Abstract
Yellowstone cutthroat trout inhabiting Yellowstone Lake have declined substantially over the past 25 years as a result of predation by invasive lake trout, the presence of whirling disease, and periods of persistent drought. We used otolith microchemistry to assess whether cutthroat trout recruitment patterns have changed in response to these environmental stressors. Though water chemistry variation among the 22 sampled spawning tributaries was low, we identified 9 distinct spawning stream clusters. Random forest models were developed for assessing relative recruitment, yielding a high classification accuracy of 84.4% for known-origin cutthroat trout fry otoliths and 79.0% for simulated otolith signatures based on water chemistry. Proportion of recruitment varied significantly between pre- and post-stressor samples (X2 = 15.40, P = 0.03). The majority of pre- (0.84) and post-stressor (0.77) recruitment occurred in the same three stream clusters, but there was a notable decrease in recruitment in streams with high whirling disease prevalence, and a notable increase from the tributary cluster without whirling disease and with low lake trout predation risk. Conservation efforts should be focused on protecting important spawning tributaries and improving cutthroat trout recruitment in spawning streams that in the past likely contributed much greater numbers of fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
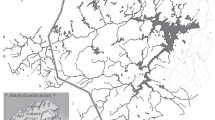
Conservation of migratory salmonids (genera Oncorhynchus, Salmo, and Salvelinus) is complex due to the wide range of habitat types (e.g. marine, freshwater, fluvial, lacustrine) occupied by a diversity of life-history forms (Klemetsen et al., 2003; Schindler et al., 2003). A driving behavior shared by these taxa is homing, where adults produced in a stream tend to return there to spawn (Ueda et al., 1998; Keefer & Caudill, 2014), resulting in reproductive isolation of populations (metapopulations) with differential productivity (Horrall, 1981; Stewart et al., 2003). Variation in productivity may be caused by local genetic adaptation to different physical conditions (e.g. flows, temperatures or spawning substrates) or biotic factors (e.g. competitors or predators) at spawning streams distributed across large spatial areas (Quinn, 2018). Assessing the relative contributions of natal tributaries to overall recruitment is vital for prioritizing protection and restoration of critical spawning and rearing habitats (Brennan et al., 2015; Brennan & Schindler, 2017; Phillis et al., 2018). Cutthroat trout [Oncorhynchus clarkii (Richardson, 1836)] subspecies of the western United States are among the salmonids with migratory life histories (Behnke, 2002). The Yellowstone cutthroat trout [O. clarkii subsp. bouvieri (Jordan & Gilbert, 1883)] in Yellowstone Lake, northwestern Wyoming, is a large genetically-pure population (al-Chokhachy et al., 2018) that exhibits a lacustrine-adfluvial life-history strategy. These Yellowstone cutthroat trout, hereafter cutthroat trout, make spawning migrations during spring into > 60 small tributaries around the lake (Gresswell et al., 1994; Varley & Schullery, 1998), move downstream to spawn in the Yellowstone River outlet (Kaeding & Boltz, 2001), or make long-distance spawning migrations upstream into the remote headwaters of the upper Yellowstone River inlet and its numerous large tributaries in the Thorofare region of the Bridger-Teton Wilderness (Ertel et al., 2017). Like other salmonids they show high spawning fidelity to natal streams (Ball, 1955; Gresswell et al., 1994). Adults of this population are iteroparous and commonly skip-spawn (Cope, 1956, 1957; Jones et al., 1986; Ertel et al., 2017). After a brief spawning period (few weeks) in tributaries, adults return to the relatively stable environment of Yellowstone Lake where they spend most of the year. After emergence, the fry produced in small tributaries generally emigrate to the lake prior to winter (Varley & Gresswell, 1988). In large tributaries (e.g., Pelican Creek) or streams in the remote upper Yellowstone River watershed (Fig. 1), fry may overwinter and return to the lake later as juveniles (Varley & Gresswell, 1988).
Map of study area and underlying geology in Yellowstone National Park, Wyoming. Symbols are the types of samples collected and the stream sample locations numbered: 1. Arnica Creek, 2. Beaverdam Creek, 3. Big Thumb Creek, 4. Bridge Creek, 5. Cabin Creek, 6. Chipmunk Creek, 7. Clear Creek. 8. Columbine Creek, 9. Cub Creek, 10. Flat Mountain Creek, 11. Grouse Creek, 12. Little Arnica Creek, 13. Little Thumb Creek, 14, 15. Thorofare Creek, 16. Mountain Creek, 17. Pelican Creek, 18. Sewer Creek, 19, 25. Upper Yellowstone River, 20. Solution Creek, 21. Trail Creek, 22. Creek 1138, 26, 27. Lower Yellowstone River
Within Yellowstone National Park, cutthroat trout are considered a keystone species with at least 4 mammal and 16 bird species depending upon them as prey (Bergum et al., 2017). Cutthroat trout abundance in Yellowstone Lake has varied among years but declined over several decades (1980s–2000s; Koel et al., 2005). The decline was largely caused by the unintentional introduction of nonnative lake trout [Salvelinus namaycush (Walbaum, 1792)] (Kaeding et al., 1996; Ruzycki et al., 2003; Koel et al., 2020a) which added a novel 4th piscivorous trophic level to the aquatic food web (Tronstad et al., 2010). Cutthroat trout loss was also caused by the introduction of [Myxobolus cerebralis (Hofer, 1903)] the causative agent of whirling disease (Koel et al., 2006), and persistent drought conditions which isolated some streams (Koel et al., 2005; Kaeding, 2020). Cascading interactions throughout the aquatic and associated terrestrial ecosystems of Yellowstone Lake were driven by the cutthroat trout decline (Tronstad et al., 2010; Koel et al., 2019a). Plankton assemblages within the lake were altered, and nutrient transport to tributary streams was reduced (Tronstad et al., 2015). Cutthroat trout once composed a large portion of the spring diet of many grizzly bears [Ursus arctos (Linnaeus, 1758)] and black bears [U. americanus (Pallas, 1780)], however, in recent years fewer bears have been observed feeding on spawning fish (Middleton et al., 2013). Similarly, once-abundant osprey [Pandion haliaetus (Linnaeus, 1758)] nesting near the lake were displaced, and bald eagles [Haliaeetus leucocephalus (Linnaeus 1766)] shifted their diet to compensate for the cutthroat trout loss (Koel et al., 2019a).
To mitigate for threats to cutthroat trout, Yellowstone National Park implemented a large-scale lake trout suppression program that over 25 years drove the invasive population into decline (Koel et al., 2020b). Assessment of fish populations and monitoring for persistent effects of drought and whirling disease in spawning streams also routinely occurs. Prevalence and severity of whirling disease has stabilized and remains high only in Pelican Creek and the Yellowstone River downstream of Yellowstone Lake, two of the largest spawning streams (Koel et al., 2019b). Previously, counts of adult spawning cutthroat trout in tributaries have been used as a metric for assessing the status of the population each year to determine which spawning streams are most important to recruitment, and how recruitment may change over time (Koel et al., 2020b). However, these counts were largely confined to small tributaries on the western side of Yellowstone Lake where fish could be easily observed and counted, while many important spawning streams in remote locations (Reinhart & Mattson, 1990; Ertel et al., 2017) were not routinely monitored due to inaccessibility. Thus, although juvenile cutthroat trout recruitment to Yellowstone Lake has improved in recent years (Koel et al., 2019a, 2020b), little is known about which tributaries are most strongly contributing to this recruitment or how these recruitment patterns may have changed over time.
We hypothesized that spawning tributaries located in lake regions with the highest lake trout densities (streams flowing into West Thumb, Flat Mountain Arm, and South Arm; Fig. 1; Bigelow et al., 2017), streams with the highest whirling disease severity (Koel et al., 2019b), and the streams that are most susceptible to drought (Koel et al., 2005), may have reduced recruitment of juvenile cutthroat trout. The majority of lake trout netted from Yellowstone Lake have been netted in the West Thumb and western side of the main basin of the lake (Bigelow et al., 2017), thus it appears that lake trout densities tend to be higher in some lake regions than others. As a result of the uneven distribution of lake trout throughout the lake, the effects of their predation on cutthroat trout may be more pronounced in relation to spawning streams flowing into portions of the lake with higher lake trout densities. Additionally, the effects of drought and whirling disease is not uniform among spawning tributaries or within Yellowstone Lake (Koel et al., 2006, 2019b), therefore, we suspect that recruitment patterns among tributaries may have shifted considerably during the past 25 years as a result of large and cumulative stressors on this population.
One method for reconstructing the lives of migratory salmonids is by using natural isotopic and trace elemental variation (Martin et al., 2013; Zimmerman et al., 2013; Phillis et al., 2018). The varying chemical composition of spawning tributaries, reflective of differences in underlying geology (Kennedy et al., 2000; Wells et al., 2003; Brennan & Schindler, 2017), impart unique chemical signatures to otoliths during early rearing, allowing for retrospective identification of natal origins and recruitment by matching water and otolith composition (Campana, 1999; Muhlfeld et al., 2012; Brennan et al., 2015; Brennan & Schindler, 2017).
However, low variation in water chemistry in some environments can limit the potential success of this methodology for assessing natal origin and movement patterns (Pangle et al., 2010; Stewart et al., 2021). In this study, our aim was to use a combination of Sr isotopes (87Sr:86Sr) and elemental ratios (Sr:Ca, Ba:Ca, Mg:Ca, Mn:Ca) in otoliths to assess potential shifts in cutthroat trout recruitment among spawning streams in the Yellowstone Lake watershed in response to significant environmental and biotic changes. The spawning stream contributions were compared for two time periods: (1) a ‘pre’ period (in 1997), prior to the significant negative effects of drought, whirling disease, and the full manifestation of lake trout population expansion and high predation pressure on cutthroat trout, and (2) and a ‘post’ period, 16 years later (in 2013), which reflected very low cutthroat trout abundance following the invasion of lake trout, drought, and whirling disease (Koel et al., 2020b). The analysis of microchemical records in otoliths offers a unique approach for assessing fish recruitment patterns over time in response to harvest, climate change, and other environmental stressors (Miller et al., 2011; Brennan et al., 2015, 2016, 2019a, 2019b; Walsworth et al. 2020).
Methods
Study area
The study area encompassed the Yellowstone Lake drainage upstream of the Upper Falls on the Yellowstone River (Figure 1). Yellowstone Lake is the largest high elevation lake (2376 m) in North America (352 km2; Kaplinski, 1991). The drainage has an area of 2616 km2 (Benson, 1961) with 124 tributaries, 68 of which have documented spawning by cutthroat trout (Jones et al. 1986). Underlying bedrock in the drainage is primarily Tertiary andesite and Quaternary rhyolite, and Tertiary sedimentary deposits are present in several tributary drainages in the southern portion of the basin (USGS 1972). At lower elevations, post-glacial deltaic deposits are common, likely as a result of historically higher lake levels (Richmond, 1976).
Two species of fish are native in the Yellowstone Lake drainage, the longnose dace [Rhinichthys cataractae (Valenciennes, 1842)] and cutthroat trout. Besides lake trout, there were four other non-native fishes introduced to Yellowstone Lake, presumably by anglers as baitfish, including redside shiner [Richardsonius balteatus (Richardson, 1836)], lake chub [Couesius plumbeus (Agassiz, 1850)], longnose sucker [Catostomus catostomus (Forster, 1773)] (Varley and Schullery 1998), and cisco [Coregonus artedi (Lesueur, 1818)] (Koel et al. 2020b). Although rarely studied, there is no evidence these fishes influenced recruitment or otherwise negatively affected the native cutthroat trout (Brown & Graham, 1954; Gresswell & Varley, 1988; Furey et al., 2020).
Water chemistry
Water chemistry used to identify natal origins was determined for 22 different tributaries. Study streams were selected by two criteria. First, we included streams known to be important for spawning based on observations of greater than 100 spawning trout observed (Reinhart, 1990; Haroldson et al., 2005; Ertel et al., 2017). Second, to ensure adequate spatial representation of tributaries likely differing in chemical composition, we also added tributaries that represented the range of underlying geology present, which has been shown to be important predictor of isotopic and elemental composition of surface waters (Hegg et al., 2013; Garcez et al., 2014; Humston et al., 2017).
Water samples were collected during summer base flow conditions in 2013 (August and September), which coincided with the post-emergence period of juvenile rearing prior to outmigration to the lake. Due to the large size of three tributaries, two water samples were collected at different locations at these sites (Fig. 1). To test for annual variation in water chemistry, a subset of 5 tributary streams were resampled in 2014. At each stream, 50 ml water samples were collected in the thalweg by inserting a sterile 50 ml syringe vertically into the upstream water column. Samples were filtered with a sterile 0.2 µm Whatman filter and then stored in acid-washed polyethylene bottles containing two drops of nitric acid.
Water samples were analyzed at Woods Hole Oceanographic Institution (WHOI) using solution-based inductively coupled plasma mass spectrometry (ICPMS) for the following isotopic and elemental ratios: 87Sr:86Sr, Sr:Ca, Ba:Ca, Mg:Ca, and Mn:Ca following the methods of Muhlfeld et al. (2012). Isotopic and elemental ratios were measured separately. For 87Sr:86Sr ratios, a portion of each water sample was evaporated and dissolved in 50% HNO3 then eluted through a Sr-specific cation exchange resin to separate out Sr. The remaining filtrate was evaporated and re-dissolved in 1 ml of 5% HNO3 and then analyzed with a Thermo Finnigan Neptune multiple collector ICPMS (Muhlfeld et al., 2012). Strontium isotope ratios were calculated by correcting for interferences of 87Rb on 87Sr and 86Kr on 86Sr using methods described by Jackson & Hart (2006). Lastly, all Sr isotope ratios were normalized to the NIST SRM987 standard. For elemental ratios, water samples were first diluted 10-fold using a 2% HNO3 solution prior to ICPMS measurement of 48Ca, 88Sr, 138Ba, 25Mg and 55Mn. Liquid standards and instrument blanks of 2% HNO3 were run every six samples. Instrument mass bias was corrected using certified values from a river water standard (SLRS-4, NRC), and an internal laboratory river water standard was used to assess measurement precision. External precision (relative standard deviation) of element:Ca ratios for the laboratory standard (n = 20) ranged between 1 and 6%.
Otolith sampling
We used otoliths from known-origin fish to correlate natal stream water chemistry to chemical composition of cutthroat trout otoliths following the methods of Muhlfeld et al. (2012) and Olley et al. (2011). Cutthroat trout fry (~1–2 months post emergence, n = 5–12 per site) were collected by dipnet from a subset of 7 natal streams (Beaverdam Creek, Columbine Creek, Cub Creek, Grouse Creek, Little Thumb Creek, Sewer Creek, and Creek 1138 during base flow conditions in August and September 2013 coincident with water sampling (Table 2). Sample sites were selected based on the underlying geology to represent the range of variation in chemical signatures among streams. Otoliths were removed under a dissecting microscope using nonmetallic forceps and processed and stored using the methods described below.
Natal recruitment during the two study periods was assessed using cutthroat trout captured during annual lakewide gillnetting assessments conducted in 1997 and 2013 (Kaeding & Koel 2011; Syslo et al., 2014). During both assessments, gillnets were distributed in the littoral zone around Yellowstone Lake to ensure sampling effort occurred throughout shallow waters of the lake. Gill nets were multi- mesh (19–51 mm bar measure), and were 38 m long by 1.5 m deep in 1997 and 90-m long by 2.4 m deep in 2013. Although the number of sampling sites differed, 11 sites in 1997 compared to 24 sites in 2013, many of the sites sampled in 2013 were in the same locations as those sampled in 1997. In addition, we know from telemetry studies that adult cutthroat trout move extensively throughout the lake (Ertel et al., 2017), ensuring that fish sampled during both years were from the same sampling population.
Sagittal otoliths from fish collected in 2013 gillnet sampling were removed using non-metallic forceps, and scrubbed, triple-rinsed, and soaked in Milli-Q water overnight prior to air-drying and storage in acid washed microcentrifuge vials. Otoliths from the 1997 sampling had been stored dry in envelopes but the instruments used in their removal were unknown; however, the processing we performed likely removed possible contaminants. Because there was a limited selection of otoliths collected in 1997 (n = 107), all were used for microchemical analysis. For the 2013 sampling, a subset of otoliths was selected for microchemical analysis using a stratified random design. One hundred otoliths were randomly selected for each of three length classes (< 200, 200–400, and > 400 mm) to ensure sampling fish across all age and size classes. For both sampling years, one otolith of each pair was randomly selected for further analysis. Some otoliths were damaged during processing and were removed from analysis; final sample sizes were n = 89 for the 1997 sample and n = 236 for the 2013 sample.
Prior to analysis, stored otoliths were rinsed with Milli-Q water and scrubbed with a nylon brush, dried under a laminar flow hood for 24 h, mounted on petrographic slides (sulcus side up) using cyanoacrylate glue, and then sanded using 600- and 1500-grit sandpaper and polished using 0.5- and 0.1-μm diamond lapping film until the plane of the nucleus became visible. After a final scrubbing and soaking with Milli-Q water, otoliths were remounted on a new petrographic slide. Finally, otoliths were aged independently by two experienced readers to assess whether the proportion of age classes among both past and recent samples were similar.
Trout age composition was similar between the two samples, ranging from age 1 to age 9, representing recruitment years 1988–1996 for the 1997 sample, and recruitment years 2004–2012 for the 2013 sample. Ages 3–6 comprised most of both samples (1997, 74.2%; 2013, 66.5%), with age 2 comprising the next predominant age group (0.1% and 13.1%, respectively).
Remaining age groups (1, 7, 8, and 9) collectively comprised 15.7% and 20.3% of the past and recent trout samples, respectively.
Otolith microchemistry
Otolith microchemistry was performed using laser ablation ICPMS equipped with a 213-mm laser. Otolith 87Sr:86Sr, Sr:Ca, and Ba:Ca ratios were obtained using a Thermo Finnigan Neptune multiple collector ICPMS, whereas elemental Mg:Ca and Mn:Ca ratios were obtained in a separate assay using a Thermo Finnigan Element single collector ICPMS, both of which were coupled to the same laser ablation system (Walther et al., 2008). Both juvenile and adult otoliths were ablated along a 450-μm, curved transect for both assays using a beam diameter of 75 μm, a repetition rate 20 Hz, and a scan speed 5 μm s−1. The length of the transect was chosen to insure an adequate sample was ablated to reduce potential variation from spot measurements.
Transects were positioned adjacent to the core to minimize interference from a maternal signal and within the narrow region corresponding to the early growth period right after emergence but prior to outmigration to the lake. This region, ~ 100 μm from the core, was determined from preliminary transects run across otoliths from the core to the edge, showing a different chemical signature from the maternal core and the lake entry and rearing phase. Sample processing was randomized to minimize potential systematic bias from instrument drift. For quality assurance, a certified reference material (MACS-3; Wilson et al., 2008) was run every 10 samples (Muhlfeld et al., 2012) to assess instrument drift and changes in mass bias. The mean 87Sr:86Sr ratio (± 1 SD) for the certified reference material run throughout the analysis was 0.707601 ± 0.00006, which is within 1 standard deviation of the accepted value of MACS-3 (0.70759). External precision (relative SDs) for Sr:Ca and Ba:Ca ratios based on repeated measurements (n = 34) of a certified reference material (FEBS-1; Sturgeon et al., 2005) was 4.0% for Sr:Ca, 0.1% for Ba:Ca, 1.5% for Mg:Ca, and 4.1% for Mn:Ca. Isotopic interference was corrected following methods described above for water analyses. Lastly, similar to Blair & Hicks (2012), all element:Ca readings that that fell below the detection limits of the LA-ICPMS and outliers greater than 5 standard deviations from the mean were excluded from further analysis.
Statistical analyses
Statistical analyses were conducted using R version 3.1.1 (R Core Team, 2014). We first examined relations between water and otolith chemistry for each isotope (87Sr:86Sr ) and elemental ratio (Sr:Ca, Ba:Ca, Mg:Ca, and Mn:Ca) with known-origin fry using simple linear regression. Initial examination of diagnostic quantile-quantile plots, box plots, and scatter plots found no violations of the parametric assumptions of normality, homoscedasticity, linearity, and equal variances. There was a near 1:1 relation between otolith and water Sr isotopes [(87Sr:86Sr)otolith = 0.98(87Sr:86Sr)water + 0.017; r2 = 0.99; p < 0.001] (Fig 2); otolith and water Sr:Ca ratios were also strongly related [(Sr:Ca)otolith = 0.21(Sr:Ca)water + 0.20; r2 = 0.74; p = 0.029) (Fig. 2). In contrast, there were no clear linear relations between otolith and water Ba:Ca (r2 = 0.21; p = 0.36), Mg:Ca (r2 = − 0.21; p = 0.76), and Mn:Ca r2 = − 0.22; p = 0.78) ratios. Thus, only 87Sr:86Sr and Sr:Ca ratios were retained for further analyses.
Studies that seek to identify the natal origin of fish using otolith microchemistry typically collect known-origin fish from a variety of recruitment sources to develop a model for classifying the natal source of unknown-origin fish (e.g. Munro et al., 2005; Muhlfeld et al., 2012; Sousa et al., 2016). In the case of Yellowstone Lake, where many potential recruitment tributaries exist, the collection of known-origin fish over many potential sources was impractical.
We therefore used associations between otolith and water chemistries to create a model to predict otolith Sr isotopic and elemental signatures from water chemistry data for all 22 study streams. A 3-step process was used to create a range of expected otolith 87Sr:86Sr and Sr:Ca ratios for each stream. First, otolith Sr:Ca ratios were predicted by applying a partition coefficient based on the relationship between water and otolith Sr:Ca derived from known-origin cutthroat trout fry.
Partition coefficients reflect the degree that elemental uptake in otoliths reflects that found in source waters; the relationship observed for Yellowstone cutthroat trout in our study based on the slope of the linear regression for Sr:Ca (DSr:Ca = 0.21) is similar to that found for cutthroat trout in other studies (Wells et al., 2003; Muhlfeld et al., 2012). In contrast, predicted otolith 87Sr:86Sr were estimated directly from the measured water 87Sr:86Sr because of the near 1:1 relationship.
Uncertainty in predicted otolith 87Sr:86Sr and Sr:Ca values was estimated by averaging the standard deviation of known-origin otolith 87Sr:86Sr and Sr:Ca calculated for each stream (n = 6; Beaverdam Creek was excluded because only one otolith remained after preparation). Second, similar to the bootstrapping appproach used by Mikheev et al. (2020) to increase sample size, we then generated 50 expected otolith 87Sr:86Sr and Sr:Ca values for each stream from a normal distribution via the “rnorm” function in R, applying the predicted mean otolith 87Sr:86Sr and Sr:Ca value and standard deviation of otolith isotopic and elemental signatures of known-origin fish as above. Due to the higher variation in incorporation of elemental Sr into otoliths from stream water, two standard deviations were used when generating expected values for Sr:Ca. Comparisons of the expected and observed otolith signatures from known-origin cutthroat trout fry were used to assess the accuracy of the simulated otolith chemistry values (see below).
Bivariate plots of stream water 87Sr:86Sr and Sr:Ca revealed considerable overlap in chemical signatures among some spawning tributaries, making it difficult to classify fish to individual streams with high confidence. Therefore, for the third step, we grouped study streams according to their isotopic and elemental signatures and then assigned fish to their putative natal “stream group” using a cluster-then-predict approach (Booker, 2016). The general approach to this process is to first apply clustering methods to assign streams into groups; visual assessment of cluster separation is used to select the number of clusters for initial grouping. Next, a classification method is applied to assign known-origin fish to a stream group (cluster) based on their otolith 87Sr:86Sr and Sr:Ca. This process is repeated with different numbers of clusters, and classification accuracy is used as the criteria for determining the maximum number of clusters that provides acceptable classification error.
Streams were grouped according to similarity of water chemistry using nearest-neighbor cluster analysis (Mächler et al., 2014). Multiple samples of water chemistry from large streams were pooled by averaging, and stream 87Sr:86Sr and Sr:Ca ratios were standardized to a mean of zero and a standard deviation of 1.0 to accommodate for the different scales of isotopic and elemental values. Variables in the cluster analysis were also weighted to reflect the higher precision of isotopic Sr as a predictor of natal origins than elemental Sr (Kennedy et al., 2000).
Standardized stream Sr:Ca ratios were multiplied by 0.50 to reduce the variance by 50% and to reflect additional weight given to Sr isotopes (Kaufman & Rousseeuw, 2005).
A random forest model was then constructed for the classification of cutthroat trout to their natal ‘cluster’ based on otolith signatures. Mercier et al., (2011) found that random forest modeling had the highest classification accuracy and least violation of statistical assumptions among discrimination methods used for otolith microchemistry studies. Because we were not able to collect fish from each stream in the study area, the ‘simulated’ otolith signatures generated from water-otolith Sr:86Sr and Sr:Ca regressions were used for the training data set.
Random forest classification trees were constructed using a random subset of 75% of the simulated otolith signatures using bootstrap resampling with replacement. For each tree, a random predictor variable was searched at each node to find the one that maximized the within group homogeneity until further splitting resulted in no gain in within-group homogeneity (Mercier et al., 2011). Our sample of known-origin cutthroat trout otoliths and the remaining 25% of the simulated otolith signatures were then used to classify the prediction accuracy of the model using a ‘confusion’ or error matrix (Tharwat, 2018). This process was applied iteratively to determine the optimum number of stream clusters. The final combination of clusters and classification model was used to classify unknown-origin fish to a putative natal stream cluster based on ensemble predictions of 500 bootstrapped random forests each with 2000 trees (see also Mikheev et al., 2020).
To assess whether there were differences in the proportional contributions of stream clusters to recruitment represented in 1997 and 2013 fish samples, differences in the assigned natal origins for each group were quantified using a Pearson’s chi-squared test with Monte-Carlo simulation of P values. Due to insufficient sample sizes (< 5) in some clusters, 2000 random p-value replicates were simulated to create a reference distribution for comparison to satisfy the assumptions of the analysis (Hope, 1968).
Results
Water chemistry
Yellowstone Lake tributaries showed some distinctive differences in 87Sr:86Sr and Sr:Ca ratios among streams but also displayed a substantial amount of overlap. Sr isotope ratios ranged from 0.705824 to 0.709242, and Sr:Ca ratios ranged from 0.92 to 6.22 mmol/mol (Table 1).
Streams in close proximity generally exhibited similar microchemical signatures, but in some cases nearby streams exhibited very different signatures (Fig. 3). For example, Chipmunk Creek and Grouse Creek, two streams that are within 5 km of one another, exhibited similar Sr:Ca (3.40 and 3.54 mmol/mol, respectively), but much different 87Sr:86Sr (0.706757 and 0.709242, respectively, Table 1). In some instances, streams that are relatively distant from one another exhibited similar microchemical signatures, e.g., Trail Creek and Little Arnica Creek had very similar 87Sr:86Sr (0.707903 and 0.707732, respectively) despite a spatial separation of nearly 47 km (Fig. 1).
Nearest neighbor cluster analysis dendrogram of streams (n = 22) and spatial distribution of 9 distinct clusters based upon stream 87Sr:86Sr and adjusted Sr:Ca ratios. The y-axis ‘height’ is a measure of the closeness of clusters based on where they split. Clear, Cub, Columbine, and Cabin creeks had replicate samples. Colored text and numbers in the left column correspond to the cluster (and corresponding colored line) in which each stream was assigned
Annual variation in water 87Sr:86Sr and Sr:Ca among streams was low, with a mean difference between replicate samples of 0.04‰ (0.01‰–0.12‰) and 6.77% (2.03%–11.90%), respectively. In contrast, differences among streams in 87Sr:86Sr and Sr:Ca were ~ 15 and 3.5 times greater, respectively, than differences among replicate water samples.
Cluster analysis resulted in all 22 sampled streams being grouped into 9 clusters (Table 1; Fig. 3) (n = 22), with the number of streams in each cluster ranging from 1 to 7. Two clusters contained multiple streams that were all located in relatively close proximity to one another, cluster 6 (Cub and Pelican Creeks) and cluster 9 (Mountain and Thorofare Creeks). However, the remaining clusters had no clear spatial patterns; for example, streams in the largest cluster (cluster 2) were distributed throughout the West Thumb, main basin, and the South and Southeast Arms of Yellowstone Lake. Bivariate plots of 87Sr:86Sr and Sr:Ca showed that addition of Sr:Ca in the analysis improved separation of the clusters (e.g. clusters 4 and 9; see Table 1 and Fig. 4).
Classification of Natal origins
Mean classification accuracy of simulated otolith signatures for the 9-cluster random forest model across all 500 random forests was 79.0% (range, 72.3–84.5%). Classification accuracy of simulated otolith signatures varied across clusters, ranging from 31.6% to 100.0% (Table 2). Cluster 1 and cluster 4 had the lowest classification accuracy of simulated otolith signatures with 49.3% and 31.6% correct classification, respectively. Cluster 7 and cluster 8 had the highest classification accuracy with 100% correct classification. Known-origin trout fry (representing 4 of the 9 clusters) were classified with 84.4% classification accuracy (range, 77.8–100.0%; Table 3).
The proportion of both past and recent fish otolith signatures assigned to each cluster ranged from 0 to 0.33 (Table 4). The largest recruitment for both groups, totaling 84% of the 1997 sample and 77% of the 2013 sample, originated from just 3 clusters (2, 5, 6), representing some of the largest tributaries, including the Upper Yellowstone River (cluster 2), the Lower Yellowstone River (cluster 5), and Pelican Creek (cluster 6). Little or no recruitment was observed from clusters 1 (Arnica Creek and Bridge Creek), 3 (Big Thumb Creek) and 8 (Grouse Creek).
Proportional trout recruitment differed significantly among the stream clusters (X2 = 15.40; P = 0.03). Recruitment remained similar over time in 4 of the 9 clusters, with proportional differences of less than 0.1 between the two sample periods. However, three clusters, clusters 5, 6, and 7, showed a 15, 33, and 66% respective decrease in recruitment contribution, whereas cluster 2 showed an increase in recruitment of 22%. The largest change occurred in cluster 9, where recruitment increased by a factor of 4.5, from 0.02 in 1997 to 0.11 in 2013.
Discussion
Analysis of otolith chemistry shows promise for assessing changes in life history variation and fish recruitment sources over time that otherwise would be difficult using traditional recruitment assessment methods (Miller et al., 2011; Brennan & Schindler, 2017, Brennan et al., 2015,, 2019a, b; Walsworth et al., 2020). In this study, we demonstrated that cutthroat trout otolith samples collected in a large lake system between a 16-year-interval, could, with some limitations, show differences in sources of natal recruitment over time in response to significant environmental stressors. We also developed a novel method for creating simulated otoliths from natal tributary water samples when otoliths from known-origin juvenile fish used to assign natal origins of adult fish are limited.
The relatively low variation in stream microchemical signatures among the 22 Yellowstone Lake spawning tributaries we sampled limited the power of our recruitment analysis. Because the underlying bedrock geology in the northern and western portion of the drainage is primarily rhyolite, and the underlying geology in the eastern and southern portion of the drainage is primarily mixed clastic/volcanic rock, streams within these respective regions often had similarities in their microchemical signatures. Accordingly, some areas with unique bedrock types within the drainage also exhibited unique microchemical signatures. For instance, Grouse Creek was the only stream that had an abundance of conglomerate bedrock within its watershed and consequently its Sr isotopic signature was much higher than other streams. There were also some anomalies in water chemistry throughout the drainage. For example, Little Thumb Creek and Solution Creek, two streams flowing into the West Thumb region of the lake, exhibited similar microchemical signatures to Cabin Creek, Beaverdam Creek, Upper Yellowstone River, and other streams originating from areas with a different dominant bedrock type. We suspect that these similarities in microchemical signatures despite very different geologies may be due to the presence of alluvial deposits at lower elevations that may mask the signature effects from higher elevation bedrock types.
The low range in variation in 87Sr:86Sr ratios we observed also contributed to the relatively low number of unique stream microchemical signatures. For example, Muhlfeld et al. (2012) found that 87Sr:86Sr ratios varied from 0.71131 to 0.74679 among 41 sites in the Flathead River drainage, MT, an isotopic range of ~0.0355. In contrast, the 87Sr:86Sr ratios in the Yellowstone Lake drainage varied from 0.705824 to 0.709242, an isotopic range of ~0.0034, an order of magnitude lower. The stream Sr:Ca ratios in this study exhibited a wider range of variation (0.92–6.22 mmol/mol) than isotopic ratios and was similar in magnitude to that observed in other studies (0.23–3.10 mmol/mol; Muhlfeld et al., 2012); however, most of the streams (59%) in our study exhibited a much smaller range of variation (3.00–5.00 mmol/mol). Additionally, Sr:Ca ratios of otoliths are heteroscedastic (Wells et al., 2003), with fish from streams with high Sr:Ca ratios, like those found in the Yellowstone Lake drainage, showing a wider range of variability in their otolith Sr:Ca signatures, thus further limiting their discriminatory power.
The low variation in stream and otolith chemistry necessitated the clustering of streams. Clustering can improve classification accuracy for estimating natal origin (Wells et a1., 2003; Carleton, 2011; Brennan & Schindler, 2017). Carleton (2011) found that classification accuracy improved by approximately 20% when streams with similar microchemical signatures were grouped together. A limitation of our study was the relatively low number of clusters identified (9) given the number of streams sampled (22). Additionally, the number of sampled streams represented only 32% of the number of streams where spawning has been documented. Though our analysis included many of the streams with the largest known concentrations of spawners, inclusion of a larger number of streams would likely have increased the number of unique clusters and provided for a finer level of discrimination among sites. However, given the low variation in water chemistry in the watershed, even more extensive water sampling may not have increased discriminatory power.
Our analysis revealed that three stream clusters were most influential for recruitment both before (combined proportion of recruitment contribution = 0.84) and after (0.77) the expansion of lake trout, emergence of whirling disease, and persistent drought. These stream clusters (2, 5, and 6) contain some of the largest tributaries within the watershed, including the upper and lower Yellowstone rivers, and Pelican Creek, all streams that previous studies reported as having large numbers of spawning fish and multiple spawning sites (Reinhart, 1990; Kelly, 1993; Ertel et al., 2017). However, our analyses also revealed some important differences in recruitment patterns over time, reflecting likely changes in response to large-scale stressors. In support of our initial hypothesis, we found 15% and 33% declines in recruitment contribution from clusters 5 and 6, respectively, which may be attributed to high juvenile mortality resulting from very high infection risk for whirling disease in Pelican Creek, the lower Yellowstone River, and the northern region of Yellowstone Lake (Koel et al., 2006, 2019b). We hypothesize that the notable decline (66%) in recruitment contribution from cluster 7, which includes Flat Mountain and Little Arnica creeks, may be due to extremely high lake trout densities in the Flat Mountain Arm and West Thumb regions of the lake. In contrast, we speculate that the increased recruitment contribution from clusters 2 (+ 22%) and 9 (+ 4500%), wherein whirling disease has not been detected, may be due to their higher watershed elevations, relatively large stream sizes, and drainage into the southern sections of Yellowstone Lake where lake trout densities are low. These clusters include Cabin, Mountain, and Thorofare creeks and the upper Yellowstone River mainstem, located in the remote Bridger-Teton Wilderness south of Yellowstone National Park.
We speculate that these larger, higher elevation streams would also be more resilient to drought, which ostensibly reduced recruitment to many of the smaller tributaries around Yellowstone Lake in the early-2000s by reducing access for spawners migrating from the lake (Koel et al., 2005; Kaeding, 2020). The South Arm of Yellowstone Lake, where the inlet of the upper Yellowstone River is located, has the lowest densities of predatory lake trout, and the fry emerging from tributaries long distances from the lake would have a greater ability to escape their predation due to increased size from a more extended growth and rearing phase before entering the lake. An important caveat in interpreting these results is that we had only 1 year each of pre- and post- data. Thus, we were unable to identify the extent of natural variation in recruitment among sites which, in Pacific salmon populations, has been shown to shift considerably even over relatively short time periods (Schindler et al. 2010).
Previous studies have emphasized the importance of sampling water and known-origin reference fish from all areas of potential recruitment when using otolith chemistry to assess natal origins (Campana, 2005; Brennan & Schindler, 2017; Mikheev et al., 2020). However, common to ours and other studies (e.g., Brennan et al., 2015), fish sampling does not occur at all water sampling stations, necessitating the development of models for estimating the degree of spatial resolution of microchemical data. Here, we developed a method for simulating otolith signatures from water sampling alone using a combination of Sr isotope and Sr:Ca data. Our results suggest that using water samples to generate simulated otolith data in combination with clustering analyses could be a useful approach to guide sampling efforts and evaluate the feasibility and discriminatory power in otolith studies. Finally, our findings also underscore the importance of extensive water sampling prior to study initiation to estimate the likely discriminatory power available. Extensive water sampling coupled with development of dendritic network models offers a promising approach for generating isoscape maps that potentially provide a greater level of discriminatory power for apportioning natal origin and recruitment of fish in a mixed stock fishery into specific locations within a watershed (Brennan et al., 2015; Brennan & Schindler, 2017).
Our study demonstrated both the potential strengths as well as the limitations of otolith microchemistry for determining spawning stock contributions and changes in fish recruitment patterns. The analyses used in this study for estimating recruitment changes over time should be useful to other researchers aiming to optimize use of otolith analyses over large drainage basins.
Data availability
The datasets generated and analyzed during the current study are available from the authors on reasonable request.
Code availability
Not applicable.
References
Al-Chokhachy, R., B. B. Shepard, J. C. Burckhardt, D. Garren, S. Opitz, T. M. Koel, L. Nelson & R. E. Gresswell, 2018. A portfolio framework for prioritizing conservation efforts for Yellowstone cutthroat trout populations. Fisheries 43: 485–496.
Ball, O. P., 1955. Some aspects of homing in cutthroat trout. Proceedings of the Utah Academy of Science, Arts, and Letters 32: 75–80.
Behnke, R. J., 2002. Trout and salmon of North America, The Free Press, New York:
Benson N. G., 1961. Limnology of Yellowstone Lake in relation to the cutthroat trout. U.S. Fish and Wildlife Service. Research Report 56: 1–38
Bergum, D. J., K. A. Gunther & L. M. Baril, 2017. Birds and mammals that consume Yellowstone cutthroat trout in Yellowstone Lake and its tributaries. Yellowstone Science 25: 86–89.
Bigelow, P. E., P. D. Doepke, B. D. Ertel, C. S. Guy, J. M. Syslo & T. M. Koel, 2017. Suppressing non-native lake trout in Yellowstone Lake. Yellowstone Science 25: 53–59.
Blair, J. M. & B. J. Hicks, 2012. Otolith microchemistry of koi carp in Waikato region, New Zealand: a tool for identifying recruitment locations? Inland Waters 2: 109–118.
Booker, D. J., 2016. Generalized models of riverine fish hydraulic habitat. Journal of Ecohydraulics 1: 31–49.
Brennan, S. R. & D. E. Schindler, 2017. Linking otolith microchemistry and dendritic isoscapes to map heterogeneous production of fish across river basins. Ecological Applications 27: 363–377.
Brennan, S. R., C. E. Zimmerman, D. P. Fernandez, T. E. Cerling, M. V. McPhee & M. J. Wooller, 2015. Strontium isotopes delineate fine-scale natal origins and migration histories of Pacific salmon. Science Advances 1: e1400124.
Brennan, S. R., D. E. Schindler, T. J. Cline, T. E. Walsworth, G. Buck & D. P. Fernandez, 2019a. Shifting habitat mosaics and fish production across river basins. Science 364: 783–786.
Brennan, S. R., T. J. Cline & D. E. Schindler, 2019b. Quantifying habitat use of migratory fish across riverscapes using space-time isotope models. Methods in Ecology and Evolution 10: 1036–1047.
Brown, C. J. D. & R. J. Graham, 1954. Observations on the longhouse sucker in Yellowstone Lake. Transactions of the American Fisheries Society 83(1): 38–46.
Campana, S. E., 1999. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188: 263–297.
Campana, S. E., 2005. Otolith science entering the 21st century. Marine and Freshwater Research 56: 485–489.
Carleton, S. A., 2011. Identifying tributaries to Jackson Lake important for Snake River cutthroat trout recruitment. University of Wyoming National Park Service Research Center Annual Report 34: 5.
Cope, O. B., 1956. Some migration patterns in cutthroat trout. Proceedings of the Utah Academy of Science, Arts, and Letters 33: 113–118.
Cope, O. B., 1957. Races of cutthroat trout in Yellowstone lake. U.S. Fish & Wildlife Service Special Scientific Report Fisheries 208: 74–84.
Ertel, B. D., T. E. McMahon, T. M. Koel, R. E. Gresswell & J. C. Burckhardt, 2017. Life history migrations of adult Yellowstone cutthroat trout in the upper Yellowstone river. North American Journal of Fisheries Management 37: 743–755.
Furey, K. M., H. C. Glassic, C. S. Guy, T. M. Koel, J. L. Arnold, P. D. Doepke & P. E. Bigelow, 2020. Diets of longnose sucker in Yellowstone Lake, Yellowstone National Park, USA. Journal of Freshwater Ecology 35: 291–303.
Garcez, R. C. S., R. Humston, D. Harbor & C. E. C. Freitas, 2014. Otolith geochemistry in young-of-the-year peacock bass Cichla temensis for investigating natal dispersal in the Rio Negro (Amazon - Brazil) River system. Ecology of Freshwater Fish 24: 242–251.
Gresswell, R. E. & J. D. Varley, 1988. Effect of a century of human influence on the cutthroat trout of Yellowstone Lake. American Fisheries Society Symposium 4: 45–52.
Gresswell, R. E., W. J. Liss & G. L. Larson, 1994. Life-history organization of Yellowstone cutthroat trout (Oncorhynchus clarkii bouvieri) in Yellowstone Lake. Canadian Journal of Fisheries and Aquatic Sciences 51: 298–309.
Haroldson, M. A., K. A. Gunther, D. P. Reinhart, S. R. Podruzny, C. Cegelski, L. Waits, T. Wyman & J. Smith, 2005. Changing numbers of spawning cutthroat trout in tributary streams of Yellowstone Lake and estimates of grizzly bears visiting streams from DNA. Ursus 16: 167–180.
Hegg, J. C., B. P. Kennedy & A. K. Fremier, 2013. Predicting strontium isotope variation and fish location with bedrock geology: understanding the effects of geologic heterogeneity. Chemical Geology 360: 89–98.
Hope, A. C. A., 1968. A simplified Monte-Carlo significance test procedure. Journal of the Royal Statistical Society 30: 582–598.
Horrall, R. M., 1981. Behavioral stock-isolating mechanisms in Great Lakes fishes with special reference to homing and site imprinting. Canadian Journal of Fisheries and Aquatic Sciences 38: 1481–1496. https://doi.org/10.1139/f81-201.
Humston, R., S. S. Doss, C. Wass, C. Hollenbeck, S. R. Thorrold, S. Smith & C. P. Bataille, 2017. Isotope geochemistry reveals ontogeny of dispersal and exchange between main-river and tributary habitats in smallmouth bass Micropterus dolomieu. Journal of Fish Biology 90: 528–548. https://doi.org/10.1111/jfb.13073.
Jackson, M. & S. Hart, 2006. Strontium isotopes in melt inclusions from Samoan basalts: implications for heterogeneity in the Samoan Plume. Earth and Planetary Science Letters 245: 260–277.
Jones, R. D., D. G. Carty, R. E. Gresswell, C. J. Hudson, L. D. Lentsch, D.L. Mahony, 1986. Fishery and aquatic management program in Yellowstone National Park. U. S. Fish and Wildlife Service, Technical Report for 1985, Yellowstone National Park, Wyoming.
Kaeding, L. R., 2020. New climate regime started and further shaped the historic Yellowstone Lake cutthroat trout population decline commonly attributed entirely to nonnative lake trout predation. Aquatic Ecology 54: 641–652.
Kaeding, L. R. & G. D. Boltz, 2001. Spatial and temporal relations between fluvial and allacustrine Yellowstone cutthroat trout, Oncorhynchus clarki bouvieri, spawning in the Yellowstone River, outlet stream of Yellowstone Lake. Environmental Biology of Fishes 61: 395–406.
Kaeding, L. R. & T. M. Koel, 2011. Age, growth, maturity, and fecundity of Yellowstone Lake cutthroat trout. Northwest Science 85: 431–444.
Kaeding, L. R., G. D. Boltz & D. G. Carty, 1996. Lake trout discovered in Yellowstone Lake threaten native cutthroat trout. Fisheries 21: 16–20.
Kaplinski, M. A. 1991. Geomorphology and geology of Yellowstone Lake, Yellowstone National Park, Wyoming. Master’s thesis. Northern Arizona University, Flagstaff, Arizona.
Kaufman, L. & P. J. Rousseeuw, 2005. Finding groups in data: An introduction to cluster analysis, Wiley and Sons Inc., Hoboken, New Jersey:, 11.
Keefer, M. L. & C. C. Caudill, 2014. Homing and straying by anadromous salmonids: a review of mechanisms and rates. Reviews in Fish Biology and Fisheries 24: 333–368. https://doi.org/10.1007/s11160-013-9334-6.
Kelly, B. M., 1993. Ecology of Yellowstone cutthroat trout and an evaluation of potential effects of angler wading in the Yellowstone River. Master’s thesis. Montana State University, Bozeman.
Kennedy, B. P., J. D. Blum, C. L. Folt & K. H. Nislow, 2000. Using natural strontium isotopic signatures as fish markers: methodology and application. Canadian Journal of Fisheries and Aquatic Sciences 57: 2280–2292.
Klemetsen, A., P.-A. Amundsen, J. B. Dempson, B. Jonsson, N. Jonsson, M. F. O’Connell & E. Mortensen, 2003. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecology of Freshwater Fish 12: 1–59. https://doi.org/10.1034/j.1600-0633.2003.00010.x.
Koel, T. M., P. E. Bigelow, P. D. Doepke, B. D. Ertel & D. L. Mahony, 2005. Nonnative lake trout result in Yellowstone cutthroat trout decline and impacts to bears and anglers. Fisheries 30: 10–19. https://doi.org/10.1577/1548-8446(2005)30[10:NLTRIY]2.0.CO;2.
Koel, T. M., D. L. Mahony, K. L. Kinnan, C. Rasmussen, C. J. Hudson, S. Murcia & B. L. Kerans, 2006. Myxobolus cerebralis in native cutthroat trout of the Yellowstone Lake ecosystem. Journal of Aquatic Animal Health 18: 157–175. https://doi.org/10.1577/H05-031.1.
Koel, T. M., L. M. Tronstad, J. L. Arnold, K. A. Gunther, D. W. Smith, J. M. Syslo & P. J. White, 2019a. Predatory fish invasion induces within and across ecosystem effects in Yellowstone National Park. Science Advances 5: eaav1139. https://doi.org/10.1126/sciadv.aav1139.
Koel, T. M., C. R. Detjens & A. V. Zale, 2020a. Two ocean pass: an alternative hypothesis for invasion of Yellowstone Lake by lake trout, and implications for future invasions. Water 12: 1629. https://doi.org/10.3390/w12061629.
Koel, T. M., J. L. Arnold, P. E. Bigelow, T. O. Brenden, J. D. Davis, C. R. Detjens, P. D. Doepke, B. D. Ertel, H. C. Glassic, R. E. Gresswell, C. S. Guy, D. J. MacDonald, M. E. Ruhl, T. J. Stuth, D. P. Sweet, J. M. Syslo, N. A. Thomas, L. M. Tronstad, P. J. White & A. V. Zale, 2020b. Yellowstone Lake ecosystem restoration: a case study for invasive fish management. Fishes 5: 18. https://doi.org/10.3390/fishes5020018.
Koel, T. M., J. L. Arnold, P. E. Bigelow, C. R. Detjens, P. D. Doepke, B. D. Ertel, D. J. MacDonald, 2019b. Native Fish Conservation Program, Yellowstone National Park: Report 2015–2018. National Park Service, Yellowstone Center for Resources YCR-2019b-04, Yellowstone National Park, Wyoming, USA.
Mächler, M., P. Rousseeuw, A. Struyf, M. Hubert, K. Hornik, M. Studer & P. Roudier, 2014. Cluster: cluster analysis extended. R Package Version 1: 15–23.
Martin, J., G. Bareille, S. Berail, C. Pecheyran, F. Daverat, N. Bru, H. Tabouret & O. Donard, 2013. Spatial and temporal variations in otolith chemistry and relationships with water chemistry: a useful tool to distinguish Atlantic salmon Salmo salar parr from different natal streams. Journal of Fish Biology 82: 1556–1581. https://doi.org/10.1111/jfb.12089.
Mercier, L., A. M. Darnaude, O. Bruguier, R. P. Vasconcelos, H. N. Cabral, M. J. Costa, M. Lara, D. L. Jones & D. Mouillot, 2011. Selecting statistical models and variable combinations for optimal classification using otolith microchemistry. Ecological Applications 21: 1352–1364.
Middleton, A. D., T. A. Morrison, J. K. Fortin, C. T. Robbins, K. M. Proffitt, P. J. White, D. E. McWhirter, T. M. Koel, D. G. Brimeyer, W. S. Fairbanks & M. J. Kauffman, 2013. Grizzly bear predation links the loss of native trout to the demography of migratory elk in Yellowstone. Proceedings of the Royal Society b: Biological Sciences 280(1762): 20130870. https://doi.org/10.1098/rspb.2013.0870.
Mikeev, P. B., M. G. Jarvis, C. D. Matthaei, T. Ingram, M. R. Reid, A. I. Nikiforov, I. S. Chernienko & G. P. Closs, 2020. Straying of brown trout in the catchment of a large New Zealand river evaluated by otolith microchemistry. Ecology of Freshwater Fish 30: 433–443.
Miller, J. A., V. L. Butler, C. A. Simenstad, D. H. Backus & A. J. R. Kent, 2011. Life history variation in upper Columbia River Chinook salmon (Oncorhynchus tshawytscha): a comparison using modern and ~500-year-old archaeological otoliths. Canadian Journal of Fisheries and Aquatic Sciences 68: 603–617.
Muhlfeld, C. C., S. R. Thorrold, T. E. McMahon & B. Marotz, 2012. Estimating westslope cutthroat trout (Oncorhynchus clarkii lewisi) movements in a river network using strontium isoscapes. Canadian Journal of Fisheries and Aquatic Sciences 69: 906–915.
Munro, A. R., T. E. McMahon & J. R. Ruzycki, 2005. Natural chemical markers identify source and date of introduction of an exotic species: lake trout (Salvelinus namaycush) in Yellowstone lake. Canadian Journal of Fisheries and Aquatic Sciences 62: 79–87.
Olley, R., R. G. Young, G. P. Closs, E. A. Kristensen, T. O. Bickel, N. A. Deans, L. N. Davey & S. M. Eggins, 2011. Recruitment sources of brown trout identified by otolith trace element signatures. Marine and Freshwater Research 45: 395–411.
Pangle, K. L., S. A. Ludsin & B. J. Fryer, 2010. Otolith microchemistry as a stock identification tool for freshwater fishes: testing its limits in Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 67: 1475–1489.
Phillis, C. C., A. M. Sturrock, R. C. Johnson & P. K. Weber, 2018. Endangered winter-run Chinook salmon rely on diverse rearing habitats in a highly altered landscape. Biological Conservervation 217: 358–362. https://doi.org/10.1016/j.biocon.2017.10.023.
Quinn, T. P., 2018. The behavior and ecology of Pacific salmon and trout, 2nd ed. University of Washington Press, Seattle, Washington, USA:
R Core Team, 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/, Accessed 15 April 2022.
Reinhart, D. P., 1990. Grizzly bear habitat use on cutthroat trout spawning streams in tributaries of Yellowstone Lake. Master’s thesis. Montana State University, Bozeman, Montana.
Reinhart, D. P. & D. J. Mattson, 1990. Bear use of cutthroat trout spawning streams in Yellowstone National Park. Pa 343- 350: in bears: their biology and management, Vol. 8, A selection of papers from the Eighth International Conference on Bear Research and Management, Victoria, British Columbia, Canada, February 1989. International Association for Bear Research and Management, New York, New York.
Richmond, G. M., 1976. Surficial geological history of the Canyon Village quadrangle. Yellowstone National Park, Wyoming, for use with map 1–642. U.S. Geological Survey Bulletin 1427.
Ruzycki, J. R., D. A. Beauchamp & D. L. Yule, 2003. Effects of introduced lake trout on native cutthroat trout in Yellowstone Lake. Ecological Applications 13: 23–37. https://doi.org/10.1890/1051-0761(2003)013[0023:Eoilto]2.0.Co;2.
Schindler, D. E., M. D. Scheuerell, J. W. Moore, S. M. Gende, T. B. Francis & W. J. Palen, 2003. Pacific salmon and the ecology of coastal ecosystems. Frontiers in Ecology and the Environment 1: 31–37. https://doi.org/10.1890/1540-9295(2003)001[0031:Psateo]2.0.Co;2.
Schindler, D. E., R. Hilborn, B. Chasco, C. P. Boatright, T. P. Quinn, L. A. Rogers & M. S. Webster, 2010. Population diversity and the portfolio effect in an exploited species. Nature 465: 609–612.
Sousa, R. G. C., R. Humston & C. E. C. Freitas, 2016. Movement patterns of adult peacock bass Cichla temensis between tributaries of the middle Negro River basin (Amazonas–Brazil): an otolith geochemical analysis. Fisheries Management and Ecology 23: 76–87.
Stewart, I. J., T. P. Quinn & P. Bentzen, 2003. Evidence for fine-scale natal homing among island beach spawning sockeye salmon, Oncorhynchus nerka. Environmental Biology of Fishes 67: 77–85. https://doi.org/10.1023/A:1024436632183.
Stewart, K. P., T. E. McMahon, T. M. Koel & R. Humston, 2021. Use of otolith microchemistry to identify subbasin natal origin and use by invasive lake trout in Yellowstone Lake. Hydrobiologia 848: 2473–2481.
Sturgeon, R. E., S. N. Willie, L. Yang, R. Greenberg, R. O. Spatz, Z. Chen, C. Scriver, V. Clancy, J. W. Lam & S. Thorrold, 2005. Certification of a fish otolith reference material in support of quality assurance for trace element analysis. Journal of Analytical Atomic Spectrometry 20: 1067–1071.
Syslo, J. M., C. S. Guy, J. L. Arnold, T. M. Koel, B. D. Ertel, 2014. Standard operating procedures for distribution netting in Yellowstone Lake. US Geologial Survey, Montana Cooperative Fishery Research Unit, 2010–2012 final report, Yellowstone National Park, Wyoming, USA.
Tharwat, A., 2018. Classification assessment methods. Applied Computing and Informatics 17: 168–192. https://doi.org/10.1016/j.aci.2018.08.003.
Tronstad, L. M., R. O. Hall Jr., T. M. Koel & K. G. Gerow, 2010. Introduced lake trout produced a four-level trophic cascade in Yellowstone Lake. Transactions of the American Fisheries Society 139: 1536–1550. https://doi.org/10.1577/T09-151.1.
Tronstad, L. M., R. O. Hall Jr. & T. M. Koel, 2015. Introduced lake trout alter nitrogen cycling beyond Yellowstone Lake. Ecosphere 6: 224. https://doi.org/10.1890/es14-00544.1.
Ueda, H., M. Kaeriyama, K. Mukasa, A. Urano, H. Kudo, T. Shoji, Y. Tokumitsu, K. Yamauchi & K. Kurihara, 1998. Lacustrine sockeye salmon return straight to their natal area from open water using both visual and olfactory cues. Chemical Senses 23: 207–212. https://doi.org/10.1093/chemse/23.2.207.
United States Geological Survey, 1972, Geologic map of Yellowstone National Park. U.S. Geological Survey, Miscellaneous Geological Investigations Report 711.
Varley, J. D. & R. E. Gresswell, 1988. Ecology, status, and management of the Yellowstone cutthroat trout. American Fisheries Society Symposium 4: 13–24.
Varley, J. D. & P. D. Schullery, 1998. Yellowstone fishes: ecology, history, and angling in the park, Stackpole Books, Mechanicsburg, Pennsylvania:
Walsworth, T. E., J. R. Baldock, C. E. Zimmerman & D. E. Schindler, 2020. Interaction between watershed features and climate forcing affects habitat profitability for juvenile salmon. Ecosphere 11(10): e03266.
Walther, B. D., S. R. Thorrold & J. E. Olney, 2008. Geochemical signatures in otoliths record natal origins of American shad. Transactions of the American Fisheries Society 137: 57–69.
Wells, B. K., B. E. Rieman, J. L. Clayton, D. L. Horan & C. M. Jones, 2003. Relationships between water, otolith, and scale chemistries of westslope cutthroat trout from the Coeur d’Alene River, Idaho: the potential application of hard-part chemistry to describe movements in freshwater. Transactions of the American Fisheries Society 132: 409–424.
Wilson, S. A., A. E. Koenig & R. Orklid, 2008. Development of a microanalytical reference material (MACS-3) for LA-ICP-MS analysis of carbonate samples. Geochemica et Cosmochemica Acta 72: A1025.
Zimmerman, C. E., H. K. Swanson, E. C. Volk & A. J. R. Kent, 2013. Species and life history affect the utility of otolith chemical composition for determining natal stream of origin for Pacific salmon. Transactions of the American Fisheries Society 142: 1370–1380. https://doi.org/10.1080/00028487.2013.811102.
Acknowledgements
Funding for this study was provided by Yellowstone Forever grant number G-022 and the U.S. National Park Service, Yellowstone National Park. We thank Jurek Blusztajn, Woods Hole Oceanographic Institution, for his valuable technical assistance during sample analysis: A. Cheyenne Stewart for her editing, analytical support, and encouragement; Colleen Detjens and Michael Consolo for assistance with sample collection; and Allison Klein for creating the map figures.
Funding
Funding for this study was provided by Yellowstone Forever and by a grant to Montana State University (W4502) from Yellowstone National Park.
Author information
Authors and Affiliations
Contributions
KS, TM, and TK conceived and designed the study. Material preparation and data collection were performed primarily by KS as part of a Master’s thesis, with consultation from TM, TK, and RH. All authors were involved in data analysis and interpretation. All authors were involved in writing the manuscript and all authors read and pproved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Animal handling and collection followed the research permitting process of Yellowstone National Park.
Additional information
Handling editor: Michael Power
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stewart, K.P., McMahon, T.E., Koel, T.M. et al. Current and historical patterns of recruitment of Yellowstone cutthroat trout in Yellowstone Lake, Wyoming, as revealed by otolith microchemistry. Hydrobiologia 851, 7–24 (2024). https://doi.org/10.1007/s10750-023-05245-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05245-z