Abstract
This study investigated the spatial variation in total benthic algal biomass and within cyanobacteria, green algae, and diatoms in sub-Arctic ponds. Additionally to more widely used explanatory variables, snowmelt and ice duration were considered as their importance on algal communities is poorly understood. The data comprised algal biomasses from 45 sub-Arctic ponds in the Finnish Lapland. A generalized linear model and hierarchical partitioning were used to identify the significantly influential variables. Cyanobacteria were the most abundant algal group. Trace elements (e.g. Fe, Al, and Mn) were the most significant explanatory variable group in explaining algal biomasses. Macronutrients apart from K were found insignificant in all models. There were positive relationships between some algal biomasses indicating no strong competition between them. Snow and ice variables were found insignificant for all models, but they could have an important secondary role on algal communities. The results highlight the importance of trace elements in shaping algal biomasses in sub-Arctic ponds and thus their wider use in research can be advocated to better understand the productivity of nutrient poor and acidic waters in sub-Arctic regions. Focussing on benthic algal biomasses and the chemical composition of sub-Arctic freshwaters provides important information on the aquatic primary production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change creates constant pressure on Arctic ecosystems causing both direct and indirect effects on the Arctic biodiversity (Culp et al., 2021). The Arctic region has recently experienced two to three times greater warming, known as the Arctic amplification, compared to the global average (Screen, 2014). This warming is expected to affect biodiversity and ecosystem functions throughout the circumpolar Arctic (Lento et al., 2022a). A fundamental change in freshwater ecosystems has been an increase in primary production over the past 150 years (Michelutti et al., 2005) caused by the lengthening of the growing season (Wrona et al., 2006). A longer growing period will increase northern greening enhancing nutrient binding to terrestrial systems on a catchment level. Changes in vegetation, macro- and microclimate, and snowpack alter Arctic and sub-Arctic ecosystems, also affecting the influx of nutrients to waters thus influencing the chemical characteristics of sub-Arctic freshwaters (Kamenik et al., 2001). Understanding how the aquatic and terrestrial ecosystems operate simultaneously, and especially their connectivity via material and energy inputs, helps us understand the changes occurring in both ecosystems (Soininen et al., 2015). On one hand, atmospheric phosphorus from combustion-related sources has declined the deposition of atmospheric phosphorus to surface waters, which may further increase oligotrophication of already nutrient-poor sub-Arctic freshwaters (Huser et al., 2020). On the other hand, higher runoff due to increasing precipitation in the Arctic may lead to increased nutrient load to surface waters thus affecting the benthic algal community and primary production (Kahlert et al., 2020; Lakka, 2020).
The significant part of the aquatic biodiversity and production in the circumpolar is provided by small and shallow inland waters and especially their benthic algal communities (Culp et al., 2021). The benthic algal community consisting mainly of cyanobacteria (Cyanophyta), green algae (Chlorophyta), and diatoms (Bacillariophyta) is expected to change due to rising temperatures, also affecting food quality and quantity to higher trophic levels (e.g. midges, larvae, and eventually fish) (McCormick et al., 2019; Lento et al., 2022b). Despite the importance of these benthic algae, there is limited information about how different environmental factors influence the algal microflora in these regions (Richter et al., 2018). Temperature, among other abiotic variables such as nutrients and conductivity, has a strong influence on the generally amictic Arctic and sub-Arctic freshwater ecosystems (Soininen, 2007; Soininen & Meier, 2014). The limiting resources for growth (e.g. nutrients, light, or temperature) vary widely among different algal species (Agrawal, 2012; Phillips et al., 2019). In shallow and pristine sub-Arctic ponds, the benthic algal community can use efficiently the available radiation contributing 39–99% of the total primary production (Rautio et al., 2011). Thus, any change in nutrients or light can cause shifts in the productivity, species distribution patterns, and food web structure (Lau et al., 2019). However, in the sub-Arctic, it is unlikely that light would limit biomass growth due to the treeless surroundings, clear water, and shallow characteristics of the ponds unless enhanced northern greening would cause shading and murkiness to the ponds. This highlights the role of other factors like nutrient input and water chemistry to benthic algal biomass. For instance, local topography, catchment exposure, precipitation, and solar radiation influence weathering rates and therefore affect the influx of trace elements to the ponds (Kamenik et al., 2001). Additionally, the joint effects of influxes such as runoff, precipitation, and groundwater and effluxes like runoff, sedimentation, wind, and filtration alter the chemical composition of sub-Arctic ponds, similar to other freshwater lakes (Dauvalter & Kashulin, 2014). For example, vanadium and copper tend to show a strong influence of seawater via atmospheric input. Iron, zinc, and lead on the other hand are mostly present in a colloidal fraction, while manganese and copper are commonly dominated by a truly soluble fraction (Marsay et al., 2018). Thus, it can be expected that there is some seasonal variation in trace element concentrations based on the different forms of fractions having an effect on the behaviour, mobility, and bioavailability of trace elements in environmental systems.
The benthic algae form an extensive biomass in the aquatic ecosystems, which, once measured through chlorophyll a concentrations, can be used to estimate primary production especially in smaller waterbodies such as ponds or small streams (Huot et al., 2007). Overall, algal biomass effectively summarizes the growing conditions of the whole algal community but since previous research has focussed on single algal groups (e.g. Lotter et al., 2010; Virta & Teittinen, 2022), the patterns and drivers for multiple algal groups are poorly understood (Lau et al., 2019). The high variation in biodiversity patterns in the sub-Arctic may be explained by the complex topography and varying ecological conditions occurring over small spatial scales (Chiu et al., 2020). The composition of the benthic algae varies significantly throughout the year due to water temperature fluctuations caused by incoming solar radiation and meltwater from snow and ice. Early in the growing season after snowmelt, the waters are cold supporting tolerant algal groups of diatoms and cyanobacteria over green algae (Pilkaitytė & Razinkovas, 2007). With time, water temperature increases allow greater accumulation of benthic biomass also comprising of green algae. Despite this, cyanobacterial and diatom biomasses are typically constantly higher compared to green algae due to their adaptive mechanisms to grow in different temperatures (Michelutti et al., 2003; Dasauni et al., 2022). In contrast, green algae tend to occur with variable biomasses depending on water temperature (Rautio et al., 2011; Agrawal, 2012).
Cyanobacteria typically dominate the autotrophic biomass in many sub-Arctic freshwaters (Rautio et al., 2011) as they have a very high pigment content (carotenoids), protecting them from radiation (Sahoo & Seckbach, 2015). They have an evolutionary advantage over smaller algae allowing better access to nutrients in aquatic media (Ramanan et al., 2016). Their ability to form algal mats, withstand droughts and freeze–thaw cycles, and the ability to efficiently bind nitrogen gives them a further advantage to dominate the benthos (Vincent & Quesada, 2012). Moreover, generally cyanobacteria have the widest temperature tolerance and a higher temperature optimum over the other algal groups (Tang et al., 1997). Green algae appear mainly as macroscopic, multicellular masses (Stevenson, 1996) and are thus a very important food source for the higher trophic levels (Sahoo & Seckbach, 2015). Especially some genera (e.g. Chlorella) have a great influence on grazing animals in the food web due to their higher concentration of lipids and proteins (Sahoo & Seckbach, 2015). Green algae rarely show up in large masses in the sub-Arctic environment, but as they are strongly seasonal, they may produce algal mats even in oligotrophic sub-Arctic ponds (Maltais & Vincent, 1997). Diatoms are typically more diverse in cold and nutrient-poor waters than other algal groups (Lento et al., 2019) and are mainly influenced by water chemistry (Lotter et al., 2010; Heikkinen et al., 2022; Muñoz-López & Rivera-Rondón, 2022). They are widely used for biomonitoring of surface water quality due to their efficient dispersal capabilities and sensitivity to the environment (Rantala et al., 2017). Although diatoms have a wide geographical distribution, their response to nutrient conditions can vary significantly between ecoregions (Soininen & Niemelä, 2002) and levels of anthropogenic impact (Pajunen et al., 2019).
The aim of this study is to investigate the patterns of total algal biomass as well as cyanobacteria, green algae, and diatom biomasses separately and the different abiotic [i.e. (1) geographical and catchment variables, including snowmelt and ice disappearance day, (2) trace elements and (3) other physical and chemical pond variables], and (4) biotic (i.e. interactions between algal groups) drivers affecting these in sub-Arctic ponds in northern Finland. This study provides new insights into the patterns and drivers affecting benthic algal biomass in such sensitive sub-Arctic ponds, where changes caused by global warming are most likely encountered headmost.
Materials and methods
Study area
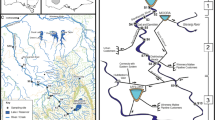
The study area is located in the Finnish Lapland and covers ca. 100 km2 (68° 55′ 39.9″–69° 06′ 40.7″ N; 20° 37′ 15.0″–21° 05′ 37.0″ E). This remote and near-pristine area provides a large variability of pond and catchment characteristics whilst minimizing differences caused by larger climatic and anthropogenic influence. Only some human disturbance may be caused by nearby hiking trails, moderate traffic from roads, and reindeer herding. The sampled ponds (n = 45) were chosen according to their variability in the surrounding catchment characteristics (i.e. vegetation and bedrock), pond surface area, and elevation (Fig. 1). The majority of the ponds are mainly rainwater fed (either entirely from rainfall or a combination of rainfall and snowmelt) while some being dominated by groundwater or snowmelt feeding systems. Most of the studied waterbodies are small in surface area (206–18,100 m2) and exhibit significant water level fluctuations caused by dry periods, whereas only one study location could be classified as a lake (25,800 m2) based on its surface area according to Bengtsson & Herschy (2012). However, as it cannot be considered a significant outlier, we included it in our data and referred to all waterbodies as ponds. The majority of the data, apart from values appearing below detection limit (see laboratory methodology), were collected in August 2021 with a monthly mean temperature of 9.6 °C and precipitation of 38.3 mm at Kilpisjärvi village centre (FMI, 2023). The mean temperature in Kilpisjärvi for the years 1991–2021 is 10.1 °C and precipitation 49.6 mm during August (FMI, 2023) indicating that August 2021 was drier compared to the average, while temperature was more typical but slightly colder for the sampling month.
a The photo shows the use of the BenthoTorch during data collection. The measurements were taken directly from ten different stones under water from a depth of ca. 10–50 cm. b A map of the studied 45 sub-Arctic ponds in northern Finland. c–f The photos show the variation of the studied sub-Arctic ponds at different elevations from low (left; 515 masl) to high (right; 874 masl). Photo credit: Heikkinen J.M. (2020–2021)
Field and laboratory methodology
At each pond, in situ measurements were taken [water pH, water temperature (°C) and conductivity (μS/cm)] with an YSI multiparameter tool (GWM_Engineering Oy, Finland). Water chemistry samples for macronutrients [anions, cations, total nitrogen (TN), and total phosphorus (TP)] were collected into a 250-ml bottle, while micronutrients (i.e. trace elements) were sampled through a 0.45 μm filter into a 10-ml tube comprising 0.05 μl of nitric acid (HNO3). The algal biomasses (i.e. cyanobacteria, green algae, and diatoms) were measured with a BenthoTorch device (bbe Moldaenke GmbH, Germany) by placing it firmly on the surface of ten boulder-sized stones underwater for 10 s each with a maximum depth of 50 cm (Fig. 1a). The median value of the ten measurements from each pond was used in the analyses. Additionally, a coefficient of variation (CV) was calculated from the ten BenthoTorch measurements at each study location in order to evaluate the potential within-pond variation of the measured chlorophyll a. As all the values for chlorophyll a were below 4 µg/cm2, they can be considered reliable based on earlier studies on the BenthoTorch device (Harris & Graham, 2015; Echenique-Subiabre et al., 2016). All water samples were stored in a fridge (4 °C) and protected from light for approximately 3 months until further analysis in the laboratory were able to be done. The pond surface areas and elevations were obtained from satellite images using Google Earth (2014–2017). All maps were created using QGIS version 3.16.2-Hannover, while the final touches for all figures were made in CorelDraw version 24.1.0.360.
All laboratory analyses were conducted at the Helsinki Geophysical, Environmental, and Mineralogical Laboratories (Hellabs) of the Department of Geosciences and Geography, University of Helsinki. TN and TP concentrations were analysed according to standardized methods (EN1189 [1996] for TP and EN ISO11905-1 [1998] for TN). An ion chromatography method (Metrohm ECO double ion chromatography system) was used to analyse the amounts of cations [sodium (Na), potassium (K), magnesium (Mg), and calcium (Ca)] and anions [fluorine (F), chlorine (Cl), nitrate (NO3−), phosphate (PO43−), and sulphate (SO42−)]. In total, 13 trace elements were measured: aluminium (Al), vanadium (V), chromium (Cr), manganese (Mn), silica (Si), iron (Fe), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), selenium (Se), molybdenum (Mo) and cadmium (Cd) with an inductively coupled plasma-mass-spectrometer (ICP-MS Agilent 7800). In order to get a more extensive dataset, a single water chemistry measurement from either 2018, 2019, or 2020 where the value was above detection limit was used, because many of the measured values in 2021 appeared below detection limit (e.g. for TP, the detection limit in 2021 was 22.3 µg/l compared to the detection limits of previous years of 5 µg/l). It is worth noting that water chemistry values have not varied substantially between years as for instance K and Mg showed no statistically significant differences in the concentrations sampled in 2015, 2018, 2020, and 2021 (F = 0.21, P = 0.692 for K and F = 0.051 and P = 0.843 for Mg).
Ice disappearance days (IDD) and days since snowmelt (DSSM) variables for each pond were calculated by utilising information from PlanetScope satellite images (3 m × 3 m resolution) from the years 2017 to 2021. Data from more than 1 year were required because individual years often have long cloudy periods when satellite observations are not available. However, the spatial patterns of snow accumulation and melting remain quite similar across years and thus inference from longer time average should not be significantly biased even when used to characterize conditions for a single year (Niittynen & Luoto, 2018). All images that were at least partly cloud-free (n = 306) over the study area were used. Clouds were manually masked and the spatial accuracy was enchained by cross-coregistering the images. Next, the randomForest model (Liaw & Wiener, 2002) was trained to separate snowy and icy pixels from melted ones from each image. Lastly, the model classification was used in a pixel-wise binomial generalised linear model which determined the average melting date for each pixel (Kemppinen & Niittynen, 2022). Finally, the ice data were calculated as the average melting date over the pixels within each pond, while snow data represent the dates when the last snow was melted in the watersheds. The values were then modified to represent the DSSM for each site, which was calculated from the 1st of August 2021, when sampling began. Negative values were changed to zero.
Data analysis
Statistical analyses were performed in R-software version 4.0.5. (R Development Core Team, 2021). Explanatory variables selected for analysis were checked for multicollinearity through Spearman’s correlation and seven variables (SO4, Na, Cl, F, Ca, Mg, and U) showing higher multicollinearity (r ≥ ± 0.7) with water pH or conductivity were removed since this restricts collinearity-driven effects and improves efficiency of the models (Brun et al., 2019; Fathi et al., 2022). Finally, the selected explanatory variables (except water pH) were logarithmically transformed (log10(x + 1)), to ensure that the data would resemble normally distributed data.
Different model-fitting techniques [least absolute shrinkage and selection operator (LASSO), stepwise Akaike information criterion (stepAIC), and generalized linear model (GLM)] were performed for which a leave-one-out cross-validation (LOOCV) was done to minimize bias and to see which model had the greatest fit without overfitting (Yates et al., 2022). The LOOCV was done through the caret-package in R by using the trainControl and train-functions (Kuhn, 2008). The Root Mean Squared Error (RMSE), r2, and Mean Absolute Error (MAE) were used to evaluate performance of the model. Based on the low RMSE and MAE values and moderate r2 values (RMSEavg = 0.041, MAEavg = 0.033, r2avg = 0.44), the final modelling method chosen to explain the different biomasses was GLM, which is a multivariate method for non-normally distributed data. In the four final GLMs with each benthic algal group biomass and total biomass, four types of explanatory variables were included: (1) geographical and catchment variables including elevation, DSSM, and IDD, (2) physical and chemical variables including surface area, conductivity, water temperature, water pH, anions, and cations, (3) trace element variables, and (4) biotic variables the latter meaning that for each algal group the biomasses of other groups were included as predictors that significantly (P < 0.05) explained the biomass variations. TP was also included in the final models despite its low significance as it has previously been found to be a limiting macronutrient for microalgae (Mischler et al., 2014; Poikane et al., 2022). In addition to the final models with abiotic and biotic variables, all biomasses were modelled through GLM without biotic variables to better understand the potential interactions between the benthic algal groups (Online Resource 1). As all data were normally distributed and continuous, biomass distributions were analysed through Gaussian distribution with only using first degree terms to improve model functioning and as variables showed linear regression according to scatterplots.
The importance of explanatory variables from the models were interpreted through hierarchical partitioning by using the hier.part-function within the hier.part-package (Walsh & Mac Nally, 2020). This method is based on z-scores and randomisation, so that the partitioning of the contributions provides an estimate of the most important predictors in a multivariate dataset. The function calculates the goodness of fit measures for regressions of each biomass using all combinations of significant explanatory variables in the GLM. However, the hier.part function allows a maximum of 12 variables in order to perform well. Thus, for cyanobacteria biomass, DSSM, Cr, and Zn were dropped due to their higher P values (P > 0.034) and lower estimates (β < 0.079) in the GLM compared to other significant explanatory variables.
Results
Our data showed a maximum snow depth at the Kilpisjärvi village centre weather station in winter 2020–2021 was 99 cm, which was slightly lower than the 1991–2021 median of 106 cm. Water temperature varied substantially among the studied ponds and had a negative correlation with elevation (r = − 0.50; Online Resource 2). Only one sample had TN above 1 mg/l. The water pH ranged from 4.32 to 7.39 with a median value of 5.93 (Table 1). Si had the highest concentration out of the measured trace elements in the study area while showing moderate correlation with conductivity (r = 0.57). Other trace element concentrations, except Fe and Al, were generally below 10 µg/l. Out of the studied macronutrients, K showed higher concentrations compared to NO3− while having a higher correlation with conductivity (r = 0.59). Generally conductivity was very low (< 40 µS/cm).
Total algal biomass varied from 0.10 to 1.58 µg/cm2 among the ponds (Fig. 2). Both diatom and green algae biomass had a minimum value of 0 but green algae biomass ranged up to 0.68 µg/cm2, while diatom biomass ranged up to 0.42 µg/cm2. Cyanobacteria biomass varied from 0.06 µg/cm2 to a maximum of 0.81 µg/cm2 within the study area. Cyanobacteria comprised most of the total biomass with 59.2%, while green algal biomass covered 33.5% and diatom biomass only 7.3% of total biomass across the ponds. The median CV of all measured chlorophyll a values for green algae and cyanobacteria were 0.5 and 0.46, respectively, showing low variance. Meanwhile, for diatoms, the CV was over 1 indicating that there is high variability in the measured chlorophyll a values between ponds. CV weakly increased for all benthic algal biomasses with elevation but was significant only for green algae showing greater variation in the measured chlorophyll a values at more pristine and harsh sub-Arctic ponds in terms of environmental conditions compared to cyanobacteria and diatom biomass.
In all biomass GLMs, TP was found insignificant. Out of all the explanatory variables for all biomass models, 60% were explained by trace elements. The significant explanatory variables in the GLM for total benthic algal biomass (explained 70.54% of the total variation including TP) consisted of only significant trace element variables of Al, Fe, Cr, As, Se, and Pb (Table 2). Diatom biomass was explained (38.13%) by two significant variables: Se and green algal biomass. Cyanobacteria biomass (78.96%) had the greatest number of significant variables (n = 14) in the model, consisting of variables from all four explanatory variable groups. The significant variables were water pH, surface area, DSSM, elevation, pond temperature, K, Al, Fe, Cr, Cu, Zn, Se, Pb, and green algal biomass. The variation in green algae was explained by variables from all variable groups apart from geographical and catchment variables and its explained variation was 73.02%. The significant variables for green algal biomass included water pH, surface area, V, Mn, Cr, Si, and cyanobacteria biomass.
According to hierarchical partitioning, the most influential variable on total algal biomass was Fe explaining roughly a third (33.2%) of the explainable variation, while As and Se each explained less than 20%, all having a positive relationship with biomass (Fig. 3a). Cyanobacteria biomass had multiple significant explanatory variables, which cannot be accurately interpreted from hierarchical partitioning due to restricting variable number by the hier.part-function. The most influential variables shown for cyanobacteria included elevation (11.2%) and K (10.4%) both with a negative relationship, and Se (9.9%) with a positive relationship but their effects were overall relatively low (Fig. 3b). For diatom biomass, green algae biomass (positive relationship) explained 59% of the explainable variation, while Se explained over 36% also with a positive relationship (Fig. 3c). For green algal biomass, Mn (positive relationship) explained over 33% of the variation, while other variables explained under 19% of the total variation (Fig. 3d).
The independent effects (%) of the significant variables from the GLMs affecting a total benthic algal biomass, b cyanobacteria biomass, c diatom biomass, and d green algal biomass using hierarchical partitioning. Next to each variable is the linear effect on the different biomasses (positive/negative). The function hier.part in R allows for a maximum of 12 variables. Thus for cyanobacteria, three weakest variables according to their estimates and P values (excluding TP) were removed in order to run the hier.part function
Discussion
Here, we investigated the patterns and drivers of benthic algal biomasses in sub-Arctic mountain ponds. Of the algal groups, cyanobacteria had the highest and diatoms had the lowest biomass. The drivers affecting benthic algal group biomasses predominantly consisted of different trace elements such as Fe, As, Se, and Pb, while geographical and catchment variables had less importance. Next, the major patterns found as well as the key drivers for the four variable groups for benthic algal biomasses are discussed.
The influence of geographical and catchment variables
Of the geographical and catchment variables, elevation was the only variable that had a significant negative relationship with cyanobacteria biomass. This can be linked to greater environmental harshness with increasing elevation as well as having a moderate negative correlation with water temperature also showing a significant negative relationship. The influence of water temperature on biomass is discussed in more detail in the paragraph about physical and chemical pond characteristics.
DSSM had a significant positive relationship with cyanobacteria suggesting that cyanobacteria biomass increases with greater number of days since a snow-free catchment. A longer snow-free period has most likely increased cyanobacteria biomass production as it may begin already in colder waters. IDD showed no significance in explaining any of the algal biomasses. Despite this, IDD could be regarded as a proxy for water temperature and light availability in sub-Arctic ponds (Wrona et al., 2006). Additionally, both IDD and DSSM may have an indirect effect on algal biomass by altering the chemical composition of the ponds through different sources (i.e. lithogenic and atmospheric) of nutrient input through catchment runoff. In general, IDD and DSSM may have a notable role in shaping algal biomasses and should thus be included in future research when investigating the key drivers in algal communities.
The influence of physical and chemical pond characteristics excluding trace elements
Of the physical variables, pond surface area had a significant positive relationship with cyanobacteria and a significant negative relationship with green algal biomass. The positive relationship on cyanobacteria can be related to greater habitat size with possibly a more stable pond ecosystem. However, our finding should be viewed with caution as samples were collected on one shore, which may not indicate accurately the biomass of larger waterbodies. Thus, a closer investigation of algal biomass variation around the ponds in the future research can be encouraged.
For the shallow, northern, and clear-watered ponds, the TP threshold for a good to moderate status is between 20 and 34 µg/l (Poikane et al., 2022). Based on this classification, over 82% of the studied ponds can be considered to be within the good–moderate status for TP, while over 93% of the ponds showed good–moderate status for TN. Of the studied macronutrients, only K showed a significant negative relationship with cyanobacteria biomass. Our finding agrees with a previous study on the toxic effect of K on the photosynthesis of marine phytoplankton (Kusk & Nyholm, 1992). However, this may be questioned as our measurements were below 1 mg/l, while in marine environments the concentrations were higher. Despite the inclusion of TP in the models, it was insignificant for all biomass models agreeing with previous research on Arctic lakes (Van Geest et al., 2007; Dranga et al., 2018). However, for high altitude lakes in Qinghai–Tibet, TP showed a positive correlation for benthic algal communities (Yang et al., 2018). As most macronutrients were insignificant in the present study, they may not be a strong limiting factor for benthic algae in the sub-Arctic unlike in lower latitudes (Sun et al., 2022). Overall, the low chlorophyll a values and oligotrophic pond conditions result from to low anthropogenic impact and productivity, as well as nutrient-poor soils (Wetzel, 2001) while for an example boreal and tropical freshwater streams typically have notably higher algal biomasses (Guo et al., 2020; Rosero-López et al., 2021).
Overall, water pH had a significant positive relationship with green algal biomass while showing a negative relationship with cyanobacteria biomass agreeing with a study from northern Canada (Dranga et al., 2018). Pond temperature showed a negative relationship with cyanobacteria biomass disagreeing with previous research (Whittaker et al., 1974; Paerl & Huisman, 2009). However, more eutrophic and larger lakes have shown no significant relationship (Havens et al., 2019), but they also indicated that cyanobacteria biomass never reached a high biomass at water temperatures of ≤ 10 °C again disagreeing with our study. In contrast, direct comparisons are difficult between these study settings due to their variable water temperatures as well as potentially different species compositions (Havens et al., 2019). Despite some studies finding that cyanobacteria tend to favour warmer waters due to a higher temperature optimum (Tang et al., 1997), they are also tolerant towards colder water temperatures even being able to withstand freezing to some extent (Vincent & Quesada, 2012). Therefore, it is likely that the drivers for cyanobacteria biomass varies substantially between different environmental conditions.
The influence of trace elements
As with the above-discussed macronutrients, the chemical composition may vary significantly between the water column and benthos (Battin et al., 2007). The benthic algae are able to utilize nutrients from the benthos more efficiently leaving the water column more nutrient poor. The significant impact of trace elements, as reported in the present study, highlights their importance on algal biomasses. It is worth noting, that as total algal biomass consists of the three biotic variable groups, the variables affecting the three individual algal biomasses will have an effect on total benthic algal biomass. Conversely, a variable influencing total algal biomass has an effect on the three algal biomass groups given that they showed a moderate to high correlation with total algal biomass in our study (Online Resource 2). Before discussing the individual influences of trace elements on algal biomasses, it is worth mentioning that each trace element originates from a different source (e.g. atmospheric, lithogenic, or anthropogenic) affecting positively or negatively algal biomasses either as growth promoters or photosynthetic rate reducers.
Chromium, zinc, and lead
High concentrations of trace elements may have an adverse effect on algal communities, such as in the case for Cr for cyanobacteria biomass, green algal biomass, and total algal biomass as found in our study. For example, in surface sea waters in the Southern Atlantic (Muse et al., 1999), Cr concentrations were relatively low (0.25 µg/l and 0.65 µg/l; mean and maximum) compared to our study (1.40 µg/l and 6.65 µg/l). In the study by Muse et al. (1999), Cr concentrations within green algae were high (0.3–4.6 µg/l) despite its potential toxic characteristics, and thus it could be considered nontoxic in the Southern Atlantic. Conversely, in our study, Cr concentrations possibly exceeded a certain threshold for toxicity towards algal biomass leading to a negative relationship. Muse et al. (1999) showed a similar response for Zn as with Cr: algal trace element concentrations were significantly higher compared to the surface water concentrations. The results here somewhat disagree with other studies on phytoplankton, where Zn concentrations had a positive or no effect on growth rates until a threshold of 38 µg/l (Zhihong et al., 2010). In our study, only cyanobacteria biomass showed a significant negative relationship with Zn despite lower concentrations (< 13.11 µg/l), which could be explained by a lower Zn requirement for prokaryotes compared to eukaryotes (reviewed by Blindauer, 2008) potentially indicating a lower threshold for Zn toxicity for cyanobacteria in sub-Arctic ponds. Pb had a significant negative effect on cyanobacteria and total algal biomass agreeing with the findings from eastern China (Gu et al., 2020). However, the lower Pb concentrations found here may still reflect the toxic effects of Pb despite measured from the water column and not from the benthos due to their potentially very different chemical compositions (Battin et al., 2007).
Manganese and copper
Mn had a significant positive relationship with green algal biomass agreeing with a previous study done in a canopy-free reach of Llano River (Texas, USA), where similar Mn concentrations were found to increase algal richness (Passy & Larson, 2019). The positive role of Mn on green algal biomass is perhaps due to solubilization of particulate Mn by photoreduction due to irradiance (Marsay et al., 2018). A cultivation experiment on cyanobacteria and green algae showed a unimodal response in growth rates with a peak Mn value of 130 µg/l (Zhihong et al., 2010). As concentrations reported here were below 10 µg/l in sub-Arctic mountain ponds, Mn enhances algal growth rates. Cu had a significant positive relationship with cyanobacteria biomass, while Passy & Larson (2019) found that Cu had a similar positive relationship than Mn on algal biomass, which would agree on the synergistic functioning of the trace elements, where Cu may work together with Mn and NO3− to lower potential toxicity caused by other elements in freshwaters (Stauber & Florence, 1985a, 1985b; Gupta, 1989). The presence of Cu in small concentrations (< 1 µg/l) can be considered a vital micronutrient (Krause et al., 2021) and as 77.8% of ponds in our study were below this, Cu may have a vital role in sub-Arctic ponds for cyanobacteria biomass. Furthermore, a culturing experiment on dominant benthic algae in moderately and lightly polluted rivers in southern Taiwan (Lai et al., 2003) revealed that dominant algal species [Oscillatoria chalybea Mertens ex Gomot, Euglena acus (O.F. Müller), and Nitzschia palea (Kützing) W. Smith] could survive high concentrations of Cu (max. 60 µg/l), indicating a high tolerance towards Cu and supporting the positive relationship of Cu with cyanobacteria found here.
Silica and aluminium
Si had the greatest concentrations out of all measured trace elements in our study and it had a negative relationship on green algal biomass, contradicting the experimental research done in Lake Michigan, USA (Carrick & Lowe, 2007), where it showed a secondary limitation after phosphorus. Despite similar Si concentrations measured here and in previous studies, no significant primary limitation (i.e. limitation of a single nutrient) was found. Moreover, an earlier study on phytoplankton limiting nutrients in Lake Champlain, USA, growth was not limited by Si despite showing similar Si concentrations (Levine et al., 1997). Furthermore, Si may be a natural blocker for Al toxicity in freshwaters for green algae at over 2.8 mg/l (Exley et al., 1993), which should promote green algal biomass despite showing a different response here. However, in the study by Exley et al. (1993), nutrient data were taken from the culture media. It may be suggested that some fraction of the measured Si concentrations may be as nanoparticles from different sources (e.g. natural environment, biological, and industrial systems) or with a different morphology (Yang et al., 2019), which have shown a negative relationship with green algae (Van Hoecke et al., 2008). Al had a significant negative relationship with total algal biomass and cyanobacteria biomass. Al is commonly used as a treatment method against cyanobacteria blooms, meaning that the addition of Al and Al-specific compounds (e.g. polyaluminium chloride) will decrease cyanobacterial biomass (Jančula et al., 2011), which is also connected with acidic waters (Havens & Heath, 1990). The negative relationship of Al with cyanobacteria biomass despite its lower concentrations compared with previous literature indicates a low toxicity threshold for Al in sub-Arctic ponds. Also, Al concentrations might help us explain the potential nutrient limitation as it enhances phosphate binding, making phosphorus less available for the benthic community constraining phosphorus-limited systems (Brönmark & Hansson, 2017) affecting phytoplankton growth and succession (Exley et al., 1993).
Selenium, arsenic, vanadium, and iron
Se had a significant positive relationship with all biomasses apart from green algae. The strong positive effect of Se on diatom biomass could be explained by its requirement for various metabolic and physiological functions, while some cyanobacteria species have not shown a similar requirement (Wake et al., 2012). Se nanoparticles (≤ 50 mg/l) boosted diatom biomass growth in marine environments (Kumar et al., 2022) as well as biomagnifying throughout the aquatic ecosystem affecting higher trophic levels (Jasonsmith et al., 2008; Kamau et al., 2014). Se concentrations were lower in sub-Arctic mountain ponds compared to previous studies, and thus the role of Se required for photosynthesis and growth is vital. However, as noted before, Se concentrations may be significantly higher in the benthos as found in Lake Wallace, Australia, where benthic algal Se concentrations were over four times higher (Jasonsmith et al., 2008). Furthermore, the correlation between Se and biomass is likely to be unimodal with a peak value of 2 µg/l (Jasonsmith et al., 2008). However, Banerjee et al. (2021) noted a decrease in cell structure at Se concentrations of above 100 mg/l, indicating very different Se concentrations measured from the benthos. Our study showed that As had a positive relationship with total algal biomass, challenging the findings on an experimental study mimicking fluvial conditions where exposure of As under oligotrophic conditions was found to affect the quality and quantity of the base of the aquatic food web (Tuulaikhuu et al., 2015). In bioremediation, As is being heavily removed from the water column by different biofilms in order to improve the quality of water (Prieto et al., 2013) leading to notably higher As concentrations in the biofilm. Here, diatom biomass showed a positive relationship with As when excluding biotic interactions (Online Resource 1) disagreeing with a study on lakes along a large As gradient (Sivarajah et al., 2019). It has been reported that in phosphorus-limited systems, As uptake and excretion by algae is enhanced (Hellweger et al., 2003). Therefore, it could be that when As is being absorbed by the benthos, it is being transported towards higher trophic levels where it may have a toxic effect, decreasing grazing and thus increasing benthic algal biomass. In sub-Arctic ponds however, As could be used rapidly within the cell by bioaccumulation or biosorption without having an effect on algae (Hellweger et al., 2003; Jahan et al., 2004). According to our research, V had a positive relationship with green algal biomass agreeing with a study on Chlorella vulgaris Beijerinck, where non-living biomass was more favourable for V absorption (Shu et al., 2022). This may suggest, that the majority of green algal species living in sub-Arctic ponds are non-living. However, it is worth noting that in the culture study, V concentrations were over 100 times greater. Diatom biomass also showed a positive relationship with V when excluding biotic interactions as found in the present study, despite being a common pollutant (Teng et al., 2006). Despite showing a positive relationship with specific diatom species such as Meridion circulare (Greville) C. Agardh (Slukovskiy & Shelekhova, 2018). According to our present findings, Fe had a positive relationship with cyanobacteria biomass agreeing with a study on Lyngbya majuscula Harvey ex Gomont from a bay (SE Queensland, Australia), where the addition of organically chelated Fe promoted growth (Ahern et al., 2008). The increase in cyanobacterial biomass was also observed to promote total algal biomass, which agrees with our findings despite lower Fe concentrations.
The influence of biotic variation
A positive relationship between cyanobacteria and green algal biomasses and vice versa as well as a positive relationship between green algal and diatom biomasses were found in the present study. Such studies about the relationships between biotic variables have received only little attention in freshwaters benthos (Holomuzki et al., 2010). When testing the different biomass models without biotic terms, these suggested that green algal biomass could potentially act as a proxy for V, Mn, and Cr for diatom biomass (Online Resource 1). This could indicate a sort of facilitation between green algae and diatoms, where species benefit from the same resources without harm (Bruno et al., 2003). Both Mn and V concentrations seem to be sufficient in order to promote green algal and diatom biomasses due to their mutual positive relationship. Because both cyanobacteria and diatom biomasses showed a positive linear relationship with green algal biomass while sharing some key abiotic drivers like water pH, surface area, Se, and Cr, it could be that there are some unmeasured variables affecting growth like TN (or some other nutrient form) which did show significance in our study. It can be suggested that due to the nitrogen fixation by especially heterocytous cyanobacteria (Watanabe & Horiike, 2021), nitrogen becomes available for the other algal groups as well (Karlson et al., 2015), which may promote growth shifting the benthic algal composition in nitrogen-limited environments (Marks and Power, 2001). Heterocytes contain an enzyme called nitrogenase, which allows these cyanobacteria to fix atmospheric molecular nitrogen (Esteves-Ferreira et al., 2017). For further information, species compositional information is required in order to know the types of cyanobacteria living in these sub-Arctic mountain ponds. Thus, we suggest future research to investigate the dominant species regarding the benthic algal groups for a more detailed description of benthic algal species assemblages. Despite the low nutrient levels and the limited extent of the growing season, the three algal groups showed no clear negative associations, suggesting that there may not be competitive interactions for the same resources (Yamamichi et al., 2018). The results presented in our research agree with studies conducted in sub-Arctic ponds in Iceland (McCormick et al., 2019) and in a shallow tropical river (Burrows et al., 2020), where algal communities are more controlled by abiotic factors. Due to the effective metabolic mechanisms of cyanobacteria, it is believed that they will dominate over the other algal groups in environments with low nutrient conditions (Beardall & Raven, 2017). It was reported in the present study that cyanobacteria biomass was the greatest out of the three benthic algal groups, and while it agrees with the previous study, it disagrees with a study conducted in an alpine stream where cyanobacteria showed the lowest chlorophyll a concentrations among benthic algal groups (Grubisic et al., 2017).
Conclusion
In order to better understand the effects of climate change on benthic algal biomass, which is the most profound contributor towards primary production in sub-Arctic ecosystems, studying mountain ponds provides indications of shifting climatic conditions and helps comprehend the vulnerability of sub-Arctic ecosystems towards climate change. Our study highlighted the importance of trace elements for benthic algal biomass instead of macronutrients suggesting that trace elements could be a useful indicator for algal biomass in ultra-oligotrophic conditions as well as in other aquatic settings (Lai et al., 2003). One additional option for future research is to measure soluble phosphorus concentrations, which however requires more efficient sample preservation methods in field conditions. Moreover, enhanced chemical data (e.g. on nanoparticles) should be collected and investigated in the future to quantify for instance micronutrient ratios in these systems as they may play an important role in determining benthic algal community structures. For a more detailed species assemblage description, a compositional analysis could also be done in the future in order to better understand the dominant species present in such habitats. An ongoing climate change is expected to promote significant changes in abiotic and biotic variables in freshwater ecosystems in the sub-Arctic, potentially increasing algal biomasses. The changes may occur within the community-level caused by changes in metabolic rates (Kraemer et al., 2017; Küfner et al., 2021) but also reflected as changes in interactions between biotic communities. To better understand these changes, the main focus should be on shallow, acidic, and oligotrophic ponds where changes occur more rapidly compared to larger freshwater bodies that buffer temperature increases and trap nutrients during the summer stratification more efficiently making them perhaps more resilient to warming. Aquatic research around the circumpolar Arctic has increased over recent years, but much more knowledge about these vulnerable aquatic environments remains to be gathered in order to better understand the effects of global change.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Agrawal, C. S., 2012. Factors controlling induction of reproduction in algae – review: the text. Folia Microbiologica 57: 287–407. https://doi.org/10.1007/s12223-012-0147-0.
Ahern, K. S., C. R. Ahern & J. W. Udy, 2008. In situ field experiment shows Lyngbya majuscule (cyanobacterium) growth stimulated by added iron, phosphorus and nitrogen. Harmful Algae 7: 389–404. https://doi.org/10.1016/j.hal.2007.08.006.
Banerjee, M., P. Kalwani, D. Chakravarty, B. Singh & A. Ballal, 2021. Functional and mechanistic insights into the differential effect of the toxicant ‘Se(IV)’ in the cyanobacterium Anabaena PCC 7120. Aquatic Toxicology. https://doi.org/10.1016/j.aquatox.2021.105839.
Battin, T. J., W. T. Sloan, S. Kjellberg, H. Daims, I. M. Head, T. P. Curtis & L. Eberl, 2007. Microbial landscapes: new paths to biofilm research. Nature Reviews Microbiology 5: 76–81. https://doi.org/10.1038/nrmicro1556.
Beardall, J. & J. A. Raven, 2017. Cyanobacteria vs green algae: which group has the edge? Journal of Experimental Botany 68: 2697–3699. https://doi.org/10.1093/jxb/erx226.
Bengtsson, L. & R. W. Herschy, 2012. Preface. In Bengtsson, L., R. W. Herschy & R. W. Fairbridge (eds), Encyclopedia of Lakes and Reservoirs. Springer, Dordrecht.
Blindauer, C. A., 2008. Zinc-handling in cyanobacteria: an update. Chemistry and Biodiversity 5: 1990–2012. https://doi.org/10.1002/cbdv.200890183.
Brönmark, C. & L.-A. Hansson, 2017. Biodiversity and environmental threats. In Brönmark, C. & L.-A. Hansson (eds), The Biology of Lakes and Ponds. Oxford University Press, Oxford.
Brun, P., W. Thuiller, Y. Chauvier, L. Pellissier, R. O. Wüest, Z. Wang & N. E. Zimmermann, 2019. Model complexity affects species distribution projections under climate change. Journal of Biogeography 47: 130–142. https://doi.org/10.1111/jbi.13734.
Bruno, J. F., J. J. Stachowicz & M. D. Bertness, 2003. Inclusion of facilitation into ecological theory. Trends in Ecology and Evolution 18: 119–125. https://doi.org/10.1016/S0169-5347(02)00045-9.
Burrows, R. M., L. Beesley, M. M. Douglas, B. J. Pusey & M. J. Kennard, 2020. Water velocity and groundwater upwelling influence benthic algal biomass in a sandy tropical river: implications for water-resource development. Hydrobiologia 847: 1207–1219. https://doi.org/10.1007/s10750-020-04176-3.
Carrick, H. J. & R. L. Lowe, 2007. Nutrient limitation of benthic algae in Lake Michigan: the role of silica. Journal of Phycology 43: 228–234. https://doi.org/10.1111/j.1529-8817.2007.00326.x.
Chiu, M.-C., S. Ao, F. He, V. H. Resh & Q. Cai, 2020. Elevation shapes biodiversity patterns through metacommunity-structuring processes. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2020.140548.
Culp, J. M., W. Goedkoop, T. Christensen, K. S. Christoffersen, E. Fefilova, P. Liljaniemi, A. A. Novichkova, J. S. Ólafsson, S. Sandøy, C. E. Zimmerma & J. Lento, 2021. Arctic freshwater biodiversity: establishing baselines, trends, and drivers of ecological change. Freshwater Biology 67: 1–13. https://doi.org/10.1111/fwb.13831.
Dasauni, K., N. Divya & T. K. Nailwal, 2022. Cyanobacteria in cold ecosystems: tolerance and adaptation. In Goel, R., R. Soni, D. C. Suyal & M. Khan (eds), Survival Strategies in Cold-adapted Microorganisms. Springer, Singapore. https://doi.org/10.1007/978-981-16-2625-8_1.
Dauvalter, V. A. & N. A. Kashulin, 2014. Flow of heavy metals (Ni and Cu) in the catchment area of a sub-Arctic lake. Contemporary Problems of Ecology 7: 375–383. https://doi.org/10.1134/S1995425514040027.
Dranga, S. A., S. Hayles & K. Gajewski, 2018. Synthesis of limnological data from lakes and ponds across Arctic and Boreal Canada. Arctic Science 4: 167–185. https://doi.org/10.1139/as-2017-0039.
Echenique-Subiabre, I., C. Dalle, C. Duval, M. W. Heath, A. Couté, S. A. Wood, J.-F. Humbert & C. Quiblier, 2016. Application of a spectrofluorimetric tool (bbe BenthoTorch) for monitoring potentially toxic benthic cyanobacteria in rivers. Water Research 15: 341–350. https://doi.org/10.1016/j.watres.2016.05.081.
Esteves-Ferreira, A. A., J. H. F. Cavalcanti, M. G. M. V. Vaz, L. V. Alvarenga, A. Nunes-Nesi & W. L. Aráújo, 2017. Cyanobacterial nitrogenases: phylogenetic diversity, regulation and functional predictions. Genetics and Molecular Biology 40: 261–275. https://doi.org/10.1590/1678-4685-GMB-2016-0050.
Exley, C., A. Tollervey, G. Gray, S. Roberts & D. Birchall, 1993. Silicon, aluminium and the biological availability of phosphorus in algae. Proceedings of the Royal Society of Biological Sciences 253: 93–99. https://doi.org/10.1098/rspb.1993.0086.
Fathi, P., E. E. Dorche, M. Z. Shahraki, J. Stribling, O. B. Kashkooli, A. E. Ofogh & A. Bruder, 2022. Revised Iranian Water Quality Index (RIWQI): a tool for the assessment and management of water quality in Iran. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-022-10121-9.
FMI, 2023. Havaintojen lataus [available on internet at: https://www.ilmatieteenlaitos.fi/havaintojen-lataus].
Grubisic, M., G. Singer, M. C. Bruno, R. H. A. van Grunsven, A. Manfrin, M. T. Monaghan & F. Hölker, 2017. Artificial light at night decreases biomass and alters community composition of benthic primary producers in a sub-alpine stream. Limnology and Oceanography 62: 2799–2810. https://doi.org/10.1002/lno.10607.
Gu, P., Q. Li, W. Zhang, Z. Zheng & X. Luo, 2020. Effects of different metal ions (Ca, Cu, Pb, Cd) on formation of cyanobacterial blooms. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2019.109976.
Guo, K., N. Wu, P. Manolaki, A. Baattrup-Pedersen & T. Riis, 2020. Short-period hydrological regimes override physico-chemical variables in shaping stream diatom traits, biomass and biofilm community functions. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2020.140720.
Gupta, S. L., 1989. Interactive effects of nitrogen and copper on growth of cyanobacterium Microcystis. Bulletin of Environmental Contamination and Toxicology 42: 270–275. https://doi.org/10.1007/BF01699410.
Harris, T. D. & J. L. Graham, 2015. Preliminary evaluation of an in vivo fluorometer to quantify algal periphyton biomass and community composition. Lake and Reservoir Management 31: 127–133. https://doi.org/10.1080/10402381.2015.1025153.
Havens, K. E. & R. T. Heath, 1990. Phytoplankton succession during acidification with and without increasing aluminium levels. Environmental Pollution 68: 129–145. https://doi.org/10.1016/0269-7491(90)90017-7.
Havens, K. E., G. Ji, J. R. Beaver, R. S. Fulton & C. E. Teacher, 2019. Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change. Hydrobiologia 829: 43–59. https://doi.org/10.1007/s10750-017-3425-7.
Heikkinen, J. M., J. Aalto, O. Rantamäki, T. Ruikkala, J. Soininen & V. Pajunen, 2022. Observing diatom diversity and community composition along environmental gradients in sub-Arctic mountain ponds. Freshwater Biology 67: 731–741. https://doi.org/10.1111/fwb.13877.
Hellweger, F. L., K. J. Farley, U. Lall & D. M. Di Toro, 2003. Greedy algae reduce arsenate. Limnology and Oceanography 48: 2275–2288. https://doi.org/10.4319/lo.2003.48.6.2275.
Holomuzki, J. R., J. W. Feminella & M. E. Power, 2010. Biotic interactions in freshwater benthic habitats. Journal of the North American Benthological Society 29: 220–244. https://doi.org/10.1899/08-044.1.
Huot, Y., M. Babin, F. Bruyant, C. Grob, M. S. Twardowski & H. Claustre, 2007. Does chlorophyll a provide the best index of phytoplankton biomass for primary productivity studies? Biogeosciences Discussions 4: 707–745. https://doi.org/10.5194/bgd-4-707-2007.
Huser, B. J., M. N. Futter, D. Bogan, J. E. Brittain, J. M. Culp, W. Goedkoop, I. Gribovskaya, J. Karlsson, D. C. P. Lau, K. M. Rühland, A. K. Schartau, R. Shaftel, J. P. Smol, T. Vrede & J. Lento, 2020. Spatial and temporal variation in Arctic freshwater chemistry – reflecting climate-induced landscape alterations and a changing template for biodiversity. Freshwater Biology 67: 14–29. https://doi.org/10.1111/fwb.13645.
Jahan, K., P. Mosto, C. Mattson, R. Frey & L. Derchak, 2004. Metal uptake by algae. In Popov, V., H. Itoh, C. A. Brebbia & S. Kungolos (eds), Waste Management and the Environment II. WIT Press, Southampton.
Jančula, D., P. Mikula & B. Maršálek, 2011. Effects of polyaluminium chloride on the freshwater invertebrate Daphnia magna. Chemistry and Ecology 27: 351–357. https://doi.org/10.1080/02757540.2011.575373.
Jasonsmith, J. F., W. Maher, A. C. Roach & F. Krikowa, 2008. Selenium bioaccumulation and biomagnification in Lake Wallace, New South Wales, Australia. Marine and Freshwater Research 59: 1048–1060. https://doi.org/10.1071/MF08197.
Kahlert, M., K. M. Rühland, I. Lavoie, F. Keck, E. Saulinier-Talbot, D. Bogan, R. B. Brua, S. Campeau, K. S. Christoffersen, J. M. Culp, S. M. Karjalainen, J. Lento, S. C. Schneider, R. Shaftel & J. P. Smol, 2020. Biodiversity patterns of Arctic diatom assemblages in lakes and streams: current reference conditions and historical context for biomonitoring. Freshwater Biology 67: 116–140. https://doi.org/10.1111/fwb.13490.
Kamau, J. N., A. Gachanja, C. Ngila, J. M. Kazungu & M. Zhai, 2014. The seasonal influence on the spatial distribution of dissolved selected metals in Lake Naivasha, Kenya. Physics and Chemistry of the Earth 67–69: 111–116. https://doi.org/10.1016/j.pce.2013.10.003.
Kamenik, C., R. Schmidt, G. Kum & R. Psenner, 2001. The influence of catchment characteristics on the water chemistry of mountain lakes. Arctic, Antarctic, and Alpine Research 33: 404–409. https://doi.org/10.2307/1552549.
Karlson, A. M. L., J. Duberg, N. H. Motwani, H. Hogfors, I. Klawonn, H. Ploug, J. B. Svedén, A. Garbaras, B. Sundelin, S. Hajdu, U. Larsson, R. Elmgren & E. Gorokhova, 2015. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio 44: 413–426. https://doi.org/10.1007/s13280-015-0660-x.
Kemppinen, J. & P. Niittynen, 2022. Microclimate relationships of intraspecific trait variation in sub-Arctic plants. Oikos. https://doi.org/10.1111/oik.09507.
Kraemer, B. M., T. Mehner & R. Adrian, 2017. Reconciling the opposing effects of warming on phytoplankton biomass in 188 large lakes. Scientific Reports. https://doi.org/10.1038/s41598-017-11167-3.
Krause, J., M. J. Hopwood, J. Höfer, S. Kirsch, E. P. Achterberg, E. Alarcón, D. Carroll, H. E. González, T. Juul-Pedersen, T. Liu, P. Lodeiro, L. Meire & M. T. Rosing, 2021. Trace element (Fe Co, Ni and Cu) dynamics across the salinity gradient in Arctic and Antarctic glacier fjords. Frontiers in Earth Science. https://doi.org/10.3389/feart.2021.725279.
Kuhn, M., 2008. Building predictive models in R using the caret package. Journal of Statistical Software. https://doi.org/10.18637/jss.v028.i05.
Kumar, C. M. V., V. Karthick, D. Inbakandan, V. G. Kumar, E. R. Rene, T. S. Dhas, M. Ravi, P. Sowmiya & C. G. A. Das, 2022. Effect of selenium nanoparticles induced toxicity on the marine diatom Chaetoceros gracilis. Process Safety and Environmental Protection 163: 200–209. https://doi.org/10.1016/j.psep.2022.05.021.
Küfner, W., A. M. Hofmann, J. Geist, N. Dubois & U. Raeder, 2021. Algal community change in mountain lakes of the Alps reveals effects of climate warming and shifting treelines. Journal of Phycology 57: 1266–1283. https://doi.org/10.1111/jpy.13163.
Kusk, K. O. & N. Nyholm, 1992. Toxic effects of chlorinated organic compounds and potassium dichromate on growth rate and photosynthesis of marine phytoplankton. Chemosphere 25: 875–886. https://doi.org/10.1016/0045-6535(92)90079-7.
Lai, S.-D., P.-C. Chen & H.-K. Hsu, 2003. Benthic algae as monitors of heavy metals in various polluted rivers by energy dispersive X-ray spectrometer. Journal of Environmental Science and Health 38: 855–866. https://doi.org/10.1081/ESE-120018596.
Lakka, H.-K., 2020. Environmental changes in Arctic freshwaters: the response of indicator species to global warming and acidification in the Arctic. PhD Thesis, University of Jyväskylä.
Lau, D. C. P., K. S. Christoffersen, J. Erkinaro, B. Hayden, J. Heino, S. Hellsten, K. Holmgren, K. K. Kahilainen, M. Kahlert, S. M. Karjalainen, J. Karlsson, L. Forsström, J. Lento, M. Mjelde, J. Ruuhijärvi, S. Sandøy, A. K. Schartau, M.-A. Svenning, T. Vrede & W. Goedkoop, 2019. Multitrophic biodiversity patterns and environmental descriptors of sub-Arctic lakes in northern Europe. Freshwater Biology 67: 30–48. https://doi.org/10.1111/fwb.13477.
Lento, J., W. Goedkoop, J. Culp, K. S. Christoffersen, K. F. Lárusson, E. Fefilova, G. Guðbergsson, P. Liljaniemi, J. S. Ólafsson, S. Sandøy, C. Zimmerman, T. Christensen, P. Chambers, J. Heino, S. Hellsten, M. Kahlert, F. Keck, S. Laske, D. Chun Pong Lau, I. Lavoie, B. Levenstein, H. Mariash, K. Rühland, E. Saulnier-Talbot, A. K. Schartau & M. Svenning, 2019. State of the Arctic Freshwater Biodiversity. Conservation of Arctic Flora and Fauna International Secretariat, Akureyri [available on internet at: https://www.caff.is/freshwater/freshwater-monitoring-publications/488-state-of-the-arctic-freshwater-biodiversity-report-full-report].
Lento, J., J. M. Culp, B. Levenstein, J. Aroviita, M. A. Baturina, D. Bogan, J. E. Brittain, K. Chin, K. S. Christoffersen, C. Docherty, N. Friberg, F. Ingimarsson, D. Jacobsen, D. C. P. Lau, O. A. Loskutova, A. Milner, H. Mykrä, A. A. Novichkova, J. S. Ólafsson, A. K. Schartau, R. Shafterl & W. Goekoop, 2022a. Temperature and spatial connectivity drive patterns in freshwater macroinvertebrate diversity across the Arctic. Freshwater Biology 67: 159–175. https://doi.org/10.1111/fwb.13805.
Lento, J., S. M. Laske, I. Lavoie, D. Bogan, B. Brua, S. Campeau, K. Chin, J. M. Culp, B. Levenstein, M. Power, É. Saulnier-Talbot, R. Shaftel, H. Swanson, M. Whitman & C. E. Zimmerman, 2022b. Diversity of diatoms, benthic macroinvertebrates, and fish varies in response to different environmental correlates in Arctic rivers across North America. Freshwater Biology 67: 95–115. https://doi.org/10.1111/fwb.13600.
Levine, S. N., A. D. Shambaugh, S. E. Pomeroy & M. Braner, 1997. Phosphorus, nitrogen, and silica as controls on phytoplankton biomass and species composition in Lake Champlain (USA–Canada). Journal of Great Lakes Research 23: 131–148. https://doi.org/10.1016/S0380-1330(97)70891-8.
Liaw, A. & M. Wiener, 2002. Classification and regression by randomForest. R News 2: 18–22.
Lotter, A. F., R. Pienitz & R. Schmidt, 2010. Diatoms as indicators of environmental change in sub-Arctic and alpine regions. In Smol, J. P. & E. F. Stroemer (eds), The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge University Press, Cambridge.
Maltais, M. J. & W. F. Vincent, 1997. Periphyton community structure and dynamics in a sub-Arctic lake. Canadian Journal of Botany 75: 1556–1569. https://doi.org/10.1139/b97-868.
Marks, J. C. & M. E. Power, 2001. Nutrient induced changes in the species composition of epiphytes on Cladophora glomerata Kütz. (Chlorophyta). Hydrobiologia 450: 187–196. https://doi.org/10.1023/A:1017596927664.
Marsay, C. M., A. Aguilar-Islas, J. N. Fitzsimmons, M. Hatta, L. T. Jensen, S. G. John, D. Kadko, W. M. Landing, N. T. Lanning, P. L. Morton, A. Pasqualini, S. Rauschenberg, R. M. Sherrell, A. M. Shiller, B. S. Twining, L. M. Whitmore, R. Zhang & C. S. Buck, 2018. Dissolved and particulate trace elements in late summer Arctic melt ponds. Marine Chemistry 204: 70–85. https://doi.org/10.1016/j.marchem.2018.06.002.
McCormick, A. R., J. S. Phillips & A. R. Ives, 2019. Responses of benthic algae to nutrient enrichments in a shallow lake: linking community production, biomass, and composition. Freshwater Biology 64: 1833–1847. https://doi.org/10.1111/fwb.13375.
Michelutti, N., A. J. Holtham, S. V. Douglas & J. P. Smol, 2003. Periphytic diatom assemblages from ultra-oligotrophic and UV transparent lakes and ponds on Victoria Island and comparisons with other diatom surveys in Canadian Arctic. Journal of Phycology 39: 465–480. https://doi.org/10.1046/j.1529-8817.2003.02153.x.
Michelutti, N., A. P. Wolfe, R. D. Vinebrooke, B. Rivard & J. P. Briner, 2005. Recent primary production increases in Arctic lakes. Geophysical Research Letters. https://doi.org/10.1029/2005GL023693.
Mischler, J. A., P. G. Taylor & A. R. Townsend, 2014. Nitrogen limitation of pond ecosystems on the plains of eastern Colorado. PLoS ONE. https://doi.org/10.1371/journal.pone.0095757.
Muñoz-López, C. L. & C. A. Rivera-Rondón, 2022. Diatom response to environmental gradients in the high mountain lakes of the Colombia’s Eastern Range. Aquatic Sciences. https://doi.org/10.1007/s00027-021-00838-z.
Muse, J. O., J. D. Stripeikis, F. M. Fernández, L. d’Huicque, M. B. Tudino, C. N. Carducci & O. E. Troccoli, 1999. Seaweeds in the assessment of heavy metal pollution in the Gulf San Jorge, Argentina. Environmental Pollution 104: 315–322. https://doi.org/10.1016/S0269-7491(98)00096-7.
Niittynen, P. & M. Luoto, 2018. The importance of snow in species distribution models of Arctic vegetation. Ecography 41: 1024–1037. https://doi.org/10.1111/ecog.03348.
Paerl, H. & J. Huisman, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports 1: 27–37. https://doi.org/10.1111/j.1758-2229.2008.00004.x.
Pajunen, V., J. Jyrkänkallio-Mikkola, M. Luoto & J. Soininen, 2019. Are drivers of microbial diatom distributions context dependent in human impacted and pristine environments? Ecological Applications 29: 1–12. https://doi.org/10.1002/eap.1917.
Passy, S. I. & C. A. Larson, 2019. Niche dimensionality and herbivory control stream algal biomass via shifts in guild composition, richness, and evenness. Ecology. https://doi.org/10.1002/ecy.2831.
Phillips, J. S., A. R. McCormick, Á. Einarsson, S. N. Grover & A. R. Ives, 2019. Spatiotemporal variation in the sign and magnitude of ecosystem engineer effects on lake ecosystem production. Ecosphere. https://doi.org/10.1002/ecs2.2760.
Pilkaityté, R. & A. Razinkovas, 2007. Seasonal changes in phytoplankton composition and nutrient limitation in a shallow Baltic lagoon. Boreal Environment Research 12: 551–559 [available on internet at: http://hdl.handle.net/10138/235513].
Poikane, S., M. G. Kelly, G. Várbíró, G. Borics, T. Erős, S. Hellsten, A. Kolada, B. Lukács, A. L. Solheim, J. P. López, N. J. Willby, G. Wolfram & G. Phillips, 2022. Estimating nutrient thresholds for eutrophication management: novel insights from understudied lake types. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2022.154242.
Prieto, D. M., R. Devesa-Rey, D. A. Rubinos, F. Díaz-Fierros & M. T. Barral, 2013. Arsenate retention by epipsammic biofilms developed on streambed sediments: influence of phosphate. BioMed Research International. https://doi.org/10.1155/2013/591634.
R Development Core Team, 2021. R: A Language and Environmental for Statistical Computing. R Foundation for Statistical Computing, Vienna [available on internet at: http://www.R-project.org].
Ramanan, R., B.-H. Kim, D.-H. Cho, H.-M. Oh & H.-S. Kim, 2016. Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnology Advances 34: 14–29. https://doi.org/10.1016/j.biotechadv.2015.12.003.
Rantala, M. V., T. P. Luoto, J. Weckström, M. Rautio & L. Nevalainen, 2017. Climate drivers of diatom distribution in shallow sub-Arctic lakes. Freshwater Biology 62: 1971–1985. https://doi.org/10.1111/fwb.13042.
Rautio, M., F. Dufresne, I. Laurion, S. Bonilla, W. F. Vincent & K. S. Christoffersen, 2011. Shallow freshwater ecosystems of the circumpolar Arctic. Écoscience. https://doi.org/10.2980/18-3-3463.
Richter, D., J. Matuła, M. Pietryka, B. Wojtuń, A. Zwolicki, K. Zmudczyńska-Skarbek & L. Stempniewicz, 2018. Cyanobacterial and green algal assemblages in various tundra habitats in the high Arctic (West Spitsbergen, Norway). Acta Soietatis Botanicorum Poloniae. https://doi.org/10.5586/asbp.3605.
Rosero-López, D., M. T. Walter, A. S. Flecker, D. F. Ontaneda & O. Dangles, 2021. Standardization of instantaneous fluoroprobe measurements of benthic algal biomass and composition in streams. Ecological Indicators. https://doi.org/10.1016/j.ecolind.2020.107185.
Sahoo, D. & J. Seckbach, 2015. Cellular Origin, Life in Extreme Habitats and Astrobiology: The Algae World, Springer, Dordrecht:
Screen, J. A., 2014. Arctic amplification decreases temperature variance in northern mid- to high-latitudes. Nature Climate Change 4: 577–582. https://doi.org/10.1038/nclimate2268.
Shu, X., Y.-Q. Yu, Q.-M. Liu & J.-Y. Yang, 2022. Adsorptive–desorptive performance of Chlorella vulgaris for the removal of vanadium from aqueous solutions. Chemistry and Ecology 39: 24–43. https://doi.org/10.1080/02757540.2022.2138362.
Sivarajah, B., J. B. Korosi, J. M. Blais & J. P. Smol, 2019. Multiple environmental variables influence diatom assemblages across an arsenic gradient in 33 sub-Arctic lakes near abandoned gold mines. Hydrobiologia 841: 133–151. https://doi.org/10.1007/s10750-019-04014-1.
Slukovskiy, Z. I. & T. S. Shelekhova, 2018. The response of the diatom flora of a small lake to the impact of heavy metals in the urban environment of the Republic of Karelia. Translated from Russian by Google Translate. Earth Sciences [available on internet at: http://hdl.handle.net/11701/10252].
Soininen, J., 2007. Environmental and spatial control of freshwater diatoms – a review. Diatom Research 22: 473–490. https://doi.org/10.1080/0269249X.2007.9705724.
Soininen, J. & S. Meier, 2014. Phytoplankton richness is related to nutrient availability, not to pool size, in a sub-Arctic rock pool system. Hydrobiologia 740: 137–145. https://doi.org/10.1007/s10750-014-1949-7.
Soininen, J. & P. Niemelä, 2002. Inferring the phosphorus levels of rivers from benthic diatoms using weighted averaging. Archiv Für Hydrobiologie 154: 1–18. https://doi.org/10.1127/archiv-hydrobiol/154/2002/1.
Soininen, J., P. Bartels, J. Heino, M. Luoto & H. Hillebrand, 2015. Toward more integrated ecosystem research in aquatic and terrestrial environments. BioScience 65: 174–182. https://doi.org/10.1093/biosci/biu216.
Stauber, J. L. & T. M. Florence, 1985a. The influence of iron on copper toxicity to the marine diatom Nitzschia closterium (Ehrenberg) W. Smith. Aquatic Toxicology 6: 297–305. https://doi.org/10.1016/0166-445X(85)90025-6.
Stauber, J. L. & T. M. Florence, 1985b. Interactions of copper and manganese: a mechanism by which manganese alleviates copper toxicity to the marine diatom Nitzschia closterium (Ehrenberg) W. Smith. Aquatic Toxicology 7: 241–254. https://doi.org/10.1016/0166-445X(85)90042-6.
Stevenson, R. J., 1996. An introduction to algal ecology in freshwater benthic habitats. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Elsevier, San Diego.
Sun, Y., X. Yu, W. Yao & Z. Wu, 2022. Research progress in relationships between freshwater bivalves and algae. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2022.113665.
Tang, E. P. Y., R. Tremblay & W. F. Vincent, 1997. Cyanobacterial dominance of polar freshwater ecosystems: are high-altitude mat-formers adapted to low temperature? Journal of Phycology 33: 171–181. https://doi.org/10.1111/j.0022-3646.1997.00171.x.
Teng, Y., S. Ni, C. Zhang, J. Wang, X. Lin & Y. Huang, 2006. Environmental geochemistry and ecological risk of vanadium pollution in Panzhihua Mining and Smelting Area, Sichuan, China. Chinese Journal of Geochemistry 25: 379–385. https://doi.org/10.1007/s11631-006-0378-3.
Tuulaikhuu, B.-A., A. M. Romaní & H. Guasch, 2015. Arsenic toxicity effects on microbial communities and nutrient cycling in indoor experimental channels mimicking a fluvial system. Aquatic Toxicology 166: 72–82. https://doi.org/10.1016/j.aquatox.2015.07.005.
Van Geest, G. J., D. O. Hessen, P. Spierenburg, G. A. P. Dahl-Hansen, G. Christensen, P. J. Faevorig, M. Brehm, M. J. J. E. Loonen & E. Van Donk, 2007. Goose-mediated nutrient enrichment and planktonic grazer control in Arctic freshwater ponds. Oecologia 153: 653–662. https://doi.org/10.1007/s00442-007-0770-7.
Van Hoecke, K., K. A. C. De Schamphelaere, P. Van der Meeren, S. Lucas & C. R. Janssen, 2008. Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: importance of surface area. Environmental Toxicology and Chemistry 27: 1948–1957. https://doi.org/10.1897/07-634.1.
Vincent, W. F. & A. Quesada, 2012. Cyanobacteria in high latitude lakes, rivers and seas. In Whitton, B. A. (ed), Ecology of Cyanobacteria II: Their Diversity in Space and Time. Springer, Dordrecht.
Virta, L. & A. Teittinen, 2022. Threshold effects of climate change on benthic diatom communities: evaluating impacts of salinity and wind disturbance on functional traits and benthic biomass. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2022.154130.
Wake, B. D., C. S. Hassler, A. R. Bowie, P. R. Haddad & E. C. V. Butler, 2012. Phytoplankton selenium requirements: the case for species isolated from temperate and polar regions of the Southern Hemisphere. Journal of Phycology 48: 585–594. https://doi.org/10.1111/j.1529-8817.2012.01153.x.
Walsh, C. & R. Mac Nally, 2020. Package ‘hier.part’. R project for statistical computing.
Watanabe, T. & T. Horiike, 2021. The evolution of molybdenum dependent nitrogenase in cyanobacteria. Biology. https://doi.org/10.3390/biology10040329.
Wetzel, R. G., 2001. Limnology, Academic, San Diego:
Whittaker, R. H., F. H. Bormann, G. E. Likens & T. G. Siccama, 1974. The Hubbard Brook ecosystem study: forest biomass and production. Ecological Monographs 44: 233–254. https://doi.org/10.2307/1942313.
Wrona, F. J., T. D. Prowse, J. D. Reist, J. E. Hobbie, L. M. J. Lévesque & W. F. Vincent, 2006. Climate change effects on aquatic biota, ecosystem structure and function. Ambio 35: 359–369. https://doi.org/10.1579/0044-7447(2006)35[359:CCEOAB]2.0.CO;2.
Yamamichi, M., T. Kazama, K. Tokita, I. Katano, H. Doi, T. Yoshida, N. G. Hairston & J. Urabe, 2018. A shady phytoplankton paradox: when phytoplankton increases under low light. Proceedings of the Royal Society of Biological Sciences 285: 1–8. https://doi.org/10.1098/rspb.2018.1067.
Yang, J., H. Jiang, W. Liu & B. Wang, 2018. Benthic algal community structures and their response to geographic distance and environmental variables in the Qinghai-Tibetan lakes with different salinity. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2018.00578.
Yang, X., X. Liu, A. Zhang, D. Lu, G. Li, Q. Zhang, Q. Liu & G. Jiang, 2019. Distinguishing the sources of silica nanoparticles by dual isotopic fingerprinting and machine learning. Nature Communications. https://doi.org/10.1038/s41467-019-09629-5.
Yates, L. A., Z. Aandahl, S. A. Richards & B. W. Brook, 2022. Cross validation for model selection: a review with examples from ecology. Ecological Monographs. https://doi.org/10.1002/ecm.1557.
Zhihong, W., C. Shiguang & C. Xin, 2010. Micro-nutrients effects on algae colony: growth rate and biomass response to various micro-nutrients and competitive inhibitions among multi-microelements. In: 4th International Conference on Bioinformatics and Biomedical Engineering. Chengdu: China, 2010: 1–8. https://doi.org/10.1109/ICBBE.2010.5516174.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This Project was primarily funded by Societas pro Fauna et Flora Fennica Foundation and the Finnish Cultural Foundation. The work was supported by Additional Grants from the University of Helsinki, Oskar Öflunds Foundation, and the Finnish Society of Sciences and Letters. We thank Länsi-Rajan rasti/Routamap Oy for providing high quality and accurate maps of the study area for data collection. We thank Juhani Virkanen, Tuija Vaahtojärvi, and Hanna Reijola for the analysis of water chemistry. This is contribution 0012 from the Environmental and Mineralogical Laboratories (Hellabs) of the Department of Geosciences and Geography, University of Helsinki and acknowledges the funding by the Faculty of Science, University of Helsinki (Project MICROCLIM, Decision 7510145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest regarding this research for all authors.
Additional information
Handling editor: Elzbieta Wilk-Wozniak
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10750_2023_5368_MOESM1_ESM.xlsx
Supplementary file1 (XLSX 11 kb) The set of biomass models were produced where biotic interactions (i.e. cyanobacteria, green algae, and diatoms) were excluded. Similar to Table 2 in the manuscript, the tables are shown with coefficient estimates, standard errors, z-values, and statistical significances (*P < 0.05, **P < 0.01, ***P < 0.001, ns=not significant). The insignificant TP variables are bolded. Also showing the Akaike’s information criterion (AIC) and the percentual value of how well the model is explained by the variables.
10750_2023_5368_MOESM2_ESM.xlsx
Supplementary file2 (XLSX 14 kb) The table shows the correlation coefficients according to Spearman’s correlation coefficient (r) and the statistical significances (*P < 0.05, **P < 0.01, ***P < 0.001) of each measured abiotic and biotic variables used in this study. The single remaining correlation coefficient of > 0.7 was between cyanobacteria biomass and total benthic algal biomass and it is bolded in the table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heikkinen, J.M., Niittynen, P., Soininen, J. et al. Patterns and drivers for benthic algal biomass in sub-Arctic mountain ponds. Hydrobiologia 851, 689–708 (2024). https://doi.org/10.1007/s10750-023-05368-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05368-3