic consequences, whose better understanding is fundamental to inform mitigation and adaptation measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are one of the biggest threats for native biodiversity in terrestrial, freshwater, and marine ecosystems (Sala et al., 2000; Halpern et al., 2008). Invasive alien species (IAS) are any non-native species that, when introduced accidentally or deliberately into a natural environment where they are not normally found, can cause serious negative impacts on their new environment. Specifically, IAS are recognized as one of the key drivers of environmental change due to their effects on biodiversity and ecosystem services (Pejchar & Mooney, 2009). In the marine environment, the management of IAS is particularly challenging because of the difficulty in detecting, controlling, and eventually eradicating them (Gallardo et al., 2016). In addition, climate changes might quicken the time of adaptation of marine IAS in non-native habitats (Hellmann et al., 2008).
The Mediterranean Sea is valued worldwide as a biodiversity hotspot due to its richness of habitats and an outstanding diversity of biota (Coll et al., 2010). Over the past decades, this enclosed sea has suffered environmental degradation due to the increased fishing pressure, the urbanization of coastal areas, pollution, habitat destruction, coastal eutrophication, and intense maritime traffic (Rilov & Galil, 2009). An additional threat to Mediterranean biodiversity is the increasing introductions of non-indigenous marine species, from the Atlantic through the strait of Gibraltar, from the Red Sea through the Suez Canal, and through ballast waters (Azzurro et al., 2019; Dragičević et al., 2019; Giangrande et al., 2020).
The lobate ctenophore Mnemiopsis leidyi A. Agassiz, 1865, commonly named warty comb jelly or sea walnut, is included in the list of the 100 of the world’s worst invasive species by the International Union for Conservation and Nature (IUCN) (Lowe et al., 2000). This species is native of the temperate and subtropical Atlantic coast of America, and since the early 1980s, it has started to invade European seas. M. leidyi was first recorded in the Black Sea in 1982 (Vinogradov et al., 1989), probably introduced through ballast waters from the Gulf of Mexico (Reusch et al., 2010; Ghabooli et al., 2011; Bayha et al., 2015). Its first bloom was registered in 1989 near the North-Eastern coast of the Black Sea, but the biomass quickly turned so massive (around 840 million tonnes) that it spread also to the whole basin (Vinogradov et al., 1989; Shiganova et al., 2019). The species kept blooming massively in the subsequent years with some interannual fluctuations, strictly related to the water temperature of the Black Sea (Shiganova 1998). The impact of M. leidyi on the Black Sea ecosystem was destructive: the main stock that suffered from the arrival of the species was the European anchovy Engraulis encrasicolus with a resulting crisis of the fishing industry, and the entire ecosystem underwent a regime shift (Vinogradov and Shushkina, 1992). From the Black Sea, M. leidyi spread throughout Ponto-Caspian basin and Mediterranean Sea (Shiganova et al 2019). In the early 2000s, M. leidyi entered the North and Baltic Seas through further invasions from the northern part of its native distribution range (e.g., Narragansett Bay) (Reusch et al., 2010; Ghabooli et al., 2011; Bayha et al., 2015).
The species is considered eurythermal and euryhaline thanks to its large range of tolerance to salinity and temperature in native areas (salinity: 1−46; temperature: 0–32 °C) (Costello et al., 2012) as well as in Eurasian seas (salinity: 6–40; temperature − 0.8−31 °C) (Shiganova 2020) with the growth optimum regionally depending on the combination of temperature, salinity, and prey concentration. Four factors seem to regulate the abundance of this species: water temperature, salinity, food availability, and predation pressure (Costello et al., 2012). The high fecundity characterizing M. leidyi (reviewed in Shiganova 2020) represents one of the most important factors supporting its ecological success in invaded regions. The highest fecundity observed in the Mediterranean was experimentally registered in coastal areas of the northern Adriatic Sea (Malej et al., 2017). Massive blooms of M. leidyi are typically experienced in eutrophic ecosystems such as lagoons, which are highly biologically productive environments (Condon & Steinberg, 2008), as seen in the S’Ena Arrubia Lagoon (Diciotti et al., 2016), the Venice Lagoon and the Marano and Grado Lagoon (Malej et al., 2017), and in France (Marchessaux et al., 2020; Marchessaux & Belloni, 2021).
The Northern Adriatic Sea is a semi-enclosed basin, which has experienced profound ecological changes over the past decades due to multiple anthropogenic pressures and climate change. It is one of the most socio-economically important basins of the Mediterranean, but also one of the most human-impacted due to human activities taking place at sea (e.g., fishing), on the coasts (e.g., tourism), or in its catchment (e.g., large human settlements, industrial, livestock, and agricultural production and associated nutrient and pollutant emissions) (Barausse et al., 2011; Lotze et al., 2011; Giani et al., 2012; Fortibuoni et al., 2017). In the Adriatic Sea, M. leidyi was first sighted in 2005 in the Slovenian waters of the Gulf of Trieste (Shiganova & Malej, 2009) and, after that, it was not seen for a decade, i.e., until 2016, when its first bloom was registered (Malej et al., 2017). Since then, M. leidyi has massively bloomed each summer in the northern Adriatic Sea and it has also been observed in the southern Adriatic Sea (Dragičević et al., 2019; Tirelli et al., 2021).
The Venice Lagoon, located along the coast of the Northern Adriatic Sea, is the largest lagoon of the entire Mediterranean Sea. Being a highly productive ecosystem, this lagoon seems to be an ideal place for the spreading of this ctenophore as well for other IAS and non-indigenous species (in the Venice Lagoon, 71 non-indigenous species were censused in 2015 by Marchini et al., 2015) mainly arrived through ballast waters (Galil et al., 2014). The Venice Lagoon is an important area for small-scale fisheries; in particular, fyke nets have been used by fishers in this area for centuries, and the spread of gelatinous plankton such as ctenophores could have a significant impact on these traditional fisheries, which represents an ancient cultural heritage. The massive proliferation of Mnemiopsis leidyi in lagoons may have strong negative impacts on artisanal fisheries (Diciotti et al., 2016; Marchessaux et al., 2022), but an assessment of the impact of M. leidyi on fisheries in the Venice Lagoon has not yet been conducted. To fill this gap, this study uses an interdisciplinary approach involving local ecological knowledge of fishermen (LEK), analysis of landing records in the lagoon, and field sampling, with the following aims: (1) reassessing the occurrence of M. leidyi in the Venice Lagoon, (2) reconstructing past changes in its abundance in this ecosystem, and (3) assessing its impact on local small-scale fisheries.
Materials and methods
Study area
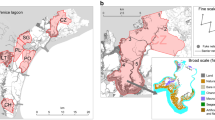
The Venice Lagoon (North-Eastern Italy) is the largest (550 km2) Mediterranean coastal lagoon. It is a shallow transitional environment with mean depth around 1 m (Molinaroli et al., 2009). It can be divided into three sectors corresponding to the three inlets which connect the lagoon to the sea: the northern (Lido inlet), central (Malamocco inlet), and southern basin (Chioggia inlet) (Fig. 1). This ecosystem experiences strong temporal and spatial variations in environmental parameters such as temperature, salinity, turbidity, and dissolved oxygen due to the combined effect of tides and the freshwater inflow of a few local rivers (Solidoro et al., 2010). The Venice Lagoon hosts a great diversity of habitats (i.e., salt marshes, seagrass meadows, subtidal and intertidal flats, and meandering creeks) that provide multiple ecosystem services (Franzoi et al., 2010; Rova et al., 2015; Roner et al., 2016). This ecosystem has been heavily impacted by several human pressures over the past decades, including: nutrient inputs due to the large human population and its activities (e.g., agriculture, industries) in the mainland; pollution by heavy metals and other contaminants mainly connected to the presence of a large industrial park (Porto Marghera) on the border between the mainland and the lagoon; aquaculture; fishing activities including impacting activities such as mechanical clam fishing; widespread erosion of tidal forms and connected loss of biodiversity due to a combination of negative sediment budget and faster currents (associated with large-scale hydraulic engineering works to allow the transit of large vessels), sediment resuspension by mechanical clam fishing, waves generated by motorboats, sea-level rise, groundwater abstraction, and the recent operation of storm-surge barriers to protect Venice from high tides (D’Alpaos, 2010; Solidoro et al., 2010; Tognin et al., 2022).
Sampling sites in the Venice Lagoon. The yellow dots indicate the sampling sites of the fyke nets belonging to lagoon fishers. The red dot indicates the Hydrobiological Station 'Umberto d’Ancona' of the University of Padova, sited in Chioggia, where water temperature was measured. Picture from Google Earth Pro (v. 7.3.4.8642)
Local ecological knowledge (LEK) surveys
A structured interview was distributed to local fishers in August 2020 to elicit their knowledge about the arrival and spread of M. leidyi and their perceptions of its impact on their own fishing activities. The interview was created using KoBoToolbox software (https://www.kobotoolbox.org; see SI 1). The interview was administrated to 10 experienced local small-scale lagoon fishers, a number which covers a large fraction (30–40%) of the active professional lagoon fishers, also considering that fishers most often have additional crew members, and is thus well representative of the artisanal fishery activity in the Venice Lagoon, where, at the time of the survey, there were about 25–30 fishers regularly using fyke nets as a fishing tool. The combined activities of the interviewed fishers covered all the three Lagoon basins, thus ensuring geographical representativeness of the survey. All the fishers use fyke nets (Fig. 2) as fishing gear and they had previously collaborated with the researchers from the University of Padova (Italy). The interview took an average of 30 min to complete and contained both closed and open-ended questions. It consisted of 14 questions divided into 3 parts facilitating the study of the relations between M. leidyi and local artisanal fishery. The first part included questions about the years when M. leidyi was first sighted and the years when the ctenophore became a problem for the respondent’s fishing activities. This information was used to reconstruct the years of appearance and spread of the ctenophore and thus divide the years into periods of absence, presence, and bloom (henceforth, periods of ‘occurrence’). The second part assessed the target species of fishing activities and whether fishers noticed a decrease in the catch of these target species after the arrival of the ctenophore. The final part of the interview was designed to explore the socioeconomic impact of the M. leidyi blooms, specifically the economic losses due to the presence of M. leidyi and how fishers attempt to avoid such losses.
Traditional fyke nets of the Venice Lagoon. a an example, seen from above, of how the single fyke nets (shown in the inset) can be deployed; arrows indicate how target species encounter the net barrier (represented by the continuous line) and are forced to move into the fyke nets (represented by shaded areas); b picture of a fyke net in the Venice Lagoon
Lagoon fishery landing records
Time series of official landing data at the wholesale fish market of Chioggia (Mazzoldi et al., 2014) were used to evaluate changes in fishery production. The dataset was kindly provided by the Chioggia fish market. The dataset reports the landings of the Chioggia’s fishing fleet and includes boats operating both at the sea and in the southern part of the Venice Lagoon. Data were available as landings in kg per day between 1997 and 2019; only data referring to the Lagoon fishery, as identifiable from the market bill records, were extracted and included in the analyses. Monthly and annual totals were calculated by summing daily records for total landings and for four main species. The four selected species are the most profitable among the fisher’s target species and represent their most abundant catches in the Venice Lagoon, as confirmed by the interviews, where they are almost exclusively caught by fyke nets. These species are as follows:
-
Grass goby (Zosterisessor ophiocephalus (Pallas, 1814)), a non-migratory bottom dwelling fish that inhabits seagrass meadows of the Lagoon (Scaggiante et al., 1999).
-
Mediterranean green crab (Carcinus aestuarii Nardo, 1847), a euryhaline and eurythermal Portunid crab that occupies the Venice Lagoon waters for its entire life cycle (Marino et al., 2010). According to the recording procedures, C. aestuarii is divided into two landing categories: C. aestuarii, referring to the ripe female (locally known as 'mazaneta'), and C. aestuarii, referring to crabs immediately after molting (or 'moeca') (Mazzoldi et al., 2014). The regular landings of the other less valuable crab life stages were not considered here.
-
Common cuttlefish (Sepia officinalis Linnaeus, 1758), a cephalopod that migrates to the Venice Lagoon to lay the eggs during spring (Lazzarini et al., 2006).
-
Big-scale sand smelt (Atherina boyeri Risso, 1810), a small euryhaline atherinid fish that inhabits shallow waters of the Lagoon and coastal marine environments (Kara & Quignard, 2019).
The total number of fishing days in the Lagoon in two seasons (winter and summer) was also calculated (SI, Fig. S1) to verify that the variations in landings over time revealed by our analyses were not simply due to changes in fishing effort.
Water temperature data
Daily lagoon surface water temperature records measured over 1997–2019 using a multiparametric probe (in degrees Celsius, with an approximation of 0.01 °C) were taken from the long-term dataset collected at the Hydrobiological Station “Umberto d’Ancona” of the University of Padova sited in Chioggia (southern part of the Venice Lagoon) (Fig. 1). We considered also the water surface temperature at sea, to test the effect of both lagoon and sea temperatures on the occurrence of M. leidyi. To do this, we extracted data from the NOAA ERSST v5 dataset (Huang et al., 2015, 2017). These data are reanalyzed sea surface temperature values expressed in degree Celsius and were estimated for the full GSA 17 (Northern and Central Adriatic). Sea surface temperatures for both the Lagoon and the sea were averaged per year and over seasons, which were defined as Winter (December of the previous year, January, February), Spring (March, April, May), Summer (June, July, August), and Autumn (September, October, November).
Statistical analyses
To verify whether the changes in landings are related to the periods of absence, presence, and bloom (i.e., occurrence) of M. leidyi reported by the fishers in the interviews, we performed a non-metric multidimensional scaling analysis (nMDS) using Euclidean distances, with landings of the four selected species (Z. ophiocephalus, C. aestuarii (“Moeca” and “Mazaneta” stages), S. officinalis, and A. boyeri) as variables (Mead, 1992). After calculating the distances between years, we grouped years using hierarchical clustering (following Ward’s distance method; Murtagh & Legendre, 2014). The resulting groups, representing different periods of change in landings, were compared with the results of the fishers’ interviews and, based on their coherence (see Results), were used as proxy indicators to define the different temporal phases of the spread of M. leidyi. From 1997 to 2019, we distinguished three periods, named as absence, presence, and bloom periods.
Since water temperature is a known driver of the population dynamics of M. leidyi (Costello et al., 2012), we tested if annual changes in sea and lagoon seasonal surface temperatures were significantly related with the periods of occurrence of M. leidyi resulting from nMDS using multinomial logistic regression. Multinomial logistic regression is a classification method used to classify a nominal-dependent variable given one or more independent variables (Menard, 2002). Multinomial logistic regression extends the logistic regression algorithm to solve multiclass problems (e.g., the nominal variable has more than two categories) (Eq. 1). Logistic regressions are usually written as follows:
where ß0 is the intercept, and ß1, …, n are the slopes, X are the control variables, and the logit function is used as the link function to transform the outcomes into log odds. The multinomial logistic regression is an extension of this model in which the outcome, instead of being the log odds, becomes the log odds of the probability of one category over the probability of a base category. We performed multiple multinomial logistic regressions using as response variable the annual M. leidyi occurrence (i.e., absence = A, presence = P, and bloom = B), as previously defined, and as annual explanatory variables different seasonal sea or lagoon temperatures (°C, indicated as T in Eqs. 2 and 3). Having 3 categories (i.e., A, P, B), our model can be solved with two equations and our reference category was set as A (Eqs. 2, 3).
We built separated models for each control variable in order to better discriminate the effects of the different seasons on the occurrence of M. leidyi. A total of 8 models were fitted, using as control variables, the surface temperature of the sea or the lagoon in spring (March to May), summer (June to August), autumn (September–November), or winter (December–February). The interpretation of the model is given by the ß coefficients and the associated P-values.
To check whether the occurrence of M. leidyi was related to changes in landings in the lagoon, we built simple linear models. We used total annual landings of the four target species as the response variable, and we tested them for temporal autocorrelation before constructing the models (SI, Fig. S2). To achieve normality, the landings data were transformed taking their natural logarithm. We used the occurrence of M. leidyi as a categorical explanatory variable and the lagoon water temperature as a continuous explanatory variable. Before starting the analyses, we run the Fligner test (Conover et al., 1981) to check for the homogeneity of variances in the three levels of the categorical variable (absence, presence, blooms) (Chi-square = 3.373, p = 0.18; therefore, the null hypothesis of equal variances was not rejected). We ran the models without and with an interaction between the effect of temperature and of the occurrence of M. leidyi (Eqs. 4, 5, respectively).
where ß0 is the coefficient of the intercept, ß1and ß2 are the coefficients of the slopes of the two predictors which are temperature TL and occurrence OCC, and ß3 is the coefficient of the interaction term.
We performed a model selection based on the Akaike Information Criterion (AIC; Cavanaugh & Neath, 2019) between these two alternative models. Finally, we checked the residuals for normality to verify the validity of the model (SI, Fig. S3). To picture the effect of M. leidyi on the single species, we created boxplots of landings versus the different occurrence periods of M. leidyi.
Data analysis was carried out with R (Vers. 4.0.5) and RStudio (Vers. 1.4.1106). The packages used were vegan for the nMDS (Dixon, 2003) and nnet for the multinomial logistic regression (Ripley et al., 2016).
Field samplings
To understand if M. leidyi can mechanically affect fishing activities, we performed 6 field sampling campaigns during peak months of the bloom period in the Venice Lagoon (Fig. 1): late spring, summer (in particular), and early autumn (Camatti et al., 2023; Schroeder et al., 2023). Nowadays, the most widespread traditional fishery in the Venice Lagoon is based on the use of fyke nets (locally called “cogolli”). Organisms enter the net when, following the flow of lagoon currents, they hit into barrier nets which drive them inside the fyke net. In the Venice Lagoon, the traditional fyke net is a funnel shaped mesh, deployed by fishers in series connected by mesh barriers (Fig. 2). In this way, organisms are forced to follow the barrier nets that direct them inside the funnel of the fyke nets at the end of the barriers. Usually, the fyke nets are deployed for 24 to 72 h, but we decided to standardize the deployment to 24 h to avoid losing some catches due to the deterioration within the net (e.g., because of predation by other captured organisms) or due to excessive clogging connected to M. leidyi biomass. In addition, during the summer, fishers tend to shorten the deployment time of the nets as the increased water temperature can degrade the quality of the catch. We sampled a total of 70 fyke nets by checking for the presence and abundance of ctenophores: 12 fyke nets in the northern lagoon (sampling dates: July 22, 2020, and September 17, 2020), 17 fyke nets in the central lagoon (sampling dates: July 21, 2020, and September 01, 2020), and 41 in the southern Lagoon (sampling dates: August 04, 2020, and September 16, 2020). After a 24-h deployment, each net was loaded aboard a boat with the help of fishers and emptied into a plastic container, from which we removed algae and any debris, which were usually negligible compared to the catch and M. leidyi. Then, the entire contents of each net (i.e., fished organisms including M. leidyi) were placed in a bucket (12 l) and weighted using a dynamometer (Dr. Meter ES-PS01 max 110 lb/50 kg with a sensitivity of 1 g). Then, the weight of M. leidyi in the sample was measured separately. If the total amount of the catch was too high (> 100 kg), the same measurements were made on a subsample equal to about one-fourth of the total amount of the total fyke net content. After that, the weight of ctenophores was transformed in fraction of catch by dividing the kg of ctenophores by the kg of total catches.
Results
Six of the total 10 fishers interviewed lived in Chioggia (southern basin of the Venice Lagoon), two fishers lived in Venice (central basin), and two fishers lived in Burano (northern basin). 90% of the fishers reported seeing M. leidyi for the first time between 2014 and 2016, with one fisher reporting seeing the ctenophore for the first time in 2009. All fishers reported no blooms before 2014, and most of them remembered that M. leidyi became a problem for their fishing activity, i.e., that the species bloomed and caused economic damage for the first time, in 2014–2016, the years when most of them first saw it. The first bloom, indeed, was in 2014 for 10% of the respondents, in 2015 for 40% of the respondents, in 2016 for 20% of the respondents, in 2017 for 20% of the respondents, and in 2018 for 10% of the respondents. All the fishers agreed that the years when M. leidyi most damaged their fishing activity were 2018 (10% of respondents) or 2019 (90% of respondents), and that ctenophore biomass increased year after year with a related decrease in the catches of target species. Based on the interviews, we identified three distinct periods of M. leidyi occurrence in the Venice Lagoon: a period of absence (A) from 1997 to 2008, a period of presence (P) from 2009 to 2013, and a period of bloom (B) from 2014 to 2019 (Fig. 3a). In the absence of direct field observations during this period, we chose 2009 as the start of the period P based on the interview of a single fisher, who is, however, very experienced and considered to be reliable based on the knowledge of the authors who regularly work with him and other fishers in the Venice Lagoon. Instead, all other fishers first spotted the ctenophore in 2014–2016. Also, the beginning of the bloom period in 2014 was reported by only one fisher, and an equally valid year for the beginning of blooms could be 2015, as given by 4 fishers. Regarding seasonality, in the years after it started blooming, M. leidyi was absent from waters or present in lower quantities in winter months according to all respondents.
nMDS of the Lagoon landings and the construction of the time series of occurrence of M. leidyi. a Temporal division of the occurrence of M. leidyi in 3 periods based on the interviews of 10 local fishers: absence (A) from 1997 to 2008, presence (P) from 2009 to 2013, and bloom (B) from 2014 to 2019. b Nonmetric multidimensional scaling (nMDS) (stress = 0.1) of the main five landing categories for the Lagoon fishery, showing three clusters based on the hierarchical clustering analysis. The clusters corresponded well to the periods indicated by the fishers and were thus indicated as absence (A) from 1997 to 2009, presence (P) from 2010 to 2013, and bloom (B) from 2014 to 2019. Species associated with each cluster (and thus more abundant) are indicated, and proximity indicates the association with the cluster. c The new temporal division in the periods A, P, and B based on the clusters found with the nMDS and compared with the fishers’ interviews
The main target species indicated by the fishers were C. aestuarii ‘Moeca’ stage, C. aestuarii ‘Mazaneta’ stage, A. boyeri, Z. ophiocephalus, and S. officinalis (SI 1). The nMDS of landings of these species showed 3 clusters with a good quality of graphical representation (stress = 0.1, Clarke et al., 2014; Fig. 3b): the first cluster includes data from 1997 to 2009 and is associated with higher landings of S. officinalis; the second cluster includes data from 2010 to 2013; Z. ophiocephalus is associated with both the first and second clusters; the last cluster includes data from 2014 until 2019 and is associated with a higher abundance of C. aestuarii. Instead, A. boyeri is associated with all the three clusters. The clusters identified by nMDS combined with hierarchical clustering corresponded almost perfectly to the periods of M. leidyi occurrence observed by the fishers. Therefore, we kept this last result as the final time series of M. leidyi occurrence (Fig. 3c), which we then used in further analyses: proxy period of absence (or A: 1997–2009), presence (or P: 2010–2013), and blooms (or B: 2014–2019). Total landings of the four key target species averaged 264049 kg/year during absence, 185864 kg/year during presence, and 157175 kg/year during bloom periods, with average landings per year decreasing by 40.47% between the absence and bloom periods (full annual trends are reported in the SI, Fig. S4). These changes in landings do not seem ascribable to changes in fishing effort, as the number of fishing days did not change systematically over time (SI, Fig. S1).
The multinomial logistic regression showed a statistically significant relationship between water temperature and the occurrence of M. leidyi. Using the lagoon temperature, we found that warmer winter, summer, and autumn water surface temperatures in the Lagoon led to a higher probability of blooms of M. leidyi (Table 1). Instead, there was no effect of temperature that could discriminate between the absence and the mere presence of the ctenophore. In the sea temperature model, we found that a warmer sea surface temperature in autumn and winter led to a higher probability of blooms of M. leidyi, and again, there was no difference between the presence and absence. The model fit was evaluated using the pseudo-R squared, calculated for the multinomial logistic regression as the McFadden R squared (pseudoRsquared = 1—(ln (Loglikelihood model) / (ln (loglikelihood null model)). A good model fit is between 0.2 and 0.4 of McFadden pseudo-R squared (Table 1). The annual mean surface water temperature in the Lagoon increased over time, from 15.47 °C during the absence period, to 16.79 °C during the presence period, and 17.22 °C during the bloom period (full interannual trends are reported in the SI, Fig. S5). The increase of annual average water temperature in the lagoon from the absence to the bloom period was statistically significant (Table 1) and equal to + 11.3%, corresponding to a strong warming of + 1.75 °C over a period of slightly more than two decades, in agreement with what reported by Camatti et al. (2023).
To evaluate the effect of the occurrence of M. leidyi on the total landings in the lagoon and account for its possible cumulative and synergistic effects with temperature, we built linear models (Eqs. 4, 5). The best model was identified based on the difference between the AIC of the two alternative models (Eqs. 4, 5). Since the difference in AIC was small (ΔAIC = 3), the best model was chosen using the principle of parsimony (Burnham & Anderson, 2002) and so it was the simplest model (without the interaction term, which could be taken to represent potential synergistic effects). The model shows a significant effect of the occurrence of M. leidyi on the total landings, while temperature was not significant (Table 2). In particular, the model identified a statistically significant decrease in landings between the absence and the bloom period but not between the absence and the presence period (Table 3, Fig. 4a). The relationship with temperature was positive over the three periods but not significant. The model has an R2 = 0.32, which is not bad given the simple adopted model but which suggests that other factors might play a role in the decline of the total landings.
Effect of M. leidyi on the Venice Lagoon landings. a Effect of M. leidyi and water temperature on the annual landings (1997–2019) of the four target species in the Venice Lagoon (the vertical axis reports the natural logarithm of the landings expressed in kg); b–e Box plots of the landings of the single species (the vertical axis reports the natural logarithm of the landings expressed in kg) and divided in the three periods A, P, and B; where b box plots for C. aestuarii (both Mazanete and Moeche), c box plots for Z. ophiocephalus, d box plots for S. officinalis, e box plots for A. boyeri. The box plots represent the mean (the wide black line) and the interquartile range (the box), while lines represent the lowest and the uppermost quartile
Looking at the effect of ctenophores on the single target species, we found that landings of Z. ophiocephalus and S. officinalis decreased with the onset of periods of presence and bloom of ctenophore (Fig. 4c, d). Instead, C. aestuarii and A. boyeri landings showed an unclear trend over time, possibly increasing or remaining stable during the presence and bloom periods of M. leidyi (Fig. 4b, e).
To understand how M. leidyi could impact the activity of the fishers, and so the landings, we performed fyke net sampling in the lagoon. In the central and the southern areas of the lagoon, we observed similar mean values of the percentage of weight in fyke nets composed by M. leidyi during our fishery sampling activities, typically representing more than 50% of the net weight (Fig. 5). In many cases (black dots), the net was completely (100% weight) filled by ctenophores (Fig. 5). The situation was partially different in the northern lagoon, where the average percentage of M. leidyi in the net was 25% and on many occasions, no M. leidyi was found in the fyke nets, although high values of filling were occasionally observed (Fig. 5).
Percentage wet weight of M. leidyi in the fyke nets in 2020. a From left to right, the results obtained in the three basins of the Venice Lagoon are shown: northern lagoon (LN), central lagoon (LC), and southern lagoon (LS). Box plots show median (dotted line), mean (the thick black line), interquartile range (the box), and raw data (dots), while lines represent the lowest and the uppermost quartile. b A fyke net completely obstructed with M. leidyi (left) and the content of a fyke net poured into a plastic container (right): the biomass of the ctenophore is predominant
Discussion
We adopted an interdisciplinary approach to assess the spread of an invasive alien species over time and its impact on a small-scale lagoon fishery. Our study relied on the local ecological knowledge of fishers in the Venice Lagoon obtained through interviews, official landings records of the Lagoon fishery from the Fish Market of Chioggia (Italy), and experimental data collected in the field by scientific observers who were on board with fishers. With this approach, we were able to confirm M. leidyi as a successful widespread invader in the Venice Lagoon and to show the clear negative impact of this ctenophore on an artisanal fishery. Our results suggest that after the arrival of M. leidyi, its blooms in the Venice Lagoon were probably favored by the warming temperature of these coastal waters. Indeed, sea surface temperature increased over time, and its value in cold periods (autumn and winter; the latter is a season when the ctenophore is rarer or absent according to our interviews with fishers) was a good predictor of the blooms after 2014, similarly to water temperatures in the lagoon whose autumn, winter, and summer values were even better bloom predictors. While of course these results represent correlations, they are in line with the scientific literature that identifies water temperature as one of the key drivers of M. leidyi abundance (Costello et al., 2006, 2012).
Traditional fishers are valuable sentinels of the Venice Lagoon ecosystem since they work daily in the lagoon visiting many areas. They are likely able to detect changes in the large and difficult to access lagoon environments as soon as they take place, often earlier than scientists. A consistent finding of our interviews, supported by 70% of the fishers, points out that M. leidyi started to bloom in the Venice Lagoon in a period between 2014 and 2016, with the reported differences about timing possibly ascribable to different memories or, also, to geographically heterogeneous and maybe relatively short-lived first blooms in the Venice Lagoon. The fishers’ local ecological knowledge therefore suggests that blooms of M. leidyi may have begun a year or two before the first bloom in 2016 reported in the scientific literature in the Northern Adriatic Sea and in the Venice Lagoon (Malej et al., 2017). This timing leads us to even suggest the hypothesis that the spread of M. leidyi into the Northern Adriatic Sea may have occurred in one or more successive waves starting from the Venice Lagoon, which is frequently visited by cruise and cargo ships that could act as an introduction vector for such invasive species, and whose biological productivity together with the observed increasingly warmer waters could have favored the wintering and reproduction of M leidyi. The connectivity of the lagoon with other areas of the Adriatic Sea may have contribute to fuel this process.
Our analyses of the Venice Lagoon landing records show three distinct groups of landings that correspond well with the periods identified by the fishers as periods of absence, presence and bloom of M. leidyi. Through statistical models and analyses, we were able to show that changes in the composition of landings of key lagoon target species clearly matched the periods of absence, presence, and blooms of the ctenophore reported by fishers. The amount of total landings also markedly declined from the absence to the bloom period. These results suggest that the different levels of M. leidyi abundance, possibly favored by long-term warming across the three A–P–B periods, could have impacted the local fishery.
The analysis of the fishery records confirmed the decrease of landings reported by 90% of the interviewed fishers, especially in the case of important fishery resources such as grass goby (Z. ophiocephalus) and cuttlefish (S. officinalis). Interestingly, landings of C. aestuarii increased during the M. leidyi bloom period compared to the period of presence, but their average was similar to the period of M. leidyi absence; this species may have been more influenced by other factors such as temperature increase, which may also affect its fishery by extending the seasonal window of its molting phase and, thus, of its sale, or it may have benefited from possible restructuring of the food web due to the presence of M. leidyi, such as release from predatory pressure.
In general, changes in fishing effort and catchability are also possible explanations that should be considered when examining landing trends, but our estimate of the average number of fishing days per fisher per year has not changed significantly over time, and we have no reason to believe that fishers’ (mostly traditional) fishing gears has changed substantially, affecting catchability, over the investigated timeframe. Alternatively, another explanation of the overall negative landing trend could be a direct impact of increased temperature over time on lagoon communities (Pranovi et al., 2016). Indeed, the fact that M. leidyi occurrence and water temperature both display clear changes over time make it hard to distinguish their effects on landings using statistical analysis of time series alone, although the effect of the ctenophore occurrence seems to be stronger than that of temperature since the latter predictor was not found to be statistically significant in our linear models. These results call for further analyses to investigate species-specific trends in landings, which would help to clarify which are the traits of resilient or vulnerable fishery resources to M. leidyi invasions and to warming in changing lagoon environments.
Although we were able to establish a clear relationship between the abundance of M. leidyi and changes in landings of Lagoon species based on local ecological knowledge and landings data, these analyses alone could not establish if this was simply a correlation or a causal relationship. Several factors may have played a role and influenced fisheries production in the Lagoon over the past two decades, such as warming, climate change (Bartolini et al., 2013; Bellafiore et al., 2014), changes in ecosystem productivity (Bertolini et al., 2021; Berti et al., 2022), and salt marsh erosion and changes in Lagoon hydrodynamics related to multiple human interventions over the past decades (to which the effect of the recent extensive construction activities and operation of the MOSE system of storm-surge barriers should be added) (Carniello et al., 2009; Umgiesser, 2020; Tognin et al., 2022). So, while the integration of landings and LEK gave us more confidence about the reliability of our temporal description of the occurrence and possible impacts on landings of M. leidyi, the samplings done in the lagoon were fundamental to clarify this causality issue by showing that M. leidyi has indeed a strong physical impact on fisheries through its blooms which can fill and clog the fishers’ nets. Based on such samplings, this study presents novel evidence of the direct negative impact of M. leidyi on artisanal fisheries in the lagoon. During the M. leidyi blooms, this invasive ctenophore species often filled 100% of fyke nets. By filling the net chambers, ctenophores prevented fish and other target species from entering the net. Moreover, fishers were not able to raise the net onboard when more than about 80% of the fyke net was filled with ctenophores, because they were too heavy. Therefore, in these cases, they had to cut the net and release the content into the water, losing all the catches of the day and damaging the nets, thus adding a further economic loss. The apparently lower percentage weight of the ctenophore in the fyke nets of the northern Lagoon warrants the investigation of possible spatial differences in the invasion and of their reason; for example, the northern Lagoon receives higher freshwater inputs from rivers, which may inhibit the invader (Jaspers et al., 2011). Although trophic interactions were not the subject of this study, we emphasize that, in addition to the observed negative mechanical effect, M. leidyi could have a direct impact on the food web functioning, as it is a voracious predator that feeds mainly on zooplankton, as also recently confirmed in the Venice Lagoon where it preferentially feeds on meroplanktonic species (e.g., decapod larvae and the slow-swimming larvae of gastropods and bivalvae) (Camatti et al., 2023; Schroeder et al., 2023). Because zooplankton is a key food source for fishery target species at larval stage and for planktivorous adults of small pelagic fish, M. leidyi has the potential to negatively impact many fishery resources, in addition to their food web, through competition. Also, important fishery resources in the Venice Lagoon such as crabs and clams have planktonic early life stages, which seem to be preferentially preyed by M. leidyi similarly to those of other meroplanktonic taxa (Schroeder et al., 2023), therefore the direct predatory impact of this ctenophore on the lagoon food web and fisheries should not be neglected.
In any case, regardless of the mechanism, we can conclude that M. leidyi has most likely had a strong negative impact on the artisanal fisheries in the Venice Lagoon over the last decade. Yet, as mentioned above, we have to take into account that numerous environmental changes have occurred in the Venice Lagoon during the last years that could be alternative or additional explanations for the landings trend, even acting synergistically with M. leidyi. These changes include water warming and other climate changes, reduced nutrient concentrations in water, morphological changes such as salt marsh erosion and deepening of lagoon bottoms, changes in hydrodynamics and the sediment transport regime including those due the ongoing works aimed to protect Venice from high tide events (Tommasini et al., 2019; Bertolini et al., 2021; Anzidei et al., 2022; Berti et al., 2022; Basili et al., 2022; Finotello et al., 2022; Mel et al., 2022; Tognin et al., 2022).
The interdisciplinary combination of LEK, landing data analysis, and field sampling allowed us to overcome the limitations of the single methodologies and highlight their remarkable consistency and, hence, the robustness of our results about the impact over time of M. leidyi on the traditional Lagoon fishery. On the one hand, for instance, the LEK of fishers, while based on many fishing days per year in the Lagoon both in summer and in winter (SI, Fig. S1), mostly relies on their own experience and memory, which can potentially lead to errors (Lima et al., 2017). Examples of this methodological issue are the identification of the start of the bloom period, on which fishers slightly disagree, or of the extent of presence period P (2009–2013): in fact, a certain degree of incertitude is inherent and well known in invasion science (Leung et al., 2012). While the definition of the presence period is consistent with the year of first sight of M. leidyi in the nearby Northern Adriatic Sea, which is 2005 (Shiganova & Malej, 2009), the limited sample size may call into question the precision of such estimate. Indeed, the ctenophore could have reached the Venice Lagoon sooner (it was first recorded in 2005 in the northern Adriatic Sea) or later. On the other hand, the statistical clusters emerged from Lagoon landings analysis are consistent with the information reported by the fishers and closely mirror the time periods derived from interviews.
To our knowledge, this work is the first demonstration of the impacts of M. leidyi in the Venice Lagoon, the largest lagoon of the Mediterranean Sea and part of the UNESCO’s World Heritage List which comprises the site “Venice and its Lagoon” in recognition of the long-term evolution of the city of Venice and the surrounding Lagoon ecosystem. The traditional small-scale fishery considered in this study is an important and still lively component of the long-term human-nature relationship in the Venice Lagoon and an example of cultural heritage. The strong negative impacts by M. leidyi on local artisanal fishery, taking place since 2014–2016 and reported here for the first time, could bring to the total loss of this traditional type of fishing in this lagoon, thus impacting both the local economy and the Venetian cultural heritage. The bloom of the invasive ctenophore, favored by the warming temperatures of the Lagoon waters, appears as one of the plausible reasons of the reduction of landings of some main fishing target species, such as Z. ophiocephalus and S. officinalis; this impact, be it due to mechanical or trophic reasons or a combination of them, takes place in a context of a Lagoon whose waters have become cleaner (Berti et al., 2022) and so less productive, making the situation for the local fishery even more complicated. The economic quantification of the damages to fisheries and of the losses in terms of ecosystem services due to this invasive ctenophore should be urgently carried out to raise awareness and contribute to a better knowledge base for mitigation and adaptation measures in the Venice Lagoon. This study demonstrates the usefulness of an interdisciplinary approach to study marine invasive species and their impacts. Further studies should clarify the direct and indirect biological impacts of M. leidyi on the Venice Lagoon food web, including their relationship to the distribution of this invader at finer spatial and temporal (e.g., seasonal) scales, which is an especially important task when considering that lagoon environments are important nursery areas for several species of fishery and conservation relevance.
Data availability
The fishery landings data from this manuscript are publicly available from the Figshare Dryad repository (https://doi.org/10.6084/m9.figshare.24009069).
Change history
30 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10750-024-05583-6
References
Anzidei, M., M. Crosetto, J. Navarro, C. Tolomei, P. Patias, C. Georgiadis, A. Vecchio, F. Doumaz, L. Trivigno, A. Falciano, M. Greco, E. Serpelloni, S. Torresan, Q. Gao, A. Barra, C. Ferrari, C. Tenderini, X. Loizidou & D. Orthodoxou, 2022. Relative sea-level rise scenarios for 2100 in the Venice Lagoon by integrated geodetic data, high-resolution topography and climate projections. New insights from the SAVEMEDCOASTS-2 Project (No. EGU22-5138). Copernicus Meetings. https://doi.org/10.5194/egusphere-egu22-5138, 2022
Azzurro, E., V. Sbragaglia, J. Cerri, M. Bariche, L. Bolognini, J. B. Souissi, G. Busoni, S. Coco, A. Chryssanthi, E. Fanelli, R. Ghanem, J. Garrabou, F. Gianni, F. Grati, J. Kolitari, G. Latterio, L. Lipej, C. Mazzoldi, N. Milone, F. Pannacciulli, A. Pešić, Y. Samuel-Rhoads, L. Saponari, J. Tomanic, N. E. Topçu, G. Vargiu & P. Moschella, 2019. Climate change, biological invasions, and the shifting distribution of Mediterranean fishes: a large-scale survey based on local ecological knowledge. Global Change Biology 25: 2779–2792. https://doi.org/10.1111/gcb.14670.
Barausse, A., A. Michieli, E. Riginella, L. Palmeri & C. Mazzoldi, 2011. Long-term changes in community composition and life-history traits in a highly exploited basin (northern Adriatic Sea): the role of environment and anthropogenic pressures. Journal of Fish Biology 79: 1453–1486. https://doi.org/10.1111/j.1095-8649.2011.03139.x.
Bartolini, F., A. Barausse, H. O. Pörtner & F. Giomi, 2013. Climate change reduces offspring fitness in littoral spawners: a study integrating organismic response and long-term time-series. Global Change Biology 19: 373–386. https://doi.org/10.1111/gcb.12050.
Bayha, K. M., M. H. Chang, C. L. Mariani, C. L. J. L. Richardson, D. L. Edwards, T. S. DeBoer, C. Moseley, E. Aksoy, M. B. Decker, P. M. Gaffney, G. R. Harbison, J. H. McDonald & A. Caccone, 2015. Worldwide phylogeography of the invasive ctenophore Mnemiopsis leidyi (Ctenophora) based on nuclear and mitochondrial DNA data. Biol Invasions 17: 827–850. https://doi.org/10.1007/s10530-014-0770-6.
Basili, M., S. M. Techtmann, L. Zaggia, G. M. Luna & G. M. Quero, 2022. Partitioning and sources of microbial pollution in the Venice Lagoon. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2021.151755.
Bellafiore, D., M. Ghezzo, D. Tagliapietra & G. Umgiesser, 2014. Climate change and artificial barrier effects on the Venice Lagoon: inundation dynamics of salt marshes and implications for halophytes distribution. Ocean & Coastal Management 100: 101–115. https://doi.org/10.1016/j.ocecoaman.2014.08.002.
Berti, M., F. Scardia, C. Carrer & F. Sorrentino, 2022. Analysis of a comprehensive monthly dataset on nitrogen, phosphorus and organic carbon in the Venice Lagoon waters (Italy). EQA-International Journal of Environmental Quality 49: 1–11. https://doi.org/10.6092/issn.2281-4485/14871.
Bertolini, C., E. Royer & R. Pastres, 2021. Multiple evidence for climate patterns influencing ecosystem productivity across spatial gradients in the Venice Lagoon. Journal of Marine Science and Engineering 9: 363. https://doi.org/10.3390/jmse9040363.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed. Springer, New York:
Camatti, E., F. Acri, A. De Lazzari, N. Nurra, M. Pansera, A. Schroeder & A. Bergamasco, 2023. Natural or anthropogenic variability? A long-term pattern of the zooplankton communities in an ever-changing transitional ecosystem. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2023.1176829.
Carniello, L., A. Defina & L. Dalpaos, 2009. Morphological evolution of the Venice lagoon: evidence from the past and trend for the future. Journal of Geophysical Research. https://doi.org/10.1029/2008JF001157.
Cavanaugh, J. E. & A. A. Neath, 2019. The Akaike information criterion: background, derivation, properties, application, interpretation, and refinements. Wiley Interdisciplinary Reviews: Computational Statistics. https://doi.org/10.1002/wics.1460.
Clarke, K. R., R. N. Gorley, P. J. Somerfield & R. M. Warwick, 2014. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, PRIMER-E, Plymouth:
Coll, M., C. Piroddi, J. Steenbeek, K. Kaschner, F. B. R. Lasram, J. Aguzzi, E. Ballesteros, C. N. Bianchi, J. Corbera, T. Dailianis, R. Danovaro, M. Estrada, C. Froglia, B. S. Galil, J. M. Gasol, R. Gertwagen, J. Gil, F. Guilhaumon, K. Kesner-Reyes, M.-S. Kitsos, A. Koukouras, N. Lampadariou, E. Laxamana, C.M.L.-F. de la Cuadra, H. K. Lotze, D. Martin, D. Mouillot, D. Oro, S. Raicevich, J. Rius-Barile, J. Ignacio, S. Salinas, C. S. Vicente, S. Somot, J. Templado, X. Turon, D. Vafidis, R. Villanueva & E. Voultsiadou, 2010. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE 5: 89. https://doi.org/10.1371/journal.pone.0011842.
Condon, R. H. & D. K. Steinberg, 2008. Development, biological regulation, and fate of ctenophore blooms in the York River estuary, Chesapeake Bay. Marine Ecology Progress Series 369: 153–168. https://doi.org/10.3354/meps07595.
Conover, W. J., M. E. Johnson & M. M. Johnson, 1981. A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics 23: 351–361. https://doi.org/10.2307/1268225.
Costello, J. H., B. K. Sullivan, D. V. Gifford, D. Van Keuren & L. J. Sullivan, 2006. Seasonal refugia, shoreward thermal amplification, and metapopulation dynamics of the ctenophore Mnemiopsis leidyi in Narragansett Bay, Rhode Island. Limnology and Oceanography 51: 1819–1831. https://doi.org/10.4319/lo.2006.51.4.1819.
Costello, J. H., K. M. Bayha, H. W. Mianzan, T. A. Shiganova & J. E. Purcell, 2012. Transitions of Mnemiopsis leidyi (Ctenophora: Lobata) from a native to an exotic species: a review. Hydrobiologia 690: 21–46. https://doi.org/10.1007/s10750-012-1037-9.
D’Alpaos, L., 2010. Fatti e misfatti di idraulica lagunare: La laguna di Venezia dalla diversione dei fiumi alle nuove opere delle bocche di porto, Grafiche editore, Lamezia Terme:
Dixon, P., 2003. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 14: 927–930. https://doi.org/10.1111/j.1654-1103.2003.tb02228.x.
Diciotti, R., J. Culurgioni, S. Serra, M. Trentadue, G. Chessa, C. T. Satta, T. Caddeo, A. Lugliè, N. Sechi & N. Fois, 2016. First record of Mnemiopsis leidyi (Ctenophora, Bolinopsidae) in Sardinia (SEna Arrubia Lagoon, Western Mediterranean): a threat to local fishery? Mediterranean Marine Science 17: 714–719. https://doi.org/10.12681/mms.1719.
Dragičević, B., O. Anadoli, D. Angel, M. Benabdi, G. Bitar, L. Castriota, F. Crocetta, A. Deidun, J. Dulčić, D. Edelist, V. Gerovasileiou, S. Giacobbe, A. Goruppi, T. Guy-Haim, E. Konstantinidis, Z. Kuplik, J. Langeneck, A. Macali, I. Manitaras, N. Michailidis, E. Michaloudi, P. Ovalis, C. Perdikaris, R. Pillon, S. Piraino, W. Renda, J. Rizgalla, A. Spinelli, J. Tempesti, F. Tiralongo, V. Tirelli, K. Tsiamis, C. Turan, N. Uygur, B. Zava & A. Zenetos, 2019. New Mediterranean Biodiversity Records (December 2019). https://doi.org/10.12681/mms.20913
Finotello, A., R. M. Capperucci, A. Bartholomä, A. D’Alpaos & M. Ghinassi, 2022. Morpho-sedimentary evolution of a microtidal meandering channel driven by 130-years of natural and anthropogenic modifications of the Venice Lagoon (Italy). Earth Surface Processes and Landforms 47: 2580–2596. https://doi.org/10.1002/esp.5396.
Fortibuoni, T., O. Giovanardi, F. Pranovi, S. Raicevich, C. Solidoro & S. Libralato, 2017. Analysis of long-term changes in a Mediterranean marine ecosystem based on fishery landings. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2017.00033.
Franzoi, P., A. Franco & P. Torricelli, 2010. Fish assemblage diversity and dynamics in the Venice Lagoon. Rendiconti Lincei 21: 269–281. https://doi.org/10.1007/s12210-010-0079-z.
Galil, B. S., A. Marchini, A. Occhipinti-Ambrogi, D. Minchin, A. Narščius, H. Ojaveer & S. Olenin, 2014. International arrivals: widespread bioinvasions in European Seas. Ethology Ecology & Evolution 26: 152–171. https://doi.org/10.1080/03949370.2014.897651.
Gallardo, B., M. Clavero, M. I. Sánchez & M. Vilà, 2016. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology 22(1): 151–163. https://doi.org/10.1111/gcb.13004.
Ghabooli, S., T. A. Shiganova, A. Zhan, M. E. Cristescu, P. Eghtesadi-Araghi & H. J. MacIsaac, 2011. Multiple introductions and invasion pathways for the invasive ctenophore Mnemiopsis leidyi in Eurasia. Biological Invasions 13: 679–690. https://doi.org/10.1007/s10530-010-9859-8.
Giangrande, A., C. Pierri, M. Del Pasqua, C. Gravili, M. C. Gambi & M. F. Gravina, 2020. The Mediterranean in check: Biological invasions in a changing sea. Marine Ecology. https://doi.org/10.1111/maec.12583.
Giani, M., T. Djakovac, D. Degobbis, S. Cozzi, C. Solidoro & S. F. Umani, 2012. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuarine, Coastal and Shelf Science 115: 1–13. https://doi.org/10.1016/j.ecss.2012.08.023.
Halpern, B. S., S. Walbridge, K. A. Selkoe, C. V. Kappel, F. Micheli, C. D’Agrosa, J. F. Bruno, K. S. Casey, C. Ebert, H. E. Fox, R. Fujita, D. Heinemann, H. S. Lenihan, E. M. P. Madin, M. T. Perry, E. R. Selig, M. Spalding, R. Steneck & R. Watson, 2008. A global map of human impact on marine ecosystems. Science 319: 948–952. https://doi.org/10.1126/science.1149345.
Hellmann, J. J., J. E. Byers, B. G. Bierwagen & J. S. Dukes, 2008. Five potential consequences of climate change for invasive species. Conservation Biology 22: 534–543. https://doi.org/10.1111/j.1523-1739.2008.00951.x.
Huang, B., V. F. Banzon, E. Freeman, J. Lawrimore, W. Liu, W. Peterson & T. C. Zhang, 2015. Extended reconstructed sea surface temperature version 4 (ERSST. v4). Part I: upgrades and intercomparisons. Journal of Climate 28: 911–930. https://doi.org/10.1175/JCLI-D-14-00006.1.
Huang, B., P. W. Thorne, V. F. Banzon, T. Boyer, G. Chepurin, J. H. Lawrimore, W. Liu, T. C. Peterson, T. M. Smith, P. W. Thorne, S. D. Woodruff & H. M. Zhang, 2017. NOAA extended reconstructed sea surface temperature (ERSST), version 5. NOAA National Centers for Environmental Information 30: 8179–8205. https://doi.org/10.1175/JCLI-D-16-0836.1.
Jaspers, C., L. F. Møller & T. Kiørboe, 2011. Salinity gradient of the Baltic Sea limits the reproduction and population expansion of the newly invaded comb jelly Mnemiopsis leidyi. PLoS ONE 6: 8. https://doi.org/10.1371/journal.pone.0024065.
Kara, M. H. & J. P. Quignard, 2019. Fishes in Lagoons and Estuaries in the Mediterranean 3A: Migratory Fish, Wiley, New York:
Lazzarini, R., J. Favretto & M. Pellizzato, 2006. Experimentation for the management of Sepia officinalis L. in the Venice Lagoon. Biologia Marina Mediterranea 13: 741–744.
Leung, B., N. Roura-Pascual, S. Bacher, J. Heikkilä, L. Brotons, M. A. Burgman, K. Dehnen-Schmutz, F. Essl, P. E. Hulme, D. M. Richardson, D. Sol & M. Vilà, 2012. TEASIng apart alien species risk assessments: a framework for best practices. Ecology Letters 15(12): 1475–1493. https://doi.org/10.1111/ele.12003.
Lima, M. S. P., J. E. L. Oliveira, M. F. de Nóbrega & P. F. M. Lopes, 2017. The use of Local Ecological Knowledge as a complementary approach to understand the temporal and spatial patterns of fishery resources distribution. Journal of Ethnobiology and Ethnomedicine 13: 1–12. https://doi.org/10.1186/s13002-017-0156-9.
Lotze, H. K., M. Coll & J. A. Dunne, 2011. Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems 14: 198–222. https://doi.org/10.1007/s10021-010-9404-8.
Lowe, S., M. Browne, S. Boudjelas & M. De Poorter, 2000. 100 of the world's worst invasive alien species: a selection from the global invasive species database). Published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), 12pp. First published as special lift-out in Aliens 12, December 2000.
Malej, A., V. Tirelli, D. Lučić, P. Paliaga, M. Vodopivec, A. Goruppi, S. Ancona, M. Benzi, N. Bettoso, E. Camatti, M. Ercolessi, C. R. Ferrari & T. Shiganova, 2017. Mnemiopsis leidyi in the northern Adriatic: here to stay? Journal of Sea Research 124: 10–16. https://doi.org/10.1016/j.seares.2017.04.010.
Marchessaux, G. & B. Belloni, 2021. Expansion of Mnemiopsis leidyi in the French Mediterranean lagoons along the Gulf of Lion. Journal of Sea Research 168: 7. https://doi.org/10.1016/j.seares.2021.101995.
Marchessaux, G., V. Faure, C. Chevalier & D. Thibault, 2020. Refugia area for the ctenophore Mnemiopsis leidyi A Agassiz 1865 in the Berre Lagoon (southeast France): the key to its persistence. Regional Studies in Marine Science 39: 8. https://doi.org/10.1016/j.rsma.2020.101409.
Marchessaux, G., D. Thibault & C. Claeys, 2022. An interdisciplinary assessment of the impact of invasive gelatinous zooplankton in a French Mediterranean lagoon. Biological Invasions 25: 499–518. https://doi.org/10.1007/s10530-022-02930-3.
Marchini, A., J. Ferrario, A. Sfriso & A. Occhipinti-Ambrogi, 2015. Current status and trends of biological invasions in the Lagoon of Venice, a hotspot of marine NIS introductions in the Mediterranean Sea. Biological Invasions 17: 2943–2962. https://doi.org/10.1007/s10530-015-0922-3.
Marino, I. A. M., F. Barbisan, M. Gennari, F. Giomi, M. Beltramini, P. M. Bisol & L. Zane, 2010. Genetic heterogeneity in populations of the Mediterranean shore crab, Carcinus aestuarii (Decapoda, Portunidae), from the Venice Lagoon. Estuarine, Coastal and Shelf Science 87: 135–144. https://doi.org/10.1016/j.ecss.2010.01.003.
Mazzoldi, C., A. Sambo & E. Riginella, 2014. The Clodia database: a long time series of fishery data from the Adriatic Sea. Scientific Data. https://doi.org/10.1038/sdata.2014.18.
Mead, A., 1992. Review of the development of multidimensional scaling methods. Journal of the Royal Statistical Society 41: 27–39. https://doi.org/10.2307/2348634.
Mel, R. A., M. Bendoni & D. Steffinlongo, 2022. Salt-marsh retreat on different time scales: issues and prospects from a 5-year monitoring campaign in the Venice Lagoon. Earth Surface Processes and Landforms 47: 1989–2005. https://doi.org/10.1002/esp.5359.
Menard, S., 2002. Applied Logistic Regression Analysis, Sage Publications, Inc., New York:
Molinaroli, E., S. Guerzoni, A. Sarretta, M. Masiol & M. Pistolato, 2009. Thirty-year changes (1970 to 2000) in bathymetry and sediment texture recorded in the Lagoon of Venice sub-basins, Italy. Marine Geology 258: 115–125. https://doi.org/10.1016/j.margeo.2008.12.001.
Murtagh, F. & P. Legendre, 2014. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? Journal of Classification 31: 274–295. https://doi.org/10.1007/s00357-014-9161-z.
Pejchar, L. & H. A. Mooney, 2009. Invasive species, ecosystem services and human well-being. Trends in Ecology & Evolution 24: 497–504. https://doi.org/10.1016/j.tree.2009.03.016.
Pranovi, F., M. Anelli Monti, D. Brigolin & M. Zucchetta, 2016. The influence of the spatial scale on the fishery landings-SST relationship. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2016.00143.
Reusch, T., S. Bolte, M. Sparwell, A. Moss & J. Javidpour, 2010. Microsatellites reveal origin and genetic diversity of Eurasian invasions by one of the world’s most notorious marine invader, Mnemiopsis leidyi (Ctenophora). Molecular Ecology 19: 2690–2699. https://doi.org/10.1111/j.1365-294X.2010.04701.x.
Rilov, G. & B. Galil, 2009. Marine bioinvasions in the Mediterranean Sea–history, distribution and ecology. Biological Invasions in Marine Ecosystems 204: 549–575. https://doi.org/10.1007/978-3-540-79236-9_31.
Ripley, B., W. Venables & M. B. Ripley, 2016. Package ‘nnet.’ R Package Version 7: 700.
Roner, M., A. D’Alpaos, M. Ghinassi, M. Marani, S. Silvestri, E. Franceschinis & N. Realdon, 2016. Spatial variation of salt-marsh organic and inorganic deposition and organic carbon accumulation: inferences from the Venice Lagoon, Italy. Advances in Water Resources 93: 276–287. https://doi.org/10.1016/j.advwatres.2015.11.011.
Rova, S., F. Pranovi & F. Müller, 2015. Provision of ecosystem services in the lagoon of Venice (Italy): an initial spatial assessment. Ecohydrology & Hydrobiology 15: 13–25. https://doi.org/10.1016/j.ecohyd.2014.12.001.
Sala, O. E., F. S. Chapin Iii, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Global biodiversity scenarios for the year 2100. Science 287(5459): 1770–1774. https://doi.org/10.1126/science.287.5459.1770.
Scaggiante, M., C. Mazzoldi, C. W. Petersen & M. B. Rasotto, 1999. Sperm competition and mode of fertilization in the grass goby Zosterisessor ophiocephalus (Teleostei: Gobiidae). Journal of Experimental Zoology 283: 81–90. https://doi.org/10.1002/(SICI)1097-010X(19990101)283:1%3c81::AID-JEZ9%3e3.0.CO;2-9.
Schroeder, A., E. Camatti, M. Pansera & A. Pallavicini, 2023. Feeding pressure on meroplankton by the invasive ctenophore Mnemiopsis leidyi. Biological Invasions 25: 2007–2021. https://doi.org/10.1007/s10530-023-03023-5.
Shiganova, T. A., 1998. Invasion of the Black Sea by the ctenophore Mnemiopsis leidyi and recent changes in pelagic community structure. Fisheries Oceanography 7: 305–310. https://doi.org/10.1046/j.1365-2419.1998.00080.x.
Shiganova, T. A., 2020. Adaptive strategies of Mnemiopsis leidyi A. Agassiz 1865 in different environments of the Eurasian seas. Marine Pollution Bulletin 161: 8. https://doi.org/10.1016/j.marpolbul.2020.111737.
Shiganova, T. & A. Malej, 2009. Native and non-native ctenophores in the Gulf of Trieste, Northern Adriatic Sea. Journal of Plankton Research 31: 61–71. https://doi.org/10.1093/plankt/fbn102.
Solidoro, C., V. Bandelj, F. Aubry Bernardi, E. Camatti, S. Ciavatta, G. Cossarini, C. Facca, P. Franzoi, S. Libralato, D. M. Canu, R. Pastres, F. Pranovi, S. Raicevich, G. Socal, A. Sfriso, M. Sigovini, D. Tagliapietra & P. Torricelli, 2010. Response of Venice Lagoon ecosystem to natural and anthropogenic pressures over the last 50 years. Coastal Lagoons: Critical Habitats of Environmental Change 8: 483–511.
Shiganova, T. A., U. Sommer, J. Javidpour, J. C. Molinero, A. Malej, A. S. Kazmin, M. Isinibilir, E. Christou, I. Siokou- Frangou, M. Marambio, V. Fuentes, Z. A. Mirsoyan, N. Gülsahin, F. Lombard, M. K. S. Lilley, D. L. Angel, B. S. Galil, D. Bonnet & F. Delpy, 2019. Patterns of invasive ctenophore Mnemiopsis leidyi distribution and variability in different recipient environments of the Eurasian seas: a review. Marine Environmental Research 152: 8. https://doi.org/10.1016/j.marenvres.2019.104791.
Tirelli, V., A. Goruppi, R. Riccamboni & M. Tempesta, 2021. Citizens’ eyes on Mnemiopsis: how to multiply sightings with a click! Diversity 13: 224. https://doi.org/10.3390/d13060224.
Tognin, D., A. Finotello, A. Dalpaos, D. P. Viero, M. Pivato, R. A. Mel, A. Defina, E. Bertuzzo, M. Marani & L. Carniello, 2022. Loss of geomorphic diversity in shallow tidal embayment promoted by storm-surge barriers. Science Advances. https://doi.org/10.1126/sciadv.abm8446.
Tommasini, L., L. Carniello, M. Ghinassi, M. Roner & A. D’Alpaos, 2019. Changes in the wind-wave field and related salt-marsh lateral erosion: inferences from the evolution of the Venice Lagoon in the last four centuries. Earth Surface Processes and Landforms 44: 1633–1646. https://doi.org/10.1002/esp.4599.
Umgiesser, G., 2020. The impact of operating the mobile barriers in Venice (MOSE) under climate change. Journal for Nature Conservation 54: 125783. https://doi.org/10.1016/j.jnc.2019.125783.
Vinogradov, M. E., E. A. Shushkina, E. I. Musayeva & P. Y. Sorokin, 1989. A newly acclimated species in the Black Sea: the ctenophore Mnemiopsis leidyi (Ctenophora: Lobata). Oceanology 29: 220–224.
Vinogradov, M. E. & E. A. Shushkina, 1992. Temporal changes in community structure in the open Black Sea. Oceanology 32: 485–491.
Acknowledgements
We thank the fishers and their representatives who made their time and knowledge available to contribute to this research, in particular “Cooperativa Pescatori San Marco di Burano” and “Cooperativa Clodiense”. We thank Andrea Sambo and Cristina Breggion for their unvaluable support in the field work.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. FPi was supported by the PhD fellowship “PON research and innovation” (funded by the European Union) on “Marine Invasive Species and fisheries in the Venice Lagoon and Northern Adriatic Sea.” CS was supported by the Marie Sklodowska Curie fellowship through the project RESET—Resilience estimation to SET management goals in marine ecosystems, grant agreement n. 101065994, under the HORIZON-MSCA-2021-PF. This work was partially carried out by AB and CM in the context of the PRID project “Don’t leave the modeller alone: integrating time series analysis with stakeholder engagement to identify causes of change in coastal ecosystems” (BIRD209409/20) funded to AB by the University of Padova—Dept. of Biology. In the case of AB, this study was also partly carried out within the RETURN Extended Partnership and received funding from the European Union NextGenerationEU (National Recovery and Resilience Plan – PNRR, Mission 4, Component 2, Investment 1.3—D.D. 1243 2/8/2022, PE00000005). AB and VT gratefully acknowledge the support of the National Biodiversity Future Center—NBFC, funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU (Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, Project title “National Biodiversity Future Center - NBFC”). This work reflects only the authors’ view; the European Commission and their executive agency are not responsible for any use that may be made of the information the work contains.
Author information
Authors and Affiliations
Contributions
FPi, FPo, CS, VT, DB, CM, and AB conceptualized the study and contributed to the design of the study. FPi and FPo carried out the field samplings and interviewed the fishers. CS, FPi, and AB contributed to analyzing and visualizing the data. AB, FPi, FPo, and CS led the writing of the manuscript with input from all co-authors. All authors contributed to writing the manuscript and approved its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Sidinei M. Thomaz, Cécile Fauvelot, Lee B. Kats, Jonne Kotta & Fernando M. Pelicice / Aquatic Invasive Species IV
The original version of this article has been revised: The Supplementary information has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piccardi, F., Poli, F., Sguotti, C. et al. Assessing the impact of the invasive ctenophore Mnemiopsis leidyi on artisanal fisheries in the Venice Lagoon: an interdisciplinary approach. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05505-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05505-6