Abstract
Since the 1980s, the freshwater apple snails, Pomacea canaliculata, Pomacea maculata, and their hybrid, have been introduced into a wide range of freshwater ecosystems in tropical to temperate regions. Although P. canaliculata has become established in temperate East Asia, P. maculata and the hybrid are rarely found in this region. To evaluate the risk of P. maculata and the hybrid particularly as rice pests in temperate regions, we compared growth rate, winter survival rate, and feeding efficiency on rice seedlings of these snails with P. canaliculata. When P. maculata and P. canaliculata hatchlings were reared under laboratory or field conditions, the adult P. maculata had larger shells than adult P. canaliculata. Neither P. maculata nor F1 hybrids could survive winter in a simulated drained paddy field, and only a few individuals of P. maculata and F1 hybrids overwintered successfully in freshwater. Pomacea maculata juveniles fed on rice seedlings at the highest rate at temperatures above 27°C. These results suggest that P. maculata becomes a serious rice pest in temperate region as P. canaliculata once it is introduced from warmer regions under global warming. Further biological examination of P. maculata is needed to evaluate the risk of this snail in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Pomacea comprises freshwater snails originally distributed in South America. Some snails in this genus have been introduced into several regions of the world since the 1980s for human consumption and for the pet trade. Shortly after their introduction, the snails escaped into freshwater ecosystems outside their native ranges and became serious agricultural pests of aquatic crops such as rice, taro, and lotus (Cowie, 2002). The snails became invasive alien species that disturb the local biota of aquatic systems (Carlsson et al., 2004; Chaichana & Sumpan, 2014). Furthermore, these snails function as intermediate hosts for the parasitic nematode Angiostrongylus cantonensis (Chen, 1935), which causes eosinophilic meningitis and eosinophilic pleocytosis in humans and animals (Lv et al., 2009).
Two species of snails in this genus, Pomacea canaliculata (Lamarck, 1822) and Pomacea maculata Perry, 1810, have been widely introduced all over the world, from tropical to temperate regions, including North America, Europe, East and Southeast Asia, and the Pacific Islands (Hayes et al., 2015). These two species are not sister taxa (Hayes et al., 2008) but can hybridize in the laboratory (Matsukura et al., 2013), and hybrid snails have been collected from both native South American (Glasheen et al., 2020) and non-native East and Southeast Asian (Matsukura et al., 2013; Yang et al., 2022; Pu et al., 2023) ranges.
The distributions of these two snail species overlap because of mixed introduction of the snails from a few distinct origins in South America (Hayes et al., 2012) but show geographical gaps (Matsukura et al., 2013). In East and Southeast Asia, where most apple snails were introduced from Argentina and Brazil via Taiwan (Mochida, 1991; Hayes et al., 2008), both species are found in tropical and subtropical regions, whereas only P. canaliculata is found in temperate regions (Matsukura et al., 2013; Yang et al., 2018). In their native South America, P. canaliculata is distributed in colder regions than P. maculata (Hayes et al., 2012), therefore the better adaptation of P. canaliculata to colder regions in non-native areas is mainly due to the inherent higher cold tolerance of this species (Matsukura et al., 2016a).
Economic loss from P. canaliculata in rice paddies in temperate Japan has gotten worse recently because global warming resulted in increased population densities in spring as well as invasions into new areas (Yoshida et al., 2022). The recent outbreaks of P. canaliculata in temperate Japan imply a novel risk in that P. maculata and a hybrid of the two species could become important rice pests in temperate regions as in tropical Southeast Asia. The risk posed by P. maculata to rice crops in temperate regions cannot be evaluated in detail because few P. maculata have been collected in such regions (Matsukura et al., 2013; Yang et al., 2018). However, adult P. maculata are larger than adult P. canaliculata, and lay larger egg masses (Hayes et al., 2012; Matsukura et al., 2013), suggesting that rice crops may be attacked by greater numbers of larger apple snails than is currently the case in temperate regions, once P. maculata becomes established in this region.
In this study, we evaluated the development, overwintering success, and feeding efficiency of P. maculata on rice seedlings under temperate field conditions and at several temperatures by comparison with P. canaliculata to estimate the ecological background and potential risk from P. maculata as an agricultural pest in temperate regions.
Materials and methods
Snail strains
We used established strains of P. canaliculata and P. maculata that were individually screened using mitochondrial COI and nuclear elongation factor 1α (EF1α) sequences as described by Matsukura et al. (2013). Progenitors of the P. canaliculata and P. maculata strains were collected in 2007, from Kitaura, Ibaraki Prefecture, Japan (36° 06′ N, 140° 22′ E) and Taketomi, Okinawa Prefecture, Japan (Iriomote Island, 24° 26′ N, 123° 47′ E), respectively. The two strains were reared in acrylic containers (40 cm × 80 cm, 40-cm depth) at 25°C under a 16:8 h light:dark regime. Artificial carp food (“Hikari”; Kyorin Ltd., Hyogo, Japan), eggplant, and cabbage were provided as basic food, and a small amount of oyster shell powder was occasionally provided as a source of calcium (Matsukura & Wada, 2007).
F1 hybrids of the two species were provided for some experiments. Our previous studies confirmed that F1 hybrids are produced after cross-mating between female P. canaliculata and male P. maculata, and vice versa (Matsukura et al., 2016a). We used only F1 hybrids obtained from female P. canaliculata and male P. maculata; biological traits of this hybrid, such as egg size, hatchability, and cold tolerance, are not significantly different from those of other hybrids obtained from reciprocal mating (Matsukura et al., 2013, 2016a).
Comparison of growth patterns of juvenile snails under laboratory conditions
We examined snail growth patterns from hatchling to juvenile stages in the laboratory at 25°C (ambient temperature) under a 16:8 h light:dark regime because tracking hatchlings in the field is difficult. Twenty hatchlings (0 days after hatching) each of P. canaliculata and P. maculata were placed in separate plastic containers (200 mm × 300 mm, 300 mm deep) filled with tap water. Oyster shells were placed on the bottom of each container to provide a calcium source for the snails. A few grains of artificial carp food were provided daily. Water was refreshed once a week. Each species had two replicates (four containers in total). Shell height at 0 (hatchlings), 4, and 8 weeks after hatching was determined using a microcaliper with 0.1-mm gradations. We compared shell height between P. canaliculata and P. maculata juveniles using nested analysis of variance (nested ANOVA) by categorizing container as a nested effect.
Comparison of snail growth patterns from mid-juvenile to adult stages in the field
We examined snail growth patterns after the mid-juvenile stage (10–15 mm in shell height) in an outdoor simulated rice paddy. We used six concrete containers (1 m × 2 m, 1 m deep) located in an experimental field of the Kyushu Okinawa Agricultural Research Center, National Agriculture Research Organization (32° 52′ 43′′ N, 130° 44′ 25′′ E). The concrete containers were filled with andosol and tap water (100–150 mm water depth). On 1 July 2011, rice seedlings (cultivar “Reiho”, 200–250 mm in plant height to avoid injury by released snails) were transplanted into the containers to simulate rice paddies, and 30 mid-juveniles were released into each container. Shell heights of the released snails were 12.8 ± 1.8 mm (mean ± SD) and 13.8 ± 1.6 mm for P. canaliculata and P. maculata, respectively. Two containers contained only P. canaliculata (n = 30), whereas two other containers contained only P. maculata (n = 30) (single treatments). Fifteen P. canaliculata and 15 P. maculata were released into each of the other two containers to evaluate the interactive effect of the two species on their growth (mixed treatment).
Shell heights of the released snails were measured on 4 August, 2 September, and 4 October 2011. Because only snails that could be seen on the surface were measured, number of snails examined for each treatment and measurement day were from 4 to 12 with an average of 8.38. Effects of species and species composition (single or mixed) on shell height at each measurement day were examined using nested two-way ANOVA by categorizing concrete container as a nested effect. After performing the nested two-way ANOVA, averaged shell heights in each container were supplied for t test to compare shell height between the two species. Averaged shell height data from the single and mixed treatments were merged in the t test.
Comparison of overwintering success under dry and flooded conditions in the field
Overwintering success (winter survival rates, % survival) of P. canaliculata, P. maculata, and their F1 hybrid were examined using the same four concrete containers as in the experiments described above. This experiment was conducted during two winter seasons: from December 2016 to March 2017 and from December 2017 to March 2018. The overwinter risks during each winter season were evaluated using a logistic regression model proposed by Yoshida et al. (2022), in which the overwinter risk (0–1, higher values for higher risks) of P. canaliculata was calculated from the cumulative low temperature below 10°C during winter. Temperature data for each winter season were obtained from the AgroMeteorological Grid Square Data System operated by the National Agricultural Research Organization (https://amu.rd.naro.go.jp/wiki_open/doku.php?id=start).
To simulate drained paddies, juvenile P. canaliculata, P. maculata, and F1 hybrids (shell heights of approximately 10 mm; n = 100 for each species/strain) were released into two of the concrete containers, which were filled with andosol and tap water (100-mm depth) on 30 November 2016 and 2017. No adults were included in the containers simulating dry conditions because adult P. canaliculata cannot overwinter in temperate regions under dry conditions such as those in drained paddies (Watanabe et al., 2000; Wada & Matsukura, 2007). Before release, the shells of all snails were marked with nail polish for species/strain identification after overwintering. Overwintering conditions in paddies were simulated by draining the water from the concrete containers on 1 December (i.e., 1 day after releasing the snails in both winter seasons) to stimulate the snails into burrowing into the soil for the winter.
To simulate flooded conditions, the other two concrete containers were filled with soil and tap water (600-mm depth) to simulate overwintering conditions in ponds and waterways. Fifty juveniles of both species and F1 hybrids (shell heights of approximately 10 mm), and 25 adult P. canaliculata and P. maculata (shell heights approximately 25 mm) were released into the containers on 1 December 2016 and 2017 after marking their shells with nail polish for identification. The numbers of surviving snails were counted on 3 April 2017 and 30 March 2018 for both dry and flooded treatments.
Effects of species/strain, winter season (2016–2017 and 2017–2018), and water condition (dry or flooded) on overwinter survival rates of juveniles were examined using paired three-way ANOVA in which species/strain was set as a corresponding factor to concrete container. After performing the paired three-way ANOVA, juvenile survival rates among treatments were compared with Tukey HSD tests, after combining the survival-rate data for the two winter seasons. We also examined effects of species and winter season on overwinter survival rates of adult P. canaliculata and P. maculata under flooded conditions using paired two-way ANOVA, followed by t-tests after combining survival-rate data for the two winter seasons. All survival data were supplied for the statistical analyses after arcsine transformation of the square root of the proportion.
Comparison of feeding rates on rice seedlings under temperature-controlled conditions
We compared feeding rates of juvenile P. canaliculata, P. maculata, and F1 hybrids on rice seedlings at a range of temperatures. Eight temperature treatments from 15 to 36°C at 3°C intervals were included. Twenty rice seedlings (cultivar “Reiho”) at the third leaf stage (approximately 150 mm in plant height) were transplanted into a plastic container (600 mm × 300 mm, 150 mm deep), which was filled with andosol (30-mm depth) and tap water (70-mm depth), and then the container was kept in a thermostatic chamber for 1 day to acclimate the soil and water to the selected temperature of each treatment. Subsequently, 10 juvenile P. canaliculata, P. maculata, or F1 hybrids (shell heights of approximately 10 mm) were released into a container. These juveniles were kept at 25°C until they were released. One day after releasing the snails, the numbers of rice seedlings completely consumed by the snails were counted. Rice seedlings partially consumed by snails were not counted in this experiment. Five replicates were included for each combination of temperature and species/strain. The effects of species/strain, temperature, and their interaction on the rate of rice seedling consumption were examined using two-way ANOVA. Consumption rates of rice seedlings among species/strains within the same temperature treatment were compared with Tukey HSD tests. Square root of the consumption rates were arcsine transformed before conducting the statistical analyses.
Statistical analyses
All statistical analyses were performed using t.test, aov, and TukeyHSD functions on R ver. 4.1.3 (R Core Team, 2022). Acquisition of climate data at the experimental site and calculation of the overwintering risk were conducted using Python 3.9.10 (https://www.python.org/downloads/release/python-3910/).
Results
Growth patterns of the two snail species from hatchling to juvenile stages
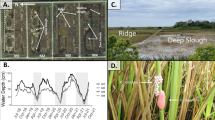
Shell heights of P. canaliculata were significantly greater than those of P. maculata at 0 (SSspecies = 12.96, SSresiduals = 3.179, dfspecies = 1, dfresiduals = 76, F = 309.9, P < 0.001, nested ANOVA) and 4 (SSspecies = 189.7, SSresiduals = 211.3, dfspecies = 1, dfresiduals = 76, F = 68.26, P < 0.001) weeks after their hatching (Fig. 1). The differences in shell height disappeared at 8 weeks (SSspecies = 0.094, SSresiduals = 613.4, dfspecies = 1, dfresiduals = 67, F = 0.010, P = 0.919), when the hatchlings grew to the mid-juvenile stage, with shell heights between 10 and 15 mm.
Average shell height of juvenile P. maculata (Pm) and P. canaliculata (Pc) at 0, 4, and 8 weeks after hatching (n = 20 for all measurements). Asterisks indicate a significant effect of species on shell height (nested Analysis of variance, P < 0.001). There were two replicates for each species (Reps. 1 and 2)
Growth patterns of the two snail species from juvenile to adult stages under field conditions
Effects of species on shell heights were not significant on 4 August, but significant on 2 September and 4 October (Table 1). Averaged shell heights of P. maculata (27.22 ± 3.29 mm (mean ± SD) on 2 September and 33.17 ± 3.27 on 4 October) were larger than those of P. canaliculata (20.50 ± 1.32 mm on 2 September and 24.60 ± 1.99 on 4 October) on 2 September (t = 3.788, df = 6, P = 0.009, t test) and 4 October (t = 4.482, df = 6, P = 0.004) (Fig. 2). Effects of species composition in each concrete container were not significant through the experimental period although growth rates from August to September seemed to vary among containers and interaction between species and species composition was significant on 4 August. These results indicate that P. maculata grows better than P. canaliculata when they occur in the same paddy field.
Changes in average shell height of P. maculata (Pm) and P. canaliculata (Pc) reared in simulated outdoor rice paddies during summer. Each value is based on 4–12 snails (average = 8.38). Asterisks indicate significant difference of shell height between the two species (t test after pooling data from alone and mixed treatments)
Overwinter survival rates of snails under dry and flooded conditions
The overwinter risks of P. canaliculata calculated using a logistic regression model were 0.791 and 0.586 in the 2016–2017 and 2017–2018 winter seasons, respectively. These values indicate a moderate to high risk of overwintering. Overwinter survival rates of juveniles were significantly affected by species/strain (SSspecies/strain = 1.109, SSresiduals = 0.099, dfspecies/strain = 2, dfresiduals = 8, F = 44.93, P < 0.001, paired three-way ANOVA) while neither winter season (SSwinter season = 0.032, SSresiduals = 0.265, dfwinter season = 1, dfresiduals = 4, F = 0.482, P = 0.526) nor water condition (SSwater condition = 0.004, SSresiduals = 0.265, dfwater condition = 1, dfresiduals = 4, F = 0.067, P = 0.809) influenced their survival. No interactions except between species/strain and water condition (SSspecies/strainwater × water condition = 2.160, SSresiduals = 0.099, dfspecies/strainwater × water condition = 2, dfresiduals = 8, F = 87.51, P < 0.001) were detected. The highest overwinter survival was for juvenile P. canaliculata under dry conditions, followed by juvenile P. canaliculata under flooded conditions (Table 2). No juvenile P. maculata or F1 hybrids survived over the winter under dry conditions, whereas fewer than 10% of both P. maculata and F1 juveniles were able to survive over the winter under flooded conditions. As seen for juveniles, overwinter survival rates of adults were affected by species SSspecies = 0.294, SSresiduals = 0.020, dfspecies = 1, dfresiduals = 2, F = 28.80, P = 0.033, paired two-way ANOVA) but not by winter season (SSwinter season = 0.011, SSresiduals = 0.078, dfwinter season = 1, dfresiduals = 2, F = 0.269, P = 0.655) with no significant interaction between species and winter season (SSspecies × winter season = 0.022, SSresiduals = 0.020, dfspecies × winter season = 1, dfresiduals = 2, F = 2.133, P = 0.282). Adult P. canaliculata survived winter better than adult P. maculata (t = 3.011, df = 7, P = 0.020, paired t test).
Snail feeding rates on rice seedlings at different temperatures
At temperatures of 27°C and above, P. maculata tended to consume rice seedlings at higher rates than P. canaliculata and F1 hybrids (Fig. 3). Both species/strain and temperature significantly affected the rate at which rice seedlings were consumed by the snails (SSspecies/strain = 0.516, SStemperature = 16.941, SSresiduals = 2.543, dfspecies/strain = 2, dftemperature = 7, dfresiduals = 96, Fspecies/strain = 9.736, Ftemperature = 91.36, P < 0.001 for species/strain and P < 0.001 for temperature) (Fig. 3). The interaction between species/strain and temperature was also significant (SSspecies×temperature = 0.916, SSresiduals = 2.543, dfspecies×temperature = 14, dfresiduals = 96, F = 2.469, P = 0.005). The feeding rate increased as the temperature increased. There were no significant differences in rates of consumption of rice plants between species/strains at the same temperature for temperatures of 24°C and below.
Efficiency of feeding on rice seedlings by juvenile P. maculata, P. canaliculata, and their F1 hybrid at different temperatures. The number of rice seedlings consumed was determined 1 day after the juveniles were released. Vertical bars indicate standard deviations. Bars labeled with the same letter indicate no significant difference between species/strains in rates of seedling consumption at that temperature (Tukey HSD test after arcsine transformation, α = 0.05). There were five replicates for each temperature and species/strain treatment
Discussion
The comparative experiments in this study found ecological characteristics of P. maculata that were different from those of P. canaliculata, and that could influence its impact as an agricultural pest. No P. maculata or F1 hybrid survived winter under dry conditions in a simulated paddy (Table 2, dry conditions) where the overwintering risk was moderate to high. Yoshida et al. (2022) reported that > 99.7% of the distribution area of P. canaliculata in Japan has an overwintering risk of moderate to high or higher, so the lack of overwintering success for P. maculata and the F1 hybrid at the experimental site, with moderate to high risk, suggests that these snails will not immediately become a rice pest as serious as P. canaliculata in temperate Japan. The survival of a few P. maculata and hybrid snails under flooded conditions (Table 2, flooded conditions) is not surprising because P. canaliculata cannot survive the winter in drained paddies but can successfully overwinter in irrigated canals at its present northernmost distribution limit in temperate Japan (Ito, 2002). The stress from low temperatures in winter is mitigated for apple snails hibernating in rivers and ponds.
Even though P. maculata poses a low immediate risk as a rice pest in temperate regions, it should be monitored as a precaution in the long term. The latest report from the Intergovernmental Panel on Climate Change (IPCC) warns that average global surface air temperature could increase 3.3–5.7°C by 2100 relative to the average air temperature from 1995 to 2014 under the highest overall emission scenario (SSP5-8.5; IPCC, 2021). Under this climate change scenario, the survival of P. maculata during winter will increase and it will expand its distribution range in temperate regions, and subsequently cause damage to rice and other aquatic crops, as occurred recently with P. canaliculata in temperate Japan (Yoshida et al., 2022).
Pomacea maculata grew into larger adults than P. canaliculata during summer in temperate paddies (Fig. 2) despite smaller body size of P. maculata at hatchling and young juvenile stage (Fig. 1). The smaller body size of P. maculata in the initial stage would be caused by smaller egg and hatchling size in this snail than P. canaliculata (Hayes et al., 2012; Matsukura et al., 2013). The better growth of P. maculata could result in serious damage to crops, because the amount of rice seedlings consumed by apple snails depends not only on snail density, but also on the body size of the snails (Wada, 2004). In addition to the difference in adult body size, the difference between P. canaliculata and P. maculata in efficiency of consuming rice seedlings in response to temperature (Fig. 3) could also promote damage to crops by P. maculata under climate change. Pomacea maculata had a higher consumption efficiency on rice seedlings than P. canaliculata at temperatures above 27°C, although consumption rates were not significantly different at temperatures between 15 and 30°C (Fig. 3). This better adaptation to higher temperature in P. maculata might be explained by the different distribution ranges of these two snails in their native South America, where P. maculata is distributed over a wide range of tropical–temperate regions, whereas P. canaliculata is restricted to temperate regions of Argentina, Paraguay, Uruguay, and southernmost Brazil (Hayes et al., 2012).
Hybridization between P. canaliculata and P. maculata is another concern when considering future risks posed by apple snails to temperate rice crops. Previous reports suggest that F1 hybrids between P. canaliculata and P. maculata are not very vigorous because the hybrids have a much lower hatching rate than the two species (Matsukura et al., 2013). Furthermore, F1 hybrids were found to have a weak cold tolerance (similar to P. maculata) in laboratory tests (Matsukura et al., 2016a). However, back-cross strains obtained from crossing F1 hybrids with P. canaliculata showed higher cold tolerance than both F1 and P. maculata (Matsukura et al., 2016a). This suggests that continuous genetic exchange between the two species would promote introgression of P. maculata in temperate regions, and that the establishment of P. maculata could alter the genetic population structure and subsequent biological characteristics of apple snails in such regions. To evaluate the risk of hybridization in the two snails, biological interaction between the two snails when they simultaneously occurs in paddy field should be clarified. For example, varied growth rates of mid-juvenile to adult snails from August to September in the mixed treatment (Fig. 2) might be caused as a result of species competition. Crossability under natural condition is also important.
To avoid future outbreaks of P. maculata in rice crops in temperate regions, the introduction of apple snails from tropical and sub-tropical regions should be restricted. In most cases, apple snails are introduced to new water ecosystems by humans (Cowie et al., 2017). Currently, most temperate regions in East Asia have only been affected by P. canaliculata, and hybrid snails are rarely recorded (Matsukura et al., 2013; Yang et al., 2018). Therefore, P. maculata cannot become established in these areas unless it is newly introduced from its native South America or from tropical or sub-tropical regions of its non-native range, such as Southeast Asia.
The prediction of future distribution ranges of apple snails on the basis of climate data is another effective approach for avoiding future expansion of apple snails into non-native ranges. It is evident that northern distribution limits of apple snails are determined by winter climates (Ito, 2002; Matsukura et al., 2016a), with a clear quantitative relationship between cumulative winter temperatures and overwinter survival rates in P. canaliculata (Yoshida et al., 2009, 2022). As with the control of several other crop pests (e.g., Biber-Freudenberger et al., 2016; Matsukura et al., 2016b; Ramos et al., 2018), visualizing and quantifying future risks of apple snail expansions at a fine geographical scale will help to implement preventive control strategies in non-native rice fields.
Data availability
All raw data obtained by the authors are available from: https://doi.org/10.6084/m9.figshare.21732098.
References
Biber-Freudenberger, L., J. Ziemacki, H. E. Z. Tonnang & C. Borgemeister, 2016. Future risks of pest species under changing climatic conditions. PLoS ONE 11: e0153237. https://doi.org/10.1371/journal.pone.0153237.
Carlsson, N. O. L., C. Brönmark & L. Hansson, 2004. Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85: 1575–1580. https://doi.org/10.1890/03-314.
Chaichana, R. & T. Sumpan, 2014. The potential impact of the exotic snail Pomacea canaliculata on the Thai native snail Pila scutata. Science Asia 40: 11–15. https://doi.org/10.2306/scienceasia1513-1874.2014.40.011.
Cowie, R. H., 2002. Apple snails as agricultural pests: their biology, impacts, and management. In Baker, G. M. (ed), Molluscs as Crop Pests CABI Publishing, Wallingford: 145–192.
Cowie, R. H., K. A. Hayes, E. E. Strong & S. C. Thiengo, 2017. Non-native apple snails: systematics, distribution, invasion history and reasons for introduction. In Joshi, R. C., R. H. Cowie & L. S. Sebastian (eds), Biology and Management of Invasive Apple Snails Philippine Rice Research Institute, Nueva Ecija: 3–32.
Glasheen, P. M., R. L. B. Burks, S. R. Campos & K. A. Hayes, 2020. First evidence of introgressive hybridization of apple snails (Pomacea spp.) in their native range. Journal of Molluscan Studies 86: 96–103. https://doi.org/10.1093/mollus/eyz035.
Hayes, K. A., R. C. Joshi, S. C. Thiengo & R. H. Cowie, 2008. Out of South America: multiple origins of non-native apple snails in Asia. Diversity and Distributions 14: 701–712. https://doi.org/10.1111/j.1472-4642.2008.00483.x.
Hayes, K. A., R. H. Cowie, S. C. Thiengo & E. E. Strong, 2012. Comparing apples with apples: clarifying the identities of two highly invasive Neotropical Ampullariidae (Caenogastropoda). Zoological Journal of the Linnean Society 166: 723–753. https://doi.org/10.1111/j.1096-3642.2012.00867.x.
Hayes, K. A., S. Burela, R. L. Burks, M. P. Cadierno, A. Castro-Vazquez, J. A. Cueto, P. L. Valentine-Darby, P. C. Darby, R. A. Dellagnola, M. S. Dreon, M. V. Frassa, M. Giraud-Billoud, M. S. Godoy, H. Heras, S. Ituarte, E. Koch, P. R. Martín, K. Matsukura, Y. Pasquevich, J. Qiu, M. C. Rodriguez, L. Saveanu, M. E. Seuffert, E. E. Strong, J. Sun, N. E. Tamburi, M. J. Tiecher, S. C. Thiengo, R. L. Turner, I. A. Vega, T. Wada, Y. Yusa & R. H. Cowie, 2015. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 58: 245–302. https://doi.org/10.4002/040.058.0209.
[IPCC] Intergovernmental Panel on Climate Change, 2021. Summary for policymakers. In Masson-Delmotte, V., P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu & B. Zhou (eds), Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge: 3–32.
Ito, K., 2002. Environmental factors influencing overwintering success of the golden apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae), in the northernmost population of Japan. Applied Entomology and Zoology 37: 655–661. https://doi.org/10.1303/aez.2002.655.
Lv, S., Y. Zhang, S. R. Chen, L. B. Wang, W. Fang, F. Chen, J. Y. Jiang, Y. L. Li, Z. W. Du & X. N. Zhou, 2009. Human angiostrongyliasis outbreak in Dali, China. PLOS Neglected Tropical Diseases 3: e520. https://doi.org/10.1371/journal.pntd.0000520.
Matsukura, K. & T. Wada, 2007. Environmental factors affecting the increase of cold hardiness in the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae). Applied Entomology and Zoology 42: 533–539. https://doi.org/10.1303/aez.2007.533.
Matsukura, K., M. Okuda, N. J. Cazzaniga & T. Wada, 2013. Genetic exchange between two freshwater apple snails, Pomacea canaliculata and Pomacea maculata invading East and Southeast Asia. Biological Invasions 15: 2039–2048. https://doi.org/10.1007/s10530-013-0431-1.
Matsukura, K., Y. Izumi, K. Yoshida & T. Wada, 2016a. Cold tolerance of invasive freshwater snails, Pomacea canaliculata, P. maculata, and their hybrids helps explain their different distributions. Freshwater Biology 61: 80–87. https://doi.org/10.1111/fwb.12681.
Matsukura, K., K. Yoshida, S. Kumashiro & M. Matsumura, 2016b. Future risk of the maize orange leafhopper, Cicadulina bipunctata, and maize wallaby ear symptom in temperate Japan. Population Ecology 58: 241–248. https://doi.org/10.1007/s10144-015-0535-9.
Mochida, O., 1991. Spread of freshwater Pomacea snails (Pilidae, Mollusca) from Argentina to Asia. Micronesica Supplement 3: 51–62.
Pu, J., P. Yang, Y. Dai, K. Tao, L. Gao, Y. Du, J. Cao, X. Yu & Q. Yang, 2023. Species identification and population genetic structure of non-native apple snails (Ampullariidea: Pomacea) in the lower reaches of the Yangtze River. Biodiversity Science 31: 22346. https://doi.org/10.17520/biods.2022346.
R Core Team, 2022. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ramos, R. S., L. Kumar, F. Shabani & M. C. Picanço, 2018. Mapping global risk levels of Bemisia tabaci in areas of suitability for open field tomato cultivation under current and future climates. PLoS ONE 13: e0198925. https://doi.org/10.1371/journal.pone.0198925.
Wada, T., 2004. Strategies for controlling the apple snail Pomacea canaliculata (Lamarck) (Gastropoda: Ampullariidae) in Japanese direct-sown paddy fields. Japan Agricultural Research Quarterly 38: 75–80.
Wada, T. & K. Matsukura, 2007. Seasonal changes in cold hardiness of the invasive freshwater apple snail, Pomacea canaliculata (Lam). Malacologia 49: 383–392. https://doi.org/10.4002/0076-2997-49.2.383.
Watanabe, T., K. Tanaka, H. Higuchi, K. Miyamoto, T. Kiyonaga, H. Kiyota, Y. Suzuki & T. Wada, 2000. Emergence of the apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae), after irrigation in a paddy. Applied Entomology and Zoology 35: 75–79. https://doi.org/10.1303/aez.2000.75.
Yang, Q., S. Liu, C. He & X. Yu, 2018. Distribution and the origin of invasive apple snails, Pomacea canaliculata and P. maculata (Gastropoda: Ampullariidae) in China. Scientific Reports 8: 1185. https://doi.org/10.1038/s41598-017-19000-7.
Yang, Q., J. C. Ip, X. Zhao, J. Li, Y. Jin, X. Yu & J. Qiu, 2022. Molecular analyses revealed three morphologically similar species of non-native apple snails and their patterns of distribution in freshwater wetlands of Hong Kong. Diversity and Distributions 28: 97–111. https://doi.org/10.1111/ddi.13443.
Yoshida, K., K. Hoshikawa, T. Wada & Y. Yusa, 2009. Life cycle of the apple snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) inhabiting Japanese paddy fields. Applied Entomology and Zoology 44: 465–474. https://doi.org/10.1303/aez.2009.465.
Yoshida, K., T. Wada, K. Matsukura & T. Shiba, 2022. Potential overwintering areas of the alien apple snail, Pomacea canaliculata, in Japan at its northern distribution limit. Aquatic Invasions 17: 402–414. https://doi.org/10.3391/ai.2022.17.3.05.
Acknowledgements
We thank Sayuri Gyotoku for maintaining the stock cultures of snails used for our experiments. We also thank Dr. Tetsuya Yasuda for reviewing an early draft manuscript. Thanks are also due to anonymous reviewers for their valuable suggestions particularly on statistical approaches. This study was funded by grants from the Research Program on Development of Innovative Technology (JPJ007097, 03022C2) to the authors from the Bio-oriented Technology Research Advancement Institution (BRAIN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsukura, K., Yoshida, K. Comparison of development and overwintering rates and feeding efficiency on rice seedlings among two invasive freshwater apple snails and their hybrid. Hydrobiologia 851, 195–203 (2024). https://doi.org/10.1007/s10750-023-05326-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05326-z