Abstract
Cyanobacterial blooms have a strong impact on the food web structure, interactions and ecosystem functioning. The aim of this study was to describe the seasonal changes in composition and abundance of heterotrophic nanoflagellates, ciliates, rotifers, and crustaceans in relation to algae and nutrients in a shallow eutrophic lake (north-eastern Poland) dominated by cyanobacteria and exposed to the strong impact of cormorants. Our results showed that algae accounted for a small part of the total phytoplankton abundance (9–40%) and biomass (10–21%) and were dominated by diatoms and cryptophytes. All of the studied groups of planktonic organisms were quite rich in species (95 algal, 79 ciliate, 44 rotifer and 25 crustacean species) and relatively abundant. Copepods formed a substantial part (45–83%) of the total zooplankton biomass during all seasons. Relatively low algal to zooplankton biomass ratio (0.8–1.1) suggests that during spring, summer, and winter algae were not sufficient food resources for metazooplankton, which supplemented its diet with protists (heterotrophic nanoflagellates and ciliates). In a shallow lake dominated by cyanobacteria, winter (ice-covered period) may be a more favourable period for the growth of some groups of algae, ciliates and rotifers than other seasons due to decreasing abundance of cyanobacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplankton species composition and size structure are regulated directly by zooplankton grazing and indirectly by nutrient regeneration by grazers (Levine et al., 1999; Mao et al., 2020). According to the concept of cascading trophic interactions, predation by fish can control the abundance and productivity of lower trophic levels, cascading down to phytoplankton (Carpenter et al., 1985). It is also documented that phytoplankton biomass is mainly regulated by bottom-up factors, while phytoplankton species composition is mainly determined by top-down factors (Lemmens et al., 2018). Expanding population of piscivorous birds, such as cormorants, can have both bottom-up (nutrient runoff from colonies increasing algal growth) and top-down impacts (predation on fish leading to a decrease in fish communities around colonies, potentially causing a trophic cascade and higher grazing pressure on phytoplankton due to higher herbivore abundances) on lower trophic levels (Gagnon et al., 2016). In some lake ecosystems, the high contribution of cyanobacteria to the total phytoplankton abundance is observed at the sites near the cormorant [Phalacrocorax carbo (Linnaeus, 1758)] roost (Klimaszyk et al., 2015; Napiórkowska-Krzebietke et al., 2020). Although the impact of cormorants on the primary producers distinctly decreases with the increasing distance from a breeding cormorant colony, it can be visible up to 1.6 km from the colony area (Napiórkowska-Krzebietke et al., 2020).
Cyanobacteria are poor-quality food for zooplankton grazers, due to their potential toxicity (Tillmanns et al., 2008), large size (Lampert, 1987) and lack of long-chained polyunsaturated fatty acids and sterols (Müller-Navarra et al., 2004). Although some zooplankton species (cladocerans and cyclopoid copepods) can ingest cyanobacteria (Ger et al., 2014; Tõnno et al., 2016), they select alternative food when available (Gebrehiwot et al., 2019). Cyanobacterial blooms have a strong impact on food web structure, interactions and ecosystem functioning (Van Wichelen et al., 2016; Josué et al., 2019). Intense summer/autumn blooms of cyanobacteria are a common phenomenon in eutrophic lakes (e.g. Krevš et al., 2010; Tõnno et al., 2016). However, some species, belonging to genera such as Microcystis (Havens et al., 2003; Ma et al., 2016; Josué et al., 2019), Pseudanabaena, Aphanizomenon, Planktothrix, and Limnothrix (Napiórkowska-Krzebietke et al., 2021) may create year-round blooms due to increasing temperature and nutrient enrichment.
Algae are composed of many diverse taxonomic groups and/or species with various environmental requirements (mainly to light and temperature) as well as with different availability and nutritional value for zooplankton (Reynolds, 2006; Napiórkowska-Krzebietke, 2017). Cryptophytes are the most preferred food resource for ciliates (Müller & Schlegel, 1999), rotifers (Bogdan & Gilbert, 1982), cladocerans (Thys et al., 2003) and copepod nauplii (Hansen & Santer, 1995), due to high content of essential fatty acids (Sommer, 1981). Chrysophytes and euglenoids may also be a food resource for some ciliate and crustacean species (Posch et al., 2015). Although diatoms contain high concentrations of highly unsaturated fatty acids (Brett et al., 2009), they are generally avoided by crustaceans because of their size and shape (Tõnno et al., 2016). Dinoflagellates are often inedible to zooplankton (Reynolds, 2006), but Ceratium hirundinella (O.F.Müller) Dujardin may be a suitable food source for advanced copepod nauplii and adult cyclopoid copepods (Sommer et al., 2003).
The interactions between cyanobacteria and different groups/species of zooplankton from temperate to tropical freshwater systems are widely discussed (e.g. Ger et al., 2014; Krztoń et al., 2017; Kosiba et al., 2019; Napiórkowska-Krzebietke et al., 2020). Relatively little is known about other phytoplankton groups, zooplankton communities, algae–zooplankton relationships, and predator–prey interactions among different groups of zooplankton in response to the prolonged blooms of cyanobacteria (Gołdyn & Kowalczewska-Madura, 2008; Esquivel et al., 2016; Tõnno et al., 2016; Kosiba et al., 2018; Kosiba & Krztoń, 2022).
The aim of this study was to describe the seasonal changes in the community composition and abundance of protozooplankton (heterotrophic nanoflagellates and ciliates) and metazooplankton (rotifers and crustaceans) in relation to algae and both physical and chemical factors in a shallow highly eutrophic lake dominated by cyanobacteria and exposed to quite strong impact of a large nesting colony of cormorants. Some results presented in this paper are based on results and methods that have already been published elsewhere in a different context; i.e. relations between cyanobacteria and both zooplankton and abiotic parameters (Napiórkowska-Krzebietke et al., 2021). In the present study, we hypothesised that, due to the intensity of cyanobacterial blooms: (1) the abundance, species composition, and relationships between algae and zooplankton would differ between seasons, (2) protists would be the main food resources for rotifers and crustaceans, mainly in summer when water bloom is the most intensive. Based on the literature data, we also assumed that: (1) high and long-lasting cyanobacterial blooms decrease the abundance and species richness of proto- and zooplankton, showing a tendency to uniformity (Kosiba et al., 2018), (2) the highest abundance of ciliates occurs during spring when cyanobacterial blooms are not fully developed, while suddenly decreasing in abundance during summer when water bloom culminates (Tirjaková et al., 2016), (3) during the winter ice-covered period, zooplankton communities (ciliates, rotifers, and crustaceans) occur at relatively low abundances (Agbeti & Smol, 1995; Ventelä et al., 1998), (4) cladocerans and copepods differ in algal diet between winter and summer (Tõnno et al., 2016), (5) the grazing impact of rotifers on ciliates and dinofagellates is extremely high during autumn (Sweeney et al., 2022), (6) cryptophytes are the main food for copepods during colder season, while diatoms are the preferred food for them in warmer seasons (Levine et al., 1999; Tailape et al., 2019), (7) cyanobacterial blooms provide a substrate for the development of heterotrophic bacteria that are food resources for heterotrophic nanoflagellates, ciliates, and rotifers (Kosiba et al., 2022), (8) the ratio of heterotrophic to autotrophic biomass decreases due to the increasing dominance of cyanobacteria (Selmeczy et al., 2019), (9) the high concentration of phosphorus conditions a decrease in abundance of high-quality algae (cryptophytes, chrysophytes, diatoms, and dinoflagellates), but not their biomass (Taipale et al., 2019).
Materials and methods
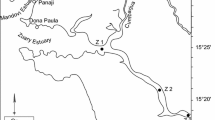
The study was carried out in the shallow, eutrophic Lake Warnołty (53° 42′ 44.5ʺ N and 21° 37′ 38.8ʺ E) situated in the Mazurian Lake District (north-eastern Poland). Lake Warnołty (area of 370.4 ha, maximum depth of 6.2 m, and mean depth of 2.5 m) is an ornithological and floral reserve. The island Warnowska, situated on the central part of the lake, is inhabited by the largest colony of the great cormorant in northeastern Poland. In the years 2009–2016, the number of cormorants fluctuated between 1000 and 1500 breeding pairs (Traczuk & Kapusta, 2017). The most frequent fish species in the diet of cormorants are Perca fluviatilis Linnaeus, 1758, Rutilus rutilus (Linnaeus, 1758), Abramis brama (Linnaeus, 1758), and Alburnus alburnus (Linnaeus, 1758) (Traczuk, unpublished data). In the last years, a high contribution (up to 38%) of Sander lucioperca (Linnaeus, 1758) in the cormorant diet has been documented (Traczuk & Kapusta, 2017). Fish surveys performed on August 10–12 with the use of the sets of Nordic multi-mesh gillnets according to the European Standard (EN 14757), showed that the lake was inhabited by 12 fish species. Perca fluviatilis was a dominant species, constituting 64% of the total abundance. Blicca bjoerkna (Linnaeus, 1758) was the second most abundant species (21% of the total abundance). The contribution of other species to the total abundance was low and did not exceed 4%. In terms of biomass, the dominant species was B. bjoerkna (30%). The important components of fish biomass were also P. fluviatilis (22%), S. lucioperca (21%) and R. rutilus (11%).
Water samples were collected once a month during spring (March–May), summer (June–August), autumn (September–November), and winter ice-covered period (December–February) 2016/17, at 0.5 m depth. On each sampling date, samples were taken from six sites located at different distances from the island (Fig. 1), because of the different effects of cormorants on primary producers (mainly cyanobacteria) and zooplankton that decrease with the increasing distance from a breeding cormorant colony (Napiórkowska-Krzebietke et al., 2020). The three sites (1, 2, and 3) were located near the island (5, 15, and 30 m from the island, respectively). Three other sites (4, 5, and 6) were located along the increasing distance from the island (70, 200, and 300 m from the island, respectively). Information on the spatial distribution of phytoplankton and zooplankton is presented in Napiórkowska-Krzebietke et al. (2020).
Lake Warnołty was covered by ice in the winter of 2016/17. The ice thickness was 1 cm in December and increased to about 20–26 cm in January and to 26–31 cm in February. A thin layer of snow was present only in January (2.0–2.5 cm) and February (1.5–4.5 cm).
Temperature and oxygen concentrations were measured using a YSI multiparameter ProDSS probe (Yellow Spring Instruments, USA). Water transparency was measured with a Secchi disc. Total phosphorus (TP) concentrations were determined colorimetrically using a Shimadzu UV 1601 spectrophotometer, after mineralization with ammonium molybdate (APHA, 1999). Nitrogen and organic carbon were measured by a Shimadzu TOC-VCSH with a nondispersive infrared NDIR analyzer. To measure total nitrogen (TN) and total organic carbon (TOC), water samples were homogenized with a magnetic stirrer, while to measure dissolved organic carbon (DOC), water samples were filtered through 0.45 μm pore size membrane filters. Particulate organic carbon (POC) was calculated as the difference between the TOC and DOC concentrations. Chlorophyll a concentrations were determined by the spectrophotometric analysis of acetone extracts of phytoplankton retained on Whatman GF/C filters according to Golterman (1969). The trophic state index (TSI) of the lake was calculated from chlorophyll a (TSICHL) and total phosphorus (TP) concentrations (TSITP), and Secchi disc visibility (TSISD) according to Carlson (1977).
Phytoplankton samples were fixed with Lugol's solution and ethanol and then enumerated by an inverted microscope according to Utermöhl (1958). Biomass was calculated from cell volume measurements, according to standard methods (Hillebrand et al., 1999; Napiórkowska-Krzebietke & Kobos, 2016).
Nanoflagellate (NF) samples were fixed with formaldehyde (final concentration 2%), stained with DAPI (Porter & Feig, 1980), filtered through 0.8 μm pore size polycarbonate membrane filters (Millipore) and enumerated by epifluorescence microscopy. The NF biomass was calculated from measurements of cell size and their approximations to simple geometric forms. Autotrophic (ANF) and heterotrophic (HNF) nanoflagellates were differentiated based on chlorophyll a autofluorescence.
Ciliate samples were fixed with Lugol’s solution and examined with a light microscope. Biomass was calculated from measurements of cell dimensions and simple geometric shapes. Species identifications of ciliates were based mainly on Foissner et al. (1999).
Rotifer and crustacean samples (25 L) were concentrated using a 30 μm mesh plankton net and preserved with Lugol’s solution and 4% ethyl alcohol. Rotifers and crustaceans were identified and enumerated under the light microscope after sedimentation. Length and length-dry mass relationships were used to determine the biomass of rotifers using Ejsmont-Karabin (1998) and the biomass of crustaceans using Bottrell et al. (1976).
The differences in the physical (water temperature), chemical (oxygen, DOC, POC, TP, TN) and biological (chlorophyll a, abundance and biomass of HNF, ciliates, rotifers, cladocerans, and copepods) parameters between seasons were analysed using the nonparametric Kruskal–Wallis test. The biological, physical and chemical variables were correlated using principal component analysis (PCA) with supplementary variables. Before PCA analysis, all data were log-transformed. Because response data were compositional and the gradient was 0.3–0.6 SD units long, the above linear method (PCA) was appropriate (ter Braak & Šmilauer, 2012). Data from the six sites for three months (n = 18 for each season) were used to perform statistical analyses in all seasons. The PCA analysis was carried out using CANOCO 5.0 (Microcomputer Power, Ithaca, NY, USA) and other statistical analyses were done using STATISTICA 10.0 (StatSoft, Inc., St Tulsa, USA).
Results
Environmental characteristics
The physical and chemical parameters are presented in Table 1. The differences in water temperature were statistically significant in most of the possible configurations of compared data (P < 0.05), with one exception (between spring and autumn). Oxygen concentrations were significantly higher in spring than in the three other seasons (P < 0.001). The concentrations of TP and TN were significantly higher in spring than in both autumn and winter (P < 0.001). In addition, there were significant differences in TN between winter and both summer (P < 0.001) and autumn (P < 0.05). DOC concentrations were very similar in spring, autumn, and winter and significantly lower in these three seasons than in summer (P < 0.05). The concentrations of POC were significantly lower in winter than in the three other seasons (P < 0.05). Chlorophyll a concentrations were significantly lower in winter than in summer (P < 0.01) and autumn (P < 0.001), while significantly higher in autumn than in spring (P < 0.001) and summer (P < 0.05).
Phytoplankton abundance and composition
The abundance and biomass of cyanobacteria were very similar in spring, summer, and autumn and significantly higher than in winter (P < 0.001; Fig. 2a). In all seasons, filamentous cyanobacteria were the dominant component of phytoplankton. Their percentage contribution both to the total abundance and biomass decreased gradually from 91.2 to 60.4% and from 90.4 to 79.0%, respectively, and was the lowest in winter under the ice cover. Cyanobacteria were mainly represented by species of the genera Pseudanabaena, Aphanizomenon, Planktothrix, and Limnothrix.
Abundance and biomass of a cyanobacteria and b algae in Lake Warnołty (Poland) during spring, summer, autumn, and winter (ice-covered period) 2016/17. Mean from six sampling sites. Lowercase letters indicate the statistically significant differences between seasons in abundance, while uppercase letters indicate the statistically significant differences in biomass (P < 0.05)
The lowest abundance of algae was noted in summer, while biomass in winter (Fig. 2b). The differences in abundance were significant (P < 0.05) only between spring and summer, while in biomass between winter and other seasons (P < 0.05). A total of 95 algal species, belonging to seven taxonomic groups, were identified. The community was dominated by diatoms and cryptophytes (Fig. 4a). Diatoms, primarily Ulnaria acus (Kützing) Aboal, U. ulna (Nitzsch) Compére, and Cyclotella sp., dominated during spring. Cryptophytes, represented mainly by Cryptomonas erosa Ehrenberg and Rhodomonas lens Pascher & Ruttner, were the dominant group in other seasons. Miozoa were the most abundant in spring (19% of the total biomass). Ochrophytes of the genera Dinobryon and Erkenia accounted for a small part of the total biomass in winter (13%). The contribution of other groups, such as chlorophytes (small-sized Scenedesmus, Chlamydomonas, Kirchneriella and Monoraphidium), euglenoids and charophytes, to the total biomass, was low and did not exceed 10%.
Protistan abundance and composition
The lowest abundance and biomass of HNF were recorded in winter, while the highest in autumn (Fig. 3a). The differences in HNF abundance were statistically significant between winter and other seasons (P < 0.05), while in HNF biomass between winter and both spring and autumn (P < 0.001) as well as between summer and both spring and autumn (P < 0.01). Small-sized (< 5 µm) HNF cells dominated in spring and winter, while medium-sized (5–10 μm) cells dominated in summer and autumn (Fig. 4b).
Abundance and biomass of a heterotrophic nanoflagellates (HNF), b ciliates, c rotifers, and d crustaceans in Lake Warnołty during spring, summer, autumn, and winter (ice-covered period) 2016/17. Mean values from six sampling sites with standard deviations. Lowercase letters indicate the statistically significant differences between seasons in abundance, while uppercase letters indicate the statistically significant differences in biomass (P < 0.05)
Percentage contribution of a algal taxonomic groups, b heterotrophic nanoflagellate (HNF) size classes, c ciliate taxonomic groups, d rotifer species, and e crustacean taxonomic groups to the total biomass in Lake Warnołty during spring, summer, autumn, and winter (ice-covered period) 2016/17. Mean values from six sampling sites. Abbreviations Rotifera: A., Asplanchna; B., Brachionus; F., Filinia; K., Keratella; P., Polyarthra; T., Trichocerca
The ciliate abundance reached a maximum in autumn and minimum in winter (Fig. 3b). In contrast, ciliate biomass was maximal in winter, while minimal in summer. The differences in abundance were significant between autumn and the three other seasons (P < 0.05). No significant differences were found in ciliate biomass (P > 0.05). A total of 79 ciliate taxa were identified. Oligotrichida, represented by species of the genera Rimostrombidium, Tintinnidium, Strombidium and Halteria, clearly dominated in spring and winter, accounting for 77% of the total biomass for both seasons (Fig. 4c). In summer, oligotrichs and prostomatids (Coleps spp. and Urotricha spp.) co-dominated, while in autumn oligotrichs and gymnostomes (mainly composed of Mesodinium spp.) were the important components of ciliate communities. It should be noted that typically bacterivorous ciliates (mainly scuticociliates and peritrichs) constituted a substantial part (21%) of the total ciliate biomass in autumn.
Rotifer abundance and composition
Rotifer abundance, biomass (Fig. 3c) and the dominance structure (Fig. 4d) changed considerably during the study. The lowest abundance was noted in spring, while the highest was both in summer and winter. The biomass was more or less similar in spring, summer, and autumn and reached a maximal value in winter. The differences in abundance were statistically significant between summer and both spring (P < 0.01) and autumn (P < 0.05) as well as between winter and both spring (P < 0.01) and autumn (P < 0.05). The differences in biomass were significant only between winter and both spring (P < 0.0001) and summer (P < 0.001). A total of 44 species were identified. The dominant species in the biomass were Asplanchna priodonta Gosse, 1850 in spring and autumn, Filinia longiseta (Ehrenberg, 1834) in summer, and Brachionus calyciflorus Pallas, 1766 in winter (Fig. 4d).
Crustacean abundance and composition
The abundance and biomass of crustaceans showed a decreasing trend throughout the year (Fig. 3d). The differences in abundance were statistically significant between winter and the three other seasons (P < 0.05) and between summer and autumn (P < 0.001). The biomass was significantly higher in summer than in autumn (P < 0.01) and winter (P < 0.001). The differences in the biomass were also found between spring and autumn (P < 0.01). A total of 25 crustacean species were identified. Copepods clearly dominated in all seasons (Fig. 4e). Cladocerans formed an important part of the total crustacean biomass (31%) only in summer. Copepods were mainly represented by Cyclops bohater Koźmiński, 1933, C. kolensis Lilljeborg, 1901, Eudiaptomus graciloides (Lilljeborg, 1888) and Thermocyclops oithonoides (G.O. Sars, 1863), while cladocerans were mainly composed of Daphnia cucullata G.O. Sars, 1862 and Chydorus sphaericus (O.F. Müller, 1776).
Relationships between algae and zooplankton
In spring, the biomass of plankton was dominated by algae and copepods (Fig. 5). In the three other seasons, algae distinctly dominated (46–65%). The ratio of algae to zooplankton biomass was about 2–3 times higher in autumn (2.1) than in other seasons (0.8, 1.1, and 0.9 in spring, summer and winter, respectively). Copepods were the dominant component of the zooplankton community, constituting 45–83% of the total biomass. The percentage contribution of cladocerans to the total zooplankton biomass was relatively high only in summer (26%). Ciliates composed an important part of zooplankton biomass in autumn (26%) and winter (23%), while rotifers in winter (32%).
In spring, cladocerans and rotifers were strongly and positively correlated with each other and positively related to ciliates, while negatively related to HNF, cryptophytes, euglenophytes, and TP (Fig. 6a). Copepods were weakly positively related to dinoflagellates (Miozoa) and negatively related to abiotic parameters, such as temperature, DOC, and POC. In turn, dinoflagellates were negatively correlated with DOC and POC. In summer, rotifers and ciliates correlated positively with each other and showed positive correlations with algal groups (among which cryptophytes and euglenophytes were the most significantly correlated) and negative correlations with cladocerans, TP, and TN (Fig. 6b). Copepods showed weak positive correlation with HNF and ciliates, while negative correlations with TP and TN. In turn, some algal groups (cryptophytes, euglenophytes, chlorophytes, and dinoflagellates) were positively related to temperature and oxygen, while negatively related to TP and TN. In autumn, cladocerans and copepods were weakly negatively related to ciliates and rotifers (Fig. 6c). In turn, rotifers were positively related to ciliates. Almost all groups of algae showed positive correlations with abiotic parameters. In winter, copepods were closely and positively related to HNF, diatoms, chlorophytes, charophytes, and dinoflagellates, while negatively related to ice/snow cover, TN, and DOC (Fig. 6d). Rotifers were weakly negatively related to euglenophytes, POC and TP. In addition, rotifers and ciliates were positively, while copepods, HNF, and some algal groups (mainly diatoms) negatively correlated with ice/snow cover and TN.
Biplot of abiotic and biotic parameters, based on principal component analysis (PCA) in a spring, b summer, c autumn, and d winter (ice-covered period) 2016/17 in Lake Warnołty. Oxy oxygen, TP total phosphorus, TN total nitrogen, DOC dissolved organic carbon, POC particulate organic carbon, Cry Cryptophyta, Och Ochrophyta, Bac Bacillariophyta, Eug Euglenozoa, Chl Chlorophyta, Cha Charophyta, Mio Miozoa. Cumulative explained variation for the two first axes in spring is 94.8 (88.6 and 6.2, respectively), in summer is 94.9 (86.9 and 8.0, respectively), in autumn is 97.2 (91.6 and 5.6, respectively), while in winter it is 91.0 (85.2 and 5.8, respectively)
Relationships between the trophic state indices calculated based on chlorophyll a and total phosphorus concentrations, and the Secchi disk are presented in Fig. 7. The values of TSI calculated for individual seasons indicated highly eutrophic characteristics (TSI above 60). The values of the TSITP varied between 71 (autumn and winter) and 74 (summer). The trophic state index based on the Secchi disk ranged from 60 in winter to 70 in summer. The values of the TSICHL varied from 67 in winter to 77 in autumn.
Relationships between the components of the trophic state index (TSI) calculated from the mean values of chlorophyll a (TSICHL), Secchi depth (TSISD) and total phosphorus (TSITP) based on the model by Carlson and Havens (2005) in Lake Warnołty during spring, summer, autumn, and winter (ice-covered period) 2016/17
Discussion
In the shallow, highly eutrophic Lake Warnołty, the phytoplankton community was mainly composed of cyanobacteria that dominated throughout the studied period (Napiórkowska-Krzebietke et al., 2020, 2021). Algae accounted for a small part of the total phytoplankton abundance and biomass (9–40% and 10–21%, respectively). Some studies have shown that cyanobacterial blooms generally contain a huge and diverse community of bacterioplankton, phytoplankton and zooplankton, among which certain species have a destructive impact on cyanobacteria (Gołdyn & Kowalczewska-Madura, 2008; Van Wichelen et al., 2016). In contrast, Kosiba et al. (2018) recorded only 15 ciliate and 54 metazooplankton taxa and indicated that in aquatic ecosystems with higher and longer-lasting toxin concentrations, the abundance and species richness of zooplankton decreased, showing a tendency to uniformity. Similarly, Tirjaková et al. (2016) and Krevš et al. (2010) stated that cyanobacterial blooms significantly lowered the diversity of ciliate communities. In this study, all of the studied groups of zooplankton were quite rich in species and relatively abundant. The ciliate abundances were very similar to those recorded in a tropical Mexican lake (Esquivel et al., 2016), but much higher than those noted by Krevš et al. (2010) in the highly eutrophic lake in Lithuania; both lakes were dominated by filamentous cyanobacteria. In lake ecosystems with summer cyanobacterial blooms, ciliates can reach maximal abundance during spring, when the cyanobacterial bloom is not fully developed, while minimal values are observed at the beginning of summer when the water bloom culminates (Tirjaková et al., 2016). In our study, ciliates were the most abundant in autumn, when maximal abundance and biomass of heterotrophic nanoflagellates were observed. In turn, the highest ciliate biomass was noted in winter, when algal biomass was the lowest and cyanobacterial bloom substantially decreased (Napiórkowska-Krzebietke et al., 2021). In winter, not only ciliates but also rotifers reached maximal biomass. At this time, the rotifer biomass was significantly higher than in spring and summer, while cryptophytes and ochrophytes reached their maximal abundance and/or biomass. The development of ciliates and rotifers was probably the result of a decrease in crustacean abundance and a visible increase in the contribution of algae to the total phytoplankton abundance (up to 40%) and biomass (to 21%). All these facts suggest that in a shallow eutrophic lake dominated by cyanobacteria, winter may be a more favourable period for the growth of some planktonic groups of organisms, especially ciliates and rotifers, despite adverse environmental conditions (quite thick ice cover in January/February and a thin layer of snow). In contrast, the ice/snow cover was a factor limiting the development of HNF, copepods, dinoflagellates, diatoms, chlorophytes, and charophytes.
Small-bodied cladocerans and cyclopoid copepods usually dominate zooplankton biomass in eutrophic lakes during cyanobacterial blooms (Zhang et al., 2013). The co-occurring cladocerans and copepods differ markedly in algal diet composition and preferences, especially between winter and summer (Tõnno et al., 2016). In the colder season, cryptophytes are the dominant food for copepods, while in warmer months (May to September), small algae (< 25 µm), such as dinoflagellates, diatoms and green algae are the preferred food for them (Levine et al., 1999; Tõnno et al., 2016). As shown by Kosiba and Krztoń (2022), algivorous ciliates as well as ciliate species feeding on bacteria and algae are consumed preferentially by copepods in the periods without and with cyanobacterial blooms, but to a greater extent during the bloom. We showed that zooplankton biomass was dominated by copepods throughout the study. In summer, rotifer and cladoceran communities showed relationships with many taxonomical groups of algae (mainly cryptophytes, euglenophytes, dinoflagellates, and chlorophytes) and ciliates. However, in the case of rotifers, these relationships were positive (the clear increase in rotifer biomass may be the result of increasing algal biomass), while in the case of cladocerans negative (strong predator–prey interactions). Our study indicated that during the studied periods, all available algal-food resources were consumed by zooplankton, except for autumn when fine detritus/organic aggregates, bacteria, and picoplankton were probably the main food for zooplankton (Gilbert, 2022). A small part of algae in summer (9 and 10% of the total phytoplankton abundance and biomass, respectively), an edible and easily available phytoplankton-food resource for zooplankton, might not be enough to maintain zooplankton communities. It is well documented that protists (HNF and ciliates) may enrich the diet of herbivorous consumers at times when the phytoplankton is deficient in nutrients, polyunsaturated fatty acids and sterols (Sommer et al., 2012), and when copepods dominate (Vargas et al., 2006). In our study, the strong relationships between crustaceans and both HNF and ciliates may indicate that in spring, summer, and winter protists could be an additional and significant food source for metazooplankton, in addition to algae (Sanders & Wickham, 1993; Jürgens et al., 1996). It is probable that at this time, nutritional value of algae was considerably reduced due to excessive concentrations of nutrients (Taipale et al., 2019). Also, rotifers supplemented their diet with HNF in spring and with ciliates in summer and autumn (Arndt, 1993; Weisse & Frahm, 2002; Gilbert, 2022).
Results of some studies showed that bacteria may be a primary food resource for rotifers, cladocerans and autotrophic flagellates, especially in a situation where the availability of algae is reduced (Sanders et al., 1989). Decaying cyanobacterial blooms are an important source of organic carbon and nutrients for heterotrophic bacteria (Engström-Öst et al., 2002). Unfortunately, bacteria were not determined in this study. However, the HNF community (the major grazers of bacteria) was characterised by relatively high biomass, but low abundance, which is consistent with the results of Krevš et al. (2010). A large proportion of medium-sized cells suggests that medium-sized bacteria could be the dominant component of bacterioplankton and the preferred food for HNF (Adamczewski et al., 2010). The presence of Hastatella radians Erlanger, 1890, bacterivorous ciliate species characteristic of waters with a high abundance of bacteria (Foissner et al., 1999), as well as the high contribution of bacterivorous ciliates to the total ciliate biomass, especially in autumn, indicates a rich, easily accessible and important source of bacterial food for heterotrophic protists. The close negative relationships between HNF and ciliates in winter confirm predator–prey coupling. In spring, summer, and winter, ciliates showed relationships with cryptophytes and additionally in the spring–summer period with euglenophytes, in autumn with charophytes, and in winter with ochrophytes. To sum up, bacteria, HNF, cryptophytes, euglenophyes, charophytes, and ochrophytes were probably fundamental food sources for ciliates, allowing them to reach high abundances in such difficult “cyanobacterial” conditions. Our results are similar to those recorded in other shallow, highly eutrophic lake dominated by filamentous cyanobacteria (Zingel et al., 2007).
The effect of fish predation on the structure of phytoplankton and zooplankton changes seasonally and is related to changes in water temperature, fish migration and reproduction (Jeppesen et al., 1997; Sommer et al., 2012). The highest predation pressure by fish is observed in summer, when fish larvae occur in the pelagial, while the lowest in winter, when low water temperature reduces the numerous metabolic processes (Sommer et al., 2012) and poor light conditions limit the visual search and capture of prey (Salonen et al., 2009). It has been shown that the food requirements of cormorants vary over seasons (Salmi et al., 2015) and are the highest during the breeding season, mainly in spring and summer (Grémillet et al., 2000). Because most fish (small-sized individuals of P. fluviatilis and B. bjoerkna) in the studied lake are an important component in the diet of cormorants (Traczuk & Kapusta, 2017) and feed mainly on crustacean zooplankton (Jeppesen et al., 1997), the relationships between cormorants-fish-zooplankton-phytoplankton may likely be more obvious in summer. Theoretically, cormorants as an opportunistic top predator may considerably decrease the abundance of fish populations, leading to the increase in zooplankton abundance that, in turn, limits algal growth. In our study, quite a large biomass of algae and the relatively high ratio (2.1) of algae to zooplankton biomass in autumn indicate that the grazing pressure of consumers on algal food was relatively low in this period. In contrast, the low ratios in spring (0.8) and winter (0.9) indicate that the impact of zooplankton on algae was exceptionally strong. It is well documented that the grazing activity of great cormorants (Phalacrocorax carbo) can increase nutrient concentrations (Klimaszyk & Rzymski, 2016) and change the abundance, species composition, and size-age structure of fish communities (Traczuk & Kapusta, 2017). A large breeding cormorant colony (3000 individuals) living on the island in Lake Warnołty can deposit a high loading of nutrients (a minimum of 2.1 and 1.3 t of N and P per year, respectively) to the soil and surrounding water, which is probably responsible for the intense cyanobacterial blooms in this lake (Napiórkowska-Krzebietke et al., 2021). The relationships between trophic state variables indicated that phytoplankton growth was mainly controlled by phosphorus during the growing season, while being restrained by zooplankton in winter. The limiting role of this parameter (positive values on the Y axis, TSICHL > TSITP) was the most important from spring to autumn, while rapidly declining in winter. At the same time, the difference between TSICHL and TSISD values increased with the development of the vegetation. The phytoplankton community probably transformed from smaller forms capable of limiting light penetration during spring, to larger forms, which disperse sunlight weakly during autumn (thus increasing X axis values). In addition, the PCA analysis showed that algal groups were related to nutrients throughout most of the study, especially in summer and autumn. Negative relationships between TN and/or TP, and both algae and zooplankton in summer indicate that the high concentrations of nutrients decrease not only the abundance of high-quality algae, e.g. cryptophytes, dinoflagellates, and euglenophytes (Taipale et al., 2019), but also the abundance and biomass of ciliates and rotifers.
Conclusions
A shallow, highly eutrophic lake dominated by cyanobacteria was characterised by the high concentrations of TP, TN and chlorophyll a, as well as a rich taxonomic composition and high abundances of ciliates, rotifers and crustaceans, indicating that cyanobacterial blooms did not limit the growth of planktonic organisms. Algae accounted for a small part of the total phytoplankton abundance and biomass, and were mainly dominated by diatoms and cryptophytes. Copepods were the dominant component of zooplankton. In spring, summer and winter, algal biomass was almost equal to zooplankton biomass. Maximal biomass of cryptophytes, ochrophytes, ciliates, and rotifers under the ice may indicate that winter may be a more favourable period for the growth of some planktonic groups of organisms than other seasons. Our study indicated that during the studied periods, all available algal-food resources were consumed by zooplankton, except for autumn when fine detritus/organic aggregates, bacteria, and picoplankton were probably the main food for zooplankton. In the warmer period (spring–autumn) algae were bottom-up controlled by nutrients.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Adamczewski, T., R. J. Chróst, K. Kalinowska & A. Skowrońska, 2010. Relationships between bacteria and heterotrophic nanoflagellates in lake water examined by different techniques controlling grazing pressure. Aquatic Microbial Ecology 60: 203–213. https://doi.org/10.3354/ame01420.
Agbeti, M. D. & J. R. Smol, 1995. Winter limnology: a comparison of physical, chemical and biological characteristics in two temperate lakes during ice cover. Hydrobiologia 304: 221–234.
American Public Health Association, 1999. Standard Methods for Examination of Water & Wastewater, American Public Health Association, New York:
Arndt, H., 1993. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates) – a review. Hydrobiologia 255(256): 231–246.
Bogdan, K. G. & J. J. Gilbert, 1982. Seasonal patterns of feeding by natural populations of Keratella, Polyarthra, and Bosmina: clearance rates, selectivities, and contributions to community grazing. Limnology and Oceanography 27: 918–934. https://doi.org/10.4319/lo.1982.27.5.0918.
Bottrell, H. H., A. Duncan, Z. M. Gliwicz, E. Grygierek, A. Herzig, A. Hillbricht-Ilkowska, H. Kurasawa, P. Larsson & T. Węgleńska, 1976. A review of some problems in zooplankton production studies. Norwegian Journal of Zoology 24: 419–456.
Brett, M. T., M. J. Kainz, S. J. Taipale & H. Seshan, 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences 106: 21197–21201. https://doi.org/10.1073/pnas.0904129106.
Carlson, R. E., 1977. A trophic state index for lakes. Limnology and Oceanography 22: 361–369.
Carlson, R. E. & K. E. Havens, 2005. Simple graphical methods for the interpretation of relationships between trophic state variables. Lake and Reservoir Management 21: 107–118. https://doi.org/10.1080/07438140509354418.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. Bioscience 35: 634–639. https://doi.org/10.2307/1309989.
Ejsmont-Karabin, J., 1998. Empirical equations for biomass calculation of planktonic rotifers. Polish Archives of Hydrobiology 45: 513–522.
Engström-Öst, J., M. Koski, K. Schmidt, M. Viitasalo, S. H. Jónasdóttir, M. Kokkonen, S. Repka & K. Sivonen, 2002. Effects of toxic cyanobacteria on a plankton assemblage: community development during decay of Nodularia spumigena. Marine Ecology Progress Series 232: 1–14. https://doi.org/10.3354/meps232001.
Esquivel, A., A. Barani, M. Macek, R. Soto-Castor & C. Bulit, 2016. The trophic role and impact of plankton ciliates in the microbial web structure of a tropical polymictic lake dominated by filamentous cyanobacteria. Journal of Limnology 75: 93–106. https://doi.org/10.4081/jlimnol.2016.1392.
Foissner, W., H. Berger & J. Schaumburg, 1999. Identification and ecology of limnetic plankton ciliates. Informationberichte des Bayer Landesamtes für Wasserwirtschaft, Heft 3/99. München, 793 p.
Gagnon, K., J. Sjöroos, J. Yli-Rosti, M. Stark, E. Rothäusler & V. Jormalainen, 2016. Nutrient enrichment overwhelms top-down control in algal communities around cormorant colonies. Journal of Experimental Marine Biology and Ecology 476: 31–40. https://doi.org/10.1016/j.jembe.2015.12.007.
Gebrehiwot, M., D. Kifle & L. Triest, 2019. Grazing and growth rate of a cyclopoid copepod fed with a phytoplankton diet constituted by a filamentous cyanobacterium. Hydrobiologia 828: 213–227. https://doi.org/10.1007/s10750-018-3813-7.
Ger, K. A., L.-A. Hansson & M. Lürling, 2014. Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwater Biology 59: 1783–1798. https://doi.org/10.1111/fwb.12393.
Gilbert, J. J., 2022. Food niches of planktonic rotifers: Diversification and implications. Limnology and Oceanography 67: 2218–2251. https://doi.org/10.1002/lno.12199.
Gołdyn, R. & K. Kowalczewska-Madura, 2008. Interactions between phytoplankton and zooplankton in the hypertrophic Swarzędzkie Lake in western Poland. Journal of Plankton Research 30: 33–42. https://doi.org/10.1093/plankt/fbm086.
Golterman, H. L., 1969. Methods for Chemical Analysis of Fresh Waters. IBP Handbook No 8. Blackwell Scientific Publications. Oxford, Edinburgh.
Grémillet, D., S. Storch & G. Peters, 2000. Determining food requirements in marine top predators: a comparison of three independent techniques in Great Cormorants, Phalacrocorax carbo carbo. Canadian Journal of Zoology 78: 1567–1579.
Hansen, A.-M. & B. Santer, 1995. The influence of food resources on the development, survival and reproduction of the two cyclopoid copepods: Cyclops vicinus and Mesocyclops leuckarti. Journal of Plankton Research 17: 631–646. https://doi.org/10.1093/plankt/17.3.631.
Havens, K. E., R. T. James, T. L. East & V. H. Smith, 2003. N: P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environmental Pollution 122: 379–390.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424. https://doi.org/10.1046/j.1529-8817.1999.3520403.x.
Jeppesen, E., T. L. Lauridsen, S. F. Mitchell & C. W. Burns, 1997. Do planktivorous fish structure the zooplankton communities in New Zealand lakes? New Zealand Journal of Marine and Freshwater Research 31: 163–173.
Josué, I. I. P., S. J. Cardoso, M. Miranda, M. Mucci, K. A. Ger, F. Roland & M. M. Marinho, 2019. Cyanobacteria dominance drives zooplankton functional dispersion. Hydrobiologia 831: 149–161. https://doi.org/10.1007/s10750-018-3710-0.
Jürgens, K., S. A. Wickham, K. O. Rothhaupt & B. Santer, 1996. Feeding rates of macro- and microzooplankton on heterotrophic nanoflagellates. Limnology and Oceanography 41(8): 1833–1839.
Klimaszyk, P. & P. Rzymski, 2016. The complexity of ecological impacts induced by great cormorants. Hydrobiologia 771: 13–30. https://doi.org/10.1007/s10750-015-2618-1.
Klimaszyk, P., R. Piotrowicz & P. Rzymski, 2015. Changes in physico-chemical conditions and macrophyte abundance in a shallow soft-water lake mediated by a Great Cormorant roosting colony. Journal of Limnology 74: 114–122. https://doi.org/10.4081/jlimnol.2015.994.
Kosiba, J. & W. Krztoń, 2022. Insight into the role of cyanobacterial bloom in the trophic link between ciliates and predatory copepods. Hydrobiologia 849: 1195–1206. https://doi.org/10.1007/s10750-021-04780-x.
Kosiba, J., W. Krztoń & E. Wilk-Woźniak, 2018. Effect of microcystins on proto- and metazooplankton is more evident in artificial than in natural waterbodies. Microbial Ecology 75: 293–302. https://doi.org/10.1007/s00248-017-1058-z.
Kosiba, J., E. Wilk-Woźniak & W. Krztoń, 2019. The effect of potentially toxic cyanobacteria on ciliates (Ciliophora). Hydrobiologia 827: 325–335. https://doi.org/10.1007/s10750-018-3783-9.
Kosiba, J., W. Krztoń, J. Koreiviené, S. Tarcz & E. Wilk-Woźniak, 2022. Interactions between ciliate species and Aphanizomenon flos-aquae vary depending on the morphological form and biomass of the diazotrophic cyanobacterium. International Journal of Environmental Research and Public Health 19: 15097. https://doi.org/10.3390/ijerph192215097.
Krevš, A., J. Koreivienė & S. Mažeikaitė, 2010. Plankton food web structure during cyanobacteria bloom in the highly eutrophic Lake Gineitiškės. Ekologija 56: 47–54. https://doi.org/10.2478/v10055-010-0007-7.
Krztoń, W., K. Pudaś, A. Pociecha, M. Strzesak, J. Kosiba, E. Walusiak, E. Szarek-Gwiazda & E. Wilk-Woźniak, 2017. Microcystins affect zooplankton biodiversity in oxbow lakes. Environmental Toxicology and Chemistry 36: 165–174. https://doi.org/10.1002/etc.3519.
Lampert, W., 1987. Laboratory studies on zooplankton-cyanobacteria interactions. New Zealand Journal of Marine and Freshwater Research 21: 483–490. https://doi.org/10.1080/00288330.1987.9516244.
Lemmens, P., S. A. J. Declerck, K. Tuytens, M. Vanderstukken & L. De Meester, 2018. Bottom-up effects on biomass versus top-down effects on identity: a multiple-lake fish community manipulation experiment. Ecosystems 21: 166–177. https://doi.org/10.1007/s10021-017-0144-x.
Levine, S. N., M. A. Borchardt, M. Braner & A. D. Shambaugh, 1999. The impact of zooplankton grazing on phytoplankton species composition and biomass in Lake Champlain (USA-Canada). Journal of Great Lakes Research 25: 61–77. https://doi.org/10.1016/S0380-1330(99)70717-3.
Ma, J., B. Qin, H. W. Paerl, J. D. Brookes, N. S. Hall, K. Shi, Y. Zhou, J. Guo, Z. Li, H. Xu, T. Wu & S. Long, 2016. The persistence of cyanobacterial (Microcystis spp.) blooms throughout winter in Lake Taihu. China. Limnology and Oceanography 61: 711–722. https://doi.org/10.1002/lno.10246.
Mao, Z., X. Gu, Y. Cao, M. Zhang, Q. Zeng, H. Chen, R. Shen & E. Jeppesen, 2020. The role of top-down and bottom-up control for phytoplankton in a subtropical shallow eutrophic lake: evidence based on long-term monitoring and modeling. Ecosystems 23: 1449–1463. https://doi.org/10.1007/s10021-020-00480-0.
Müller, H. & A. Schlegel, 1999. Responses of three freshwater planktonic ciliates with different feeding modes to cryptophyte and diatom prey. Aquatic Microbial Ecology 17: 49–60. https://doi.org/10.3354/AME017049.
Müller-Navarra, D. C., M. T. Brett, S. Park, S. Chandra, A. P. Ballantyne, E. Zorita & C. R. Goldman, 2004. Unsaturated fatty acid content in seston and tropho-dynamic coupling in lakes. Nature 427: 69–72. https://doi.org/10.1038/nature02210.
Napiórkowska-Krzebietke, A., 2017. Phytoplankton response to fish-induced environmental changes in a temperate shallow pond-type lake. Archives of Polish Fisheries 25: 211–264. https://doi.org/10.1515/aopf-2017-0020.
Napiórkowska-Krzebietke, A. & J. Kobos, 2016. Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Research 104: 532–546. https://doi.org/10.1016/j.watres.2016.08.016.
Napiórkowska-Krzebietke, A., K. Kalinowska, E. Bogacka-Kapusta, K. Stawecki & P. Traczuk, 2020. Cyanobacterial blooms and zooplankton structure in lake ecosystem under limited human impact. Water 12: 1252. https://doi.org/10.3390/w12051252.
Napiórkowska-Krzebietke, A., K. Kalinowska, E. Bogacka-Kapusta, K. Stawecki & P. Traczuk, 2021. Persistent blooms of filamentous cyanobacteria in a cormorant-affected aquatic ecosystem: Ecological indicators and consequences. Ecological Indicators 124: 107421. https://doi.org/10.1016/j.ecolind.2021.107421.
Porter, K. G. & Y. S. Feig, 1980. The use of DAPI for identifying and counting aquatic microflora. Limnology and Oceanography 25: 943–948. https://doi.org/10.4319/lo.1980.25.5.0943.
Posch, T., B. Eugster, F. Pomati, J. Pernthaler, G. Pitsch & E. M. Eckert, 2015. Network of interactions between ciliates and phytoplankton during spring. Frontiers in Microbiology 6: 1289. https://doi.org/10.3389/fmicb.2015.01289.
Reynolds, C. S., 2006. The Ecology of Phytoplankton, Cambridge University Press, Cambridge:
Salmi, J. A., H. Auvinen, J. Raitaniemi, M. Kurkilahti, J. Lilja & R. Maikola, 2015. Perch (Perca fluviatilis) and pikeperch (Sander lucioperca) in the diet of the great cormorant (Phalacrocorax carbo) and effects on catches in the Archipelago Sea, Southwest coast of Finland. Fisheries Research 164: 26–34. https://doi.org/10.1016/j.fishres.2014.10.011.
Salonen, K., M. Leppäranta, M. Viljanen & R. D. Gulati, 2009. Perspectives in winter limnology: closing the annual cycle of freezing lakes. Aquatic Ecology 43: 609–616. https://doi.org/10.1007/s10452-009-9278-z.
Sanders, R. W. & S. A. Wickham, 1993. Planktonic protozoa and metazoa: predation, food quality and population control. Marine Microbial Food Webs 7: 197–223.
Sanders, R. W., K. G. Porter, S. J. Bennett & A. E. DeBiase, 1989. Seasonal patterns of bacterivory by flagellates, ciliates, rotifers, and cladocerans in a freshwater planktonic community. Limnology and Oceanography 34: 673–687. https://doi.org/10.4319/lo.1989.34.4.0673.
Selmeczy, G. B., A. Abonyi, L. Krienitz, P. Kasprzak, P. Casper, A. Telcs, Z. Somogyvári & J. Padisák, 2019. Old sins have long shadows: climate change weakens efficiency of trophic coupling of phyto- and zooplankton in a deep oligo-mesotrophic lowland lake (Stechlin, Germany) – a causality analysis. Hydrobiologia 831: 101–117. https://doi.org/10.1007/s10750-018-3793-7.
Sommer, U., 1981. The role of r- and K-selection in the succession of phytoplankton in Lake Constance. Acta Oecologica, Oecologia Generalis 2: 327–342.
Sommer, U., F. Sommer, B. Santer, E. Zöllner, K. Jürgens, C. Jamieson, M. Boersma & K. Gocke, 2003. Daphnia versus copepod impact on summer phytoplankton: functional compensation at both trophic levels. Oecologia 135: 639–647. https://doi.org/10.1007/s00442-003-1214-7.
Sommer, U., R. Adrian, L. De Senerpont Domis, J. J. Elser, U. Gaedke, B. Ibelings, E. Jeppesen, M. Lürling, J. C. Molinero, W. M. Mooij, E. vanDonk & M. Winder, 2012. Beyond the plankton ecology group (PEG) model: mechanisms driving plankton succession. Annual Review of Ecology, Evolution, and Systematics 43: 429–448. https://doi.org/10.1146/annurev-ecolsys-110411-160251.
Sweeney, K., G. Rollwagen-Bollens & S. E. Hampton, 2022. Grazing impacts of rotifer zooplankton on a cyanobacteria bloom in a shallow temperate lake (Vancouver Lake, WA, USA). Hydrobiologia 849: 2683–2703. https://doi.org/10.1007/s10750-022-04885-x.
Taipale, S. J., K. Vuorio, S. L. Aalto, E. Peltomaa & M. Tiirola, 2019. Eutrophication reduces the nutritional value of phytoplankton in boreal lakes. Environmental Research 179: 108836. https://doi.org/10.1016/j.envres.2019.108836.
ter Braak, C. J. F., P. Šmilauer, 2012. Canoco reference manual and user's guide: software for ordination (version 5.0). Microcomputer Power (Ithaca, NY, USA).
Thys, I., B. Leporcq & J.-P. Descy, 2003. Seasonal shifts in phytoplankton ingestion by Daphnia galeata, assessed by analysis of marker pigments. Journal of Plankton Research 25: 1471–1484. https://doi.org/10.1093/plankt/fbg103.
Tillmanns, A. R., A. E. Wilson, F. R. Pick & O. Sarnelle, 2008. Meta-analysis of cyanobacterial effects on zooplankton population growth rate: species-specific responses. Fundamental and Applied Limnology 171: 285–295. https://doi.org/10.1127/1863-9135/2008/0171-0285.
Tirjaková, E., K. Krajčovičová, M. Illyová & P. Vdačny, 2016. Interaction of ciliate communities with cyanobacterial water bloom in a shallow, hypertrophic reservoir. Acta Protozoologica 55: 173–188. https://doi.org/10.4467/16890027AP.16.017.5749.
Tõnno, I., H. Agasild, T. Kõiv, R. Freiberg, P. Nõges & T. Nõges, 2016. Algal diet of small-bodied crustacean zooplankton in a cyanobacteria-dominated eutrophic lake. PLoS ONE 11: e0154526. https://doi.org/10.1371/journal.pone.0154526.
Traczuk, P. & A. Kapusta, 2017. Great cormorant (Phalacrocorax carbo) predation on pikeperch (Sander lucioperca L.) in shallow eutrophic lakes in Poland. Archives of Polish Fisheries 25: 123–130. https://doi.org/10.1515/aopf-2017-0012.
Utermöhl, H., 1958. Guidance on the quantitative analysis of phytoplankton – methods. Mitteilung Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 9: 1–38.
Van Wichelen, J., P. Vanormelingen, G. A. Codd & W. Vyverman, 2016. The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 55: 97–111. https://doi.org/10.1016/j.hal.2016.02.009.
Vargas, C. A., R. Escribo & S. Poulet, 2006. Phytoplankton food quality determines time windows for successful zooplankton reproductive pulses. Ecology 87: 2992–2999. https://doi.org/10.1890/0012-9658(2006)87[2992:pfqdtw]2.0.co;2.
Ventelä, A. M., V. Saarikari & K. Vuorio, 1998. Vertical and seasonal distributions of microorganisms, zooplankton and phytoplankton in a eutrophic lake. Hydrobiologia 363: 229–240.
Weisse, T. & A. Frahm, 2002. Direct and indirect impact of two common rotifer species (Keratella spp.) on two abundant ciliate species (Urotricha furcata, Balanion planctonicum). Freshwater Biology 47: 53–64.
Zhang, J., P. Xie, M. Tao, L. Guo, J. Chen, X. Zhang & L. Zhang, 2013. The impact of fish predation and cyanobacteria on zooplankton size structure in 96 subtropical lakes. PLoS One 8(10): e76378. https://doi.org/10.1371/journal.pone.0076378.
Zingel, P., H. Agasild, T. Nõges & V. Kisand, 2007. Ciliates as the dominant grazers on pico- and nanoplankton in a shallow, naturally highly eutrophic lake. Microbial Ecology 53: 134–142. https://doi.org/10.1007/s00248-006-9155-4.
Acknowledgements
We are grateful to Waldemar Kozłowski, Adam Mańko, and Jan Rola for help in the field sampling as well as Joanna Hutorowicz, Jakub Pyka, and Stanisław Sidorski for chemical analyses. This research was financed through the National Inland Fisheries Research Institute in Olsztyn within its statutory research activity (topics no. Z-014 and Z-016). We thank two reviewers for their valuable comments, which greatly helped us to improve the manuscript.
Author information
Authors and Affiliations
Contributions
KK, PT and DU conceived and designed the study and performed the fieldwork. KK provided data on protists and prepared the first draft of the manuscript. ANK provided data on the phytoplankton. EBK contributed rotifer and crustacean data. KS contributed chemical data. All of the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling editor: Elzbieta Wilk-Wozniak
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalinowska, K., Napiórkowska-Krzebietke, A., Bogacka-Kapusta, E. et al. Algae–zooplankton relationships during the year-round cyanobacterial blooms in a shallow lake. Hydrobiologia 851, 2025–2040 (2024). https://doi.org/10.1007/s10750-023-05435-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05435-9