Abstract
The coral reef crisis has influenced research for over two decades, during which time the capacity of corals to withstand and respond to environmental stress has been documented from the cellular to ecosystem level. Over the past decade, research is increasingly working towards uncovering the extent of coral–bacterial interactions, finding that diverse and stable microbial interactions can be indicative of the health of the coral host. However, we have yet to determine at which level of organismal organisation these interactions occur, in particular those with the coral’s photosynthetic dinoflagellate symbionts. This information is critical if we are to understand the impact of stress on meta-organism functioning. Using 16S gene amplicon sequencing, we investigated the bacterial microbiome of endosymbiotic Symbiodiniaceae from thermally stressed Acropora aspera, under 3 ecologically relevant temperature trajectories (defined as protective, repetitive and single) that are expected under a changing climate. We show that endosymbiotic Symbiodiniaceae host a distinct and diverse bacterial assemblage when compared with the A. aspera host. Alphaproteobacteria (mainly Rhodobacteraceae and Bradyrhizobiaceae), from the Rhizobiales order dominated the Symbiodiniaceae microbiome, while Gammaproteobacteria (mainly Endozoicomonadaceae) dominated the coral microbiome. The Symbiodiniaceae core microbiome also reflected the distinct microbiomes of the two partners, specifically, Rhizobiales were not present in the A. aspera core, while Endozoicomonadaceae were not present in the Symbiodiniaceae core. We show the Symbiodiniaceae-associated microbiome was highly responsive to increases in temperature, and the microbial consortium was significantly altered in the Symbiodiniaceae retained in the host exposed to different temperature. Most notably, Myxococcolaes were up to 25-fold higher relative abundance in dinoflagellate partner microbiomes under the single temperature trajectory, compared with the repetitive and control treatments. The distinct composition of bacteria associated with Symbiodiniaceae suggests a previously unrecognised, yet important functional role of these associations to overall coral health, which is increasingly important as reefs decline worldwide. Our study provides the first characterisation of Symbiodiniaceae-associated microbes from a coral host under a range of temperature trajectories occurring on the Great Barrier Reef.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic environmental change has caused severe worldwide degradation of coral reefs at an unprecedented scale (Hughes et al., 2017). One of the main drivers of coral bleaching is thermal stress, as sea surface temperatures continue to rise under a changing climate (Hughes et al., 2018). Bleaching is the visual manifestation of a loss of endosymbiotic microalgae, and/or their photosynthetic pigments. As a result of global change, mass coral bleaching events are increasing in frequency and severity (Leggat et al., 2019). Despite the wide-ranging impact of coral bleaching, not all coral reefs are equally exposed to severe temperature stress events (Hughes et al., 2017), with evidence for local and regional variation and species-specific responses to thermal stress (Safaie et al., 2018). Furthermore, pre-stress events above the maximum monthly mean temperature, but below the bleaching threshold, have the potential to reduce the severity of thermally induced bleaching events by improving thermal tolerance and reducing coral cell death (Ainsworth et al., 2016a). Such acclimation responses could provide novel insight into how corals are able to persist into the future.
The coral microbiome
Although the association between corals and Symbiodiniaceans is well described, comparatively less is known about the functional role of the coral-associated prokaryotes, collectively referred to as the coral microbiome. The coral holobiont comprises the cnidarian host, microalgal symbionts and a highly diverse bacterial community (Rohwer et al., 2001, 2002; Bourne & Munn, 2005), which in some cases form species‐specific associations with corals (Rohwer et al., 2001, 2002; Cárdenas et al., 2017). There are upwards of several thousand distinct microbes associated with corals, including viruses, fungi and archaea, and this community differs from that of the surrounding seawater (Rohwer et al., 2001; Wegley et al., 2007; Bayer et al., 2013; Ainsworth et al., 2015). These coral‐associated microbial communities have been shown to play an important role in the provisioning and cycling of carbon, anti-microbials, nitrogen and sulfur in coral reefs (Wegley et al., 2007; Raina et al., 2009; Lema et al., 2012; Rädecker et al., 2015). Furthermore, microbial communities may facilitate acclimatisation of the coral holobiont to changes in the environment through rapid restructuring of the community composition (Reshef et al., 2006; Torda et al., 2017; Ziegler et al., 2017), and studies indicate that an intact (Rosenberg et al., 2007; Krediet et al., 2013) and diverse (Hadaidi et al., 2017) coral microbiome is essential to coral immunity and health. Responses of coral-associated bacterial communities to shifts in coral health (Bourne et al., 2008; Cárdenas et al., 2012; Glasl et al., 2016; Guest et al., 2016; Ziegler et al., 2017) and environmental stressors (Vega Thurber et al., 2009, 2014; Jessen et al., 2013; Garren et al., 2015; Gardner et al., 2019) have been extensively explored and reported, with recent evidence suggesting stability of these communities may influence holobiont resistance to thermal stress (Pogoreutz et al., 2018; Dunphy et al., 2019). More recently, microbiome dynamics have been shown to be linked to patterns of coral heat tolerance (Ziegler et al., 2017), but we are still to fully understand the implications of this relationship. Numerous studies have also shown that bacteria form stable associations with host coral species (Hernandez-Agreda et al., 2016; 2017) and these associations are often species-specific. These conserved bacterial communities comprise the “core microbiome” and represent a subset of bacterial taxa universally present in all individual corals, or corals from similar environmental conditions. The composition of this core could deliver key ecological information about the fundamental aspects of a “holobiont” (see Rohwer et al., 2002; Bosch & McFall-Ngai, 2011), for instance the contribution and potential redundancy of many diverse taxa versus the contribution and abundance of the core community may influence overall organism function. The core community is suggested to play a key role in health by providing corals with access to otherwise unavailable nutrients and metabolic pathways (Ainsworth et al., 2015); identifying this core will improve our understanding of coral biology and microbial symbiosis (Kellogg, 2019).
The coral’s microbial environment
The coral host provides several distinct habitats for its microbial inhabitants, including the mucus layer on the coral surface (Ritchie & Smith, 2004; Ritchie, 2006; Klaus et al., 2007; Glasl et al., 2016), the coral tissues and gut (Ainsworth et al., 2015) and in the coral skeleton (which is open to colonisation from the surrounding environment; Rohwer et al., 2002), and evidence increasingly suggests these micro-niches have distinct communities (Sweet et al., 2011). Studies have reported variability in bacterial compositions between coral microhabitats (Weinbauer et al., 2012; Ainsworth et al., 2015; Weiler et al., 2018), yet relatively little is known about the spatial and temporal variation of the coral's prokaryotic community. For example, despite evidence that Symbiodiniaceae in culture typically also live in close association with bacterial partners (Lawson et al., 2018; Camp et al., 2020; Nitschke et al., 2020; Maire et al., 2021) little is known about the microbial communities associated with the key symbiotic partner Symbiodiniaceae, in hospite, although the presence of intracellular bacteria in in hospite Symbiodiniaceae has been confirmed in two cnidarian species (Maire et al., 2021). To date, the potential roles bacteria may play in Symbiodiniaceae physiology have been almost entirely overlooked (Matthews et al., 2020). Integration of dinoflagellate–microbial interactions into the current framework understanding of coral–microbial interactions is critical during a time when many corals and reefs are experiencing disturbances caused by climate change and multiple other anthropogenic factors, which can disrupt these interactions (Hughes et al., 2017, 2018).
Photoautotroph symbioses
There are several notable examples of symbiotic relationships between photoautotrophs and bacteria in both Embryophytes (land plants) and phytoplankton. In terrestrial systems, essential interactions between photosynthetic organisms and bacteria occur within the rhizosphere, a region surrounding plant roots that supports distinct microbial communities (Jones et al., 2007). In aquatic systems, similar interactions are theorised to occur within the phycosphere, a marine analogue of the rhizosphere (Seymour et al., 2017). Interestingly, both theoretical and empirical studies suggest that phytoplankton are surrounded by a diffusive boundary layer in which secreted molecules accumulate in excess of bulk seawater concentrations, enhancing the potential for bacterial communication and interaction with algal cells (Amin et al., 2015). Similar Symbiodiniacean-specific examples include the photosynthesis-induced microbial calcification and production of symbiolites in cultures of free-living Symbiodiniaceans (Frommlet et al., 2015). Symbiolite-producing and non-symbiolite producing Symbiodiniacean cultures have distinct microbiomes with the symbiolite-producing cultures containing a high abundance of Planctomycetes (Nitschke et al., 2020). In addition, research indicates that members of the Flavobacteriaceae may improve the thermal and light tolerances of cultured Symbiodiniaceans through the production of the carotenoid zeaxanthin, which has a potent antioxidant activity (Motone et al., 2020). These examples indicate that Symbiodiniaceae-bacterial interactions can produce surprising emergent properties and influence the algal phenotype in ways that could greatly influence their host relations. However, the role of these interactions within the coral holobiont is yet to be determined.
Drivers of the Symbiodiniaceae microbiome
As sea surface temperatures continue to rise, the frequency of thermal stress events are projected to increase, raising the probability for these events to become far more lethal for corals (Ainsworth et al., 2016a). Ainsworth et al. (2016a) previously classified three temperature trajectories that are likely to occur during heat stress events on coral reefs; (1) a trajectory where corals are exposed to periods of thermal recovery as temperatures rise (designated as a protective sea-surface temperature [SST]); (2) a trajectory of repeated bleaching level temperature stressors (repetitive trajectory) and; (3) a single bleaching trajectory, where temperatures increase from below the maximum monthly mean to remain above the local bleaching threshold (Ainsworth et al., 2016a). These trajectories result in different bleaching responses, with the protective mechanism resulting in less stress and a higher population of symbionts, however, under climate change the protective mechanism is likely to be lost from reefs. Given the recent evidence that the coral’s microbial composition is influenced by increased SST and coral bleaching (Webster & Reusch, 2017; Glasl et al., 2019; van Oppen & Blackall, 2019), these different thermal trajectories may also influence microbial interactions between the coral, and its dinoflagellate partners’. Here, we investigated the bacteria associated with Symbiodiniaceae in Acropora aspera (Dana, 1846) under three ecologically relevant thermal-stress scenarios. Using projected temperature trajectories, we exposed coral fragments to protective, repetitive and single bleaching temperature trajectories to investigate the bacterial community composition of the coral holobiont, and importantly that of the remaining Symbiodiniaceae.
Materials and methods
Acroporid corals, such as Acropora aspera used here, are some of the most important taxa in driving rapid recovery and resilience in Pacific coral reefs (Ortiz et al., 2018). This experiment was run in parallel with Ainsworth et al. (2016a), where branches of A. aspera (7 cm) were collected from three distinct large patch coral stands on the reef crest adjacent to Heron Island, separated by at least 15 m on distinct sand regions (Ainsworth et al., 2016a). Branches were transported immediately to high volume (1000 l) raceway tanks with ambient flow-through seawater and held upright for a period of 5 days to allow recovery from collection (recovery was determined based on re-growth of coral tissue over the branch base). Branches were randomly allocated to one of four temperature treatment trajectories; control (ambient temperature 26 °C), protective bleaching trajectory (32 °C), repetitive trajectory (34 °C) and single bleaching temperature trajectory (34 °C; for further information on temperature treatments see Supplementary Fig. S1, as per Ainsworth et al., 2016a). Measurements of Symbiodiniacean maximum quantum yield of photosystem II (FV/FM) and Symbiodiniacean densities have been previously reported (see Ainsworth et al., 2016a), however, remaining samples were collected for analysis in the current study.
Coral samples were randomly sampled from each of the temperature trajectories (n = 6 per trajectory, 4 temperature trajectories, for a total sample size of n = 24) and fixed in 4% DNA/RNA free PFA for 12 h at 4 °C, then stored in DNA/RNA free water prior to airbrushing to remove host tissues and symbiont cells. Symbiodiniaceae cells were separated via centrifugation from the host tissue and washed six times in DNA/RNA free 3× PBS to be used for DNA extraction from isolated symbiont cells. As part of the wash steps, cells (particulates) were spun down (3 min at 5000×g), the supernatant (including host tissue) was removed leaving the intact symbiont pellet and the pellet was resuspended in DNA/RNA free 3× PBS buffer and homogenised (Fig. 1a). These steps were repeated to ensure the remaining Symbiodiniacean cells were thoroughly rinsed to minimise contamination by host cells. The remaining pellet was snap frozen in liquid nitrogen and stored at − 80 °C until DNA extraction on the remaining Symbiodiniaceae. Entire branches of the Acropora aspera coral holobiont were also sampled from ambient conditions (as per Ogawa et al., 2013), on days 1, 4, 9, and 14, no thermal stress, total n = 24), from which they were snap frozen, and stored in − 80 °C, following which the coral was homogenised under liquid nitrogen. DNA was extracted from 20 mg homogenised coral holobiont (n = 24) and DNA extracted following Hernandez-Agreda et al. (2018).
Methods for isolating Symbiodiniaceans from airbrushed fragment of Acropora aspera for DNA extraction used in this study (a), Maximum quantum yield of photosystem II for the control, protective, repetitive and single temperature trajectories over time (days) (b), asterisks indicate timepoints where significant difference was detected between treatments and insert showing Symbiodiniacean cell density remaining at day 17 for the control, protective, repetitive and single trajectories (c), letters show significantly different post-hoc groupings. Data presented in b has been published as a dataset in Ainsworth et al. (2016b) and c in Ainsworth et al. (2016a) (as Fig. 2b) and (2016b) (as a dataset). Principal Coordinates Analysis (PCoA) of unweighted unifrac distances for Symbiodiniacean-associated bacteria under different temperature trajectories (d), weighted unifrac distances (e) and weighted unifrac distances (f) for the core bacterial taxa identified in the Symbiodiniacean temperature trajectories, with shaded areas enclosing points at alpha = 0.5. Temperature trajectories include control (black symbols), protective (blue symbols), repetitive (green symbols) and single (red symbols) for (b–f)
DNA extraction and 16S amplicon sequencing
DNA extraction on Symbiodiniaceae cells was performed using the QIAamp DNA Mini Kit (QIAGEN®, Germany) following the manufacturer’s protocol. The only modification involved bead beating each sample 3 times for 20 s using silicon beads at 6.0 m/s. The solution was transferred to clean Eppendorf tubes and the QIAamp extraction protocol was followed. Samples were eluted in 50 µl of AE buffer and the quantity of bacterial and algal DNA was determined using a Qubit Fluorometer. DNA was stored at − 20 °C before PCR amplification. Genomic template primers 27F/519R (V1-V3 region) were used to amplify bacterial 16S rRNA gene amplicons for examining bacterial assemblage structure. Gene amplicons were amplified in a single-step, 28-cycle PCR (HotStarTaq plus master mix kit; Qiagen, USA). The conditions for PCR were as follows: (i) 3 min at 94 °C; (ii) 28 cycles, with each cycle consisting of 30 s at 94 °C, 40 s at 53 °C, and 1 min at 72 °C; (iii) a final elongation step of 5 min at 72 °C. PCR products were checked in 2% agarose gels, and samples were pooled in equal proportions based on molecular weight and DNA concentrations. Pooled samples were purified using calibrated Ampure XP beads. DNA library was prepared following the Illumina TruSeq DNA library protocol. Sequencing was performed by MrDNA (Molecular Research LP; Shallowater, TX, USA) using 300-bp paired ends on an Illumina MiSeq platform following the manufacturer’s guidelines.
Sequence analysis of amplicon datasets and statistical analysis
Raw data were demultiplexed and Cutadapt was used to remove primers. Due to low quality of the reverse sequences which substantially reduced merging of paired-ends, we opted to analyse the forward reads only (spanning 260 bp and covering V1-V2 regions). We used the open-source software package DADA2 (Callahan et al., 2016) to filter and trim (truncLen = 260, maxEE = 4), denoise to produce a table of chimera-free amplicon sequence variants (ASVs). ASVs are analogous to OTUs, but have higher (single nucleotide) resolution (Callahan et al., 2017). Taxonomy was assigned to single-end reads using RDP Classifier to assign taxonomy to ASVs against the SILVA v.138 reference database. The phylogenetic tree was generated by ASV alignment with MAFFT and the tree was produced with FastTreeMP (Price et al., 2009) using RAxML-HPC2 on XSEDE (Stamatakis, 2014) on the CIPRES (v3.3) Science Gateway (Miller et al., 2010). A phyloseq object was created for analysis in R v.3.6.1. Sequences classified as chloroplast, mitochondria, eukaryota and archaea and singletons were removed before further analysis. Chloroplast sequences represented < 2% of the overall dataset (88 ASVs from 4,479 ASVs before they were removed). Potential DNA extraction kit contaminants, detected in sequenced negative blank controls in Salter et al. (2014) were subset from post-processed phyloseq object to create a separate table (Supplementary Table 1) containing the possible kit contaminants including the read/count number for each ASV shown to the taxonomic level of genus, but were not removed from our analyses. ASV abundances were analysed in R with Phyloseq v1.28 (McMurdie & Holmes, 2013) and Vegan v2.5.6 (Oksanen et al., 2007). For alpha-diversity estimates the non-transformed, raw count data was first rarefied to the minimum sample depth and Good’s Coverage scores were calculated to ensure sufficient sampling depth (Supplementary Fig. S2). The number of Observed ASVs, Chao1 richness, and Shannon diversity were computed using Phyloseq and a one-way ANOVA was run for each diversity index after checking for homogeneity and normality using Levene’s and Shapiro’s tests, respectively, using rstatix v0.7.0 (Kassambara, 2021). Alpha-diversity was also run for A. aspera separately (Supplementary Fig. S3) using a one-way ANOVA (for the number of observed ASVs and Shannon diversity) and a non-parametric analysis (Kruskal–Wallis for Chao1). To analyse community composition, ASV counts were hellinger transformed to reduce the effects of numerically large values from very abundant taxa. Permutational multivariate analysis of variance (PERMANOVA; n = 999 permutations, adonis function in Vegan) was performed to test for significant differences among temperature trajectories. Homogeneity of dispersions around group centroids (i.e., variation) was assessed using PERMDISP (betadisper function in Vegan). A principal coordinate analysis (PCoA) was used to visualise dissimilarities in microbial communities between treatments using unweighted and weighted UniFrac distance (Lozupone et al., 2011) and plotted for the whole dataset and the core. Taxonomy bar graphs were plotted to order level and represent those with a relative abundance above 1% across all samples.
Differential abundance of ASVs at the taxonomic rank of family was tested using DESeq2 v1.24 (Love et al., 2014) to determine the differences between temperature trajectories with the Wald test and a parametric fit. Core microbiota were identified based on their presence in 100% of the samples at ASV and genus level and plotted using InteractiVenn (Heberle et al., 2015).
Results
Bleaching dynamics and effects on microbiome structure
Measurements of Symbiodiniacean maximum quantum yield of photosystem II (FV/FM) and Symbiodiniacean densities (see Ainsworth et al., 2016b) show a significant decrease between control and temperature trajectories for days 15–17 [FV/FM (Day 15; F3,20 = 10.29, P < 0.001, day 16; F3,20 = 13.28, P < 0.001 and day 17; F3,20 = 19.58, P < 0.001; Fig. 1b; also see Ainsworth et al., 2016b); endosymbiont density (F3,20 = 7.44, P = 0.002; Fig. 1c; also see Ainsworth et al., 2016b)]. The microbiome data set comprised 48 16S rRNA gene libraries (n = 24 for the Acropora aspera, collected on days 1, 4, 9, and 14 and n = 24 for the Symbiodiniaceaean samples, with 4 temperature trajectories) totalling 2,196,013 sequences. After quality filtering and exclusion of chimeras, sequences annotated to chloroplasts, mitochondria, eukaryota and archaea, 1,753,162 total sequences were annotated to bacteria. DADA2 denoising produced 4,387 ASVs from 45 samples with an average of 225 ASVs per sample (Supplementary Table S2). Three samples, all from A. aspera host, were removed from the remaining analysis due to very low-sequence reads (SampleIDs 14.C.380.L1, 9.C.380.L1 and 4.C.380.L1). PCoA of Symbiodiniaceaen ASV composition showed the least dispersion around group centroids for the control, protective and single treatments (unweighted UniFrac and weighted UniFrac distance, Fig. 1d and e, respectively), however there was no significant difference in dispersion (PERMDISP; P = 0.633 and 0.798, respectively). A broad overlap across the protective, repetitive and single temperature trajectories along the primary axis (axis 1) was observed, explaining 55.5% of the total variation (Fig. 1e). PCoA for the core community, however, shows a broad overlap across all trajectories explaining 41.5% of the variation along axis 1 (Fig. 1f).
The Symbiodiniaceae microbiome under heat stress
Significant differences between the Symbiodiniaceae-associated microbiome under heat stress were found for only Shannon diversity (ANOVA; F3,20 = 4.275, P = 0.017; Fig. 2a; Supplementary Table S3). Samples were rarefied to 27,945 for the Symbiodiniacean samples and the mean Good’s coverage score for the rarefied data was 99.98% ± 0.01, indicating sequencing depth was adequate to reliably describe the bacterial microbiome. The average number of distinct ASVs ranged between 285.00 ± 61.82 for the repetitive trajectory to 434.82 ± 50.19 in the control, however, this was not significantly different (P = 0.146; Fig. 2a). Predicted species richness (Chao1) was also not significant, but ranged between 286.49 ± 62.65 in the repetitive trajectory to 436.35 ± 50.68 in the control (P = 0.151; Fig. 2a). Diversity was significantly lower in the repetitive bleaching exposed samples with the lowest Shannon Index of 3.92 ± 0.23 (ANOVA; F3,20 = 4.275, P = 0.0174; Fig. 2a) when compared with the controls maintained under ambient conditions (4.86 ± 0.17). The A. aspera samples were rarefied to 4,433 and the mean Good’s coverage score for the rarefied data were 99.83% ± 0.16. No significant differences were detected in the A. aspera samples for the observed number of ASVs (P = 0.316), Chao1 (P = 0.370) or Shannon (P = 0.623) between days (Supplementary Fig. S3).
Alpha-diversity indices for Symbiodiniacean-associated bacteria including the number of observed ASVs, Chao1 and Shannon diversity for control (grey), protective (blue), repetitive (green) and single (red) temperature trajectories (a). Letters indicate post hoc groupings (P < 0.05). Relative abundances (%) in taxonomic barplots for Acropora aspera host, Symbiodiniacean control, protective, repetitive, and single temperature trajectories at the order level (n = 6) (b)
Microbial communities varied significantly between all treatments (PERMANOVA, F = 8.56, R2 = 0.46, P < 0.001; Figs. 1e and 2b) and differences in dispersion (variance) around the centroids were detected between treatment (PERMDISP, F = 10.47, P < 0.001; Fig. 1e), with the largest variability in the A. aspera samples. The Symbiodiniaceae-associated microbiome from heat stressed corals was dominated by Alphaproteobacteria (Fig. 2b). More specifically, the control treatment was dominated by Rhodobacterales (relative read abundance of 22.48% ± 4.54), while the protective, repetitive and single trajectories were dominated by Rhizobiales (21.58% ± 2.14, 27.21% ± 3.26 and 18.51% ± 2.96, respectively, Fig. 2b). In stark contrast, the Acropora aspera holobiont was dominated by Oceanospirillales, with Endozoicomonadaceae as the most abundant family (45.45% ± 8.62; Fig. 2b).
Differential abundance testing taxon abundance between temperature trajectories
Differential abundance testing locates the ASVs that differ between groups while controlling for the false-discovery rate. DESeq2 was used to compare families (Fig. 3, Supplementary Table S4a) and ASVs (Supplementary Table S4b) that were significantly different, using all the microbiome data for each type-treatment. In the Symbiodiniacean samples the largest differences detected were between single vs. repetitive and single vs. protective groups. The greatest log2 fold increase of 34.14 ± 4.17 (P < 0.001) was from the Vicinamibacteraceae family (ASV1476) which was significantly higher in the single compared with protective temperature trajectory, followed by a log2 fold increase of 31.84 ± 4.16 (P < 0.001) of Blastocatellaceae (ASV10) in the single compared with the protective trajectory (Fig. 3, Supplementary Table S4a). Myxococcaceae (ASV997) were also significantly higher in the single compared with protective (24.42 ± 4.17 log2 fold change, P < 0.001), in the control compared with protective (19.45 ± 4.19 log2 fold change, P < 0.001) and repetitive compared with protective (17.53 ± 4.20 log2 fold change, P < 0.001; Fig. 3, Supplementary Table S4a).
Comparison of bacterial families that are significantly different in abundance between temperature trajectories; control (grey shaded area), protective (blue), repetitive (green) and single (red). Values represent log2fold changes based on differential abundance analysis (DESeq2). Letters before bacterial taxa names along the y-axis indicates if the order (o), or class (c) name is presented instead of family name
Interestingly, despite not being detected in the Symbiodiniacean core community, there was a higher relative abundance of Endozoicomonadaceae in the protective compared with the control (1.84 ± 0.62 log2 fold change), protective compared with the single (3.41 ± 0.62 log2 fold change) and the repetitive compared with the single (2.79 ± 0.62 log2 fold change; Fig. 3, Supplementary Table S4a) temperature trajectories. Another notable taxon is the Rhizobiales order which was not detected in the differential abundance testing run at the family taxonomic level, however it was detected at the ASV level with the largest difference in the repetitive versus the protective (32.03 ± 4.17 log2 fold change) and single versus the protective trajectory (31.49 ± 4.17 log3 fold change, Supplementary Table S4b). Furthermore, Labrenzia sp. (Rhizobiales) was more abundant in the repetitive and single trajectories, compared with the control, and in the single and repetitive versus the protective at the ASV level (Supplementary Table S4b).
Core microbiome
Core microbiome analyses were based on non-normalised data defined as ASVs present across all type-treatment-samples regardless of their abundance and were run for Symbiodiniaceae plus A. aspera combined and the Symbiodiniaceae-associated, and A. aspera communities separately. The top 40 taxa identified to order was used to generate a heatmap to further visualise the relative abundance of the dominant taxa (Fig. 4). Across the Symbiodiniaceae and A. aspera treatments, ASV5 from the Cutibacterium genus (Propionibacteriales order) was the only shared ASV, despite only having a small relative read abundance of 2.24% ± 0.55 (Supplementary Fig. S4a).
Heatmap of the top 40 bacterial taxa to the classification of order for Acropora aspera, control, protective, repetitive and single trajectories, based on a weighted unifrac distance. Colour scale bar showing the relative abundance as a percentage (the darker colour representing a higher relative abundance)
A main effect of treatment was detected between the core communities for Symbiodiniaceaens (PERMANOVA; F = 23.14; R2 = 0.78, P < 0.001; Supplementary Fig. S4b), yet dispersions (variance) between temperature trajectories were not different (P = 0.96). The 24 core ASVs identified for the samples represented 46.67% of the total relative read abundance within the overall Symbiodiniaceae microbiome (Supplementary Fig. S4b). For the combined Symbiodiniacean samples the most abundant ASV (ASV10) was from the Bradyrhizobium genus (order Rhizobiales; 17.63% ± 1.39 average relative abundance ± SE), followed by ASV51 (unassigned genus from the Rhizobiales order) representing 4.11% ± 0.60 relative abundance (Supplementary Fig. S4b). Of these 24 core ASVs identified in the Symbiodiniaceaen samples, 9 of these were from the Rhizobiales order, comprising 29.66% of the Symbiodiniacean core, the largest genera was Bradyrhizobium with 4 ASVs in the Symbiodiniacean core, contributing 19.77%.
When looking at the core ASVs in the Symbiodiniacean temperature treatments separately, there were 49 core ASVs in the control trajectory representing 50.82% of the overall relative abundance, 47 in the protective (representing 63.78% relative abundance), 42 in the repetitive (representing 71.28% relative abundance) and 54 in the single trajectory (representing 57.27% relative abundance, Supplementary Fig. S4b). ASV10 (order Rhizobiales) had the largest average relative read abundance of core ASVs in all temperature trajectories representing 11.99% ± 1.97 in the control, 18.97% ± 1.85 in the protective, 23.49% ± 2.87 in the repetitive and 16.07% ± 2.38 in the single trajectories.
In comparison, there were only 2 ASVs identified in the A. aspera core, ASV3 from the Endozoicomonas genus (Oceanospirillales order) representing 12.33% ± 2.61 of the relative read abundance and ASV5 from the Cutibacterium genus (Propionibacteriales order), representing 2.87% ± 1.13). These two ASVs represent 15.21% of the total relative read abundance within the overall A. aspera microbiome. Interestingly, Rhizobiales were not present in the A. aspera core, while Oceanospirillales were not present in the Symbiodiniaceaen core.
Discussion
In this study, we describe the bacterial community associated with Symbiodiniaceans in corals exposed to three ecologically relevant temperature trajectories. We provide new insight on the contribution of the Symbiodiniaceae-bacterial symbiosis to overall coral health which is crucial for ecosystem management initiatives by improving our capability of predicting responses to thermally induced coral bleaching. Importantly, we also show that the Symbiodiniaceae-associated microbiome is distinct, and the endosymbiotic dinoflagellate hosts a diverse bacterial community which differs substantially in several keyways to that observed and reported in the coral meta-organism. Our results suggest that the Symbiodiniaceae-associated microbial community appears comparable to the rhizosphere of plant roots, and is distinct from the Acropora aspera host-associated microbiome.
The Order Rhizobiales
Nitrogen-cycling microbes (diazotrophs) are reported as ubiquitous and consistent members of the coral microbiome (Rosenberg et al., 2007; Wegley et al., 2007; Fiore et al., 2010; Lema et al., 2012, 2014). Members of the Rhizobiales order (formerly Rickettsiales) however, are well-known bacteria that are capable of fixing and providing a source of organic nitrogen, and act as beneficial partners in plant–microbe interactions (Carvalho et al., 2010). The best-known interactions within the rhizosphere involve endosymbiotic associations between legumes and rhizobia, which form anoxic root nodules where they fix nitrogen (Oldroyd, 2013). We find that members of this order (including Xanthobacteraceae, Hyphomicrobiaceae and Rhizobiaceae families) are dominant members of the Symbiodiniacean microbiome with relative abundances up to 37%, while only comprising a small portion of the coral host microbiome (< 2%, Fig. 2b). These Symbiodiniaceae-associated microbes might be analogous to those on the terrestrial plant roots and the “rhizosphere”, a zone enriched in organic substrates exuded by the plant into the surrounding soil, described as one of the most complex ecological interfaces worldwide (Philippot et al., 2013). The symbiosome membrane, the host-derived vesicle where the intracellular dinoflagellate symbiont is housed, is the coral analogue of the tightly coupled interactions of the bacterial–plant rhizosphere (Lundberg et al., 2012) and symbiotic oceanic algae (Thompson et al., 2012). These Symbiodiniaceae-bacteria associations potentially provide access to additional metabolic pathways to increase the access to nitrogen during periods of limitation (Ainsworth et al., 2015). For example, the host is thought to exert population control on symbiotic Symbiodiniaceans by limiting their access to nitrogen (Xiang et al., 2020); promoting a niche for Rhizobiales could enhance the access of Symbiodiniaceans to nitrogen, limiting the effectiveness of this host control. This interaction requires further investigation given the Rhizobiales has been reported in association with corals from reefs worldwide [including the Great Barrier Reef (Ainsworth et al., 2015) and the Hawaiian Line Islands (Hester et al., 2016)], are found to increase in corals on reefs impacted by climate change, water pollution, and overfishing (see review McDevitt-Irwin et al., 2017) and also in association with corals undergoing stony coral tissue loss disease (Rosales et al., 2020). Furthermore, Lesser et al. (2018) suggest that a large proportion of nitrogenase (nifH) gene sequences that are found in coral likely cluster with those of the Rhizobiales, further indicating the importance of a coral-Symbiodiniaceae–Rhizobiales tripartite interaction.
Efforts to describe the members of niche habitats can provide compelling evidence for the potential functional significance of a coral-Symbiodiniaceae-bacteria tri-partite symbiosis. Previously, theoretical co-occurrences have been investigated using network analysis to characterise baseline patterns of Symbiodiniacean-bacterial co-occurrences (Bernasconi et al., 2019). Further experimental research also demonstrated that bacterial species of cultured Symbiodiniaceans are likely to be intimately associated with corals (Lawson et al., 2018), further suggesting meta-analyses alone are also unlikely to capture specific interactions; strengthening the case for in hospite investigations such as undertaken here. For example research by Shoguchi et al. (2013) predicted bacteria on the surfaces of Symbiodiniaceans closely matched the family Rhodobiaceae (formerly Phyllobacteriaceae) of the order Rhizobiales (Schleheck et al., 2011), as has been found in the current study. Similarly, other studies demonstrated that Labrenzia sp. are associated with Symbiodiniaceae (Lawson et al., 2018; Camp et al., 2020; Nitschke et al., 2020; Maire et al., 2021). These genera are known to synthesise dimethylsulfoniopropionate (DMSP), a compound commonly produced in Symbiodiniaceae cultures (Steinke et al., 2011), and the coral metaorganism (Gardner et al., 2017) which likely plays a role in stress tolerance (Sunda et al., 2002). Lawson et al. (2018) suggested that Labrenzia sp. could be partially responsible for some of the traits (e.g., DMSP biosynthesis) currently assigned solely to Symbiodiniaceae. Interestingly, Labrenzia sp. were in higher relative abundance in the single and repetitive trajectories in our study, and as one of the 12 bacterial genera ubiquitously associated with various reef-building corals across the globe, this is suggestive of its functional importance not only in association with Symbiodiniaceae but also in cultures and coral holobionts (Lawson et al., 2018). Taken together, these studies highlight the need for future research that investigates the functional significance coral- Symbiodiniacean-bacteria interaction.
Alphaproteobacteria
We also find that Alphaproteobacteria are dominant and prevalent bacteria associated with Symbiodiniaceans in hospite. Specifically, four of the top ten ASVs from Symbiodiniacean samples were Alphaproteobacteria, comprising 56% of the overall relative abundance. Interactions with members of the Alphaproteobacteria maybe essential for the survival of Symbiodiniaceans, as this group of bacteria are shown to promote growth, nutrient cycling, and production of bioactive compounds (e.g., vitamin B12, see Croft et al., 2005; Robbins et al., 2019). Bacterial production of vitamins is one interaction which may also benefit the Symbiodiniacean–bacterial symbiosis (Croft et al., 2005), as dinoflagellates, including Symbiodiniaceans, require a combination of vitamins B7, B12 and B1 for growth and functioning (Croft et al., 2005; Agostini et al., 2009; Cruz-Lopez & Maske, 2016). Previously, Lingulodinium polyedrum (CCMP 1936, previously Gonyaulax polyedra) was found to acquire both vitamin B1 and B12 from the bacterial community located on its cell surface (Cruz-Lopez & Maske, 2016) and Amphidinium operculatum (Klebs, 1884) grown in axenic cultures not supplemented with B12 were found to have a significantly reduced cell density (Agostini et al., 2009). These primary metabolites provided by the bacterial community were proven to be essential in sustaining maximum growth rate (Cruz-Lopez & Maske, 2016), therefore, the ability to ingest bacteria and retain a functional synthetic pathway would likely provide an evolutionary advantage (Croft et al., 2005).
Symbiodiniaceae microbiome shifts during heat stress
We also report that the microbial community of the endosymbiotic dinoflagellate differs in corals experiencing a decline in Symbiodiniacean photosynthetic performance and density, in response to the three temperature trajectory treatments. This is most evident for the members of the Symbiodiniacean microbiome. We find that the most abundant members of the ambient (control) trajectory were the Rhodobacterales, whereas for the protective, repetitive and single trajectories, were all dominated by the Rhizobiales, compared with Oceanospirillales in A. aspera. Ainsworth et al. (2015) also reported that Rhizobiales were only associated with the symbiotic microbiome (isolated from the tissue layer hosting endosymbionts) but not detected in the whole coral colony community (using 454 technology). While Rhodobacteraceae (order Rhodobacterales) were in the top three highly abundant ASVs for all temperature trajectories, they have been shown to demonstrate environmental flexibility (Röthig et al., 2016) and are regularly reported as a component of the coral holobiont for both healthy and stressed/diseased corals (Kellogg et al., 2013; Li et al., 2014; Gardner et al., 2019). The lowest relative abundance of Rhodobacteraceae in the repetitive trajectory may indicate that this treatment had the largest impact on overall Symbiodiniacean health.
Here, we also find that bacteria from the order Myxococcales (Fig. 4; Supplementary Table S3) differed significantly between the heat-stressed treatments. Myxococcales are known as ‘micro-predators’ that are metabolically active in the soil microbial food web (Zhou et al., 2014) and these taxa can lyse other bacteria and grow on the nutrients released (Berleman & Kirby, 2007; Keane & Berleman, 2016). Interestingly, this role in coral may also be supported by the findings that these groups are in higher relative abundance in corals experiencing low rates of disease, in higher relative abundance in healthy coral compared to white disease affected corals, and disease resistant corals (Garcia et al., 2013; Rosales et al., 2019). This group has also been widely reported in healthy corals, though generally were reported in the meta-organism in low relative abundance. Importantly, almost all Myxococcales grow well at 30 °C (Reichenbach, 1999) and we provide support for this with a 25-log fold increase in Myxococcales between the single and repetitive trajectories compared to the protective temperature trajectory. The largest increase was between the single and repetitive groups, which may suggest the thermally induced stress in the repetitive trajectory (although temporally inconsistent) was enough to provide ideal growth conditions for this taxon, as was the single trajectory, whereas the protective trajectory may not have provided long enough continuous heat exposure for growth of this group. However, taken together, this provides compelling support for the frequency and severity of bleaching stress exposure playing a significant role in microbiome structure. Previous work on soils has shown that the abundance of Myxococcales communities were not only correlated with site temperature, but also the carbon‐to‐nitrogen ratio (Zhou et al., 2014), indicating a potentially vital role in the turnover of carbon in soil ecosystems (Reichenbach, 1999; Lueders et al., 2006). Our results indicate the potential for an equally important role in Symbiodiniaceae-bacteria associations for this group, such as where the temporal and/or cumulative exposure to increased sea surface temperatures may allow thermal pre-conditioning that could prevent destabilisation of the microbiome through the growth of predatory bacteria (Ainsworth et al., 2016a). Combined with the dominance of nitrogen-fixing Rhizobiales, these results suggest these taxa are functionally important in the Symbiodiniaceae-associated microbiome that should be the focus of further investigation. Future work incorporating taxonomy based functional profiling will improve our understanding of these changes and should also be coupled with a greater understanding of the bacterial function, and functional contribution to the meta-organism.
Studies of the Symbiodiniacean-bacterial interaction in culture show that Symbiodiniacean culture-associated bacterial communities change with heat stress, however, Camp et al. (2020), report no Symbiodiniacean-independent signs of bacterial community reorganisation (across three Symbiodiniacean genera) and find a generally stable bacterial community under heat stress. In contrast, studies of the coral-microbiome under stress consistently find an increase in diversity under stress, referred to as the Anna Karenina Principle (Zaneveld et al., 2017) and are likely reflective of the high microbial diversity of the natural environment compared to culture conditions. However, Camp et al. (2020) found all Symbiodiniacean strains examined were dominated by multiple bacteria genera in the Alphaproteobacteria class. This is consistent with our findings of a dominant Alphaproteobacteria in association with Symbiodiniaceans, and other Symbiodiniacean cultures (Lawson et al., 2018). Notably, together these studies suggest a resilient interaction between Symbiodiniaceans and Alphaproteobacteria.
How could coral-dinoflagellate–bacterial interactions arise?
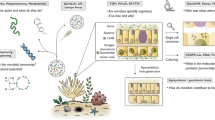
One important and significantly understudied aspect of understanding the coral meta-organism is how interactions may establish and be maintained throughout the life of a coral. Corals uptake endosymbionts via endocytosis early in their life history during larval and settlement stages, at which point the juvenile coral has established its tissue layers, gut structure and, after settlement, begun calcification. Symbiont uptake occurs via the mouth into the gastrodermal (endodermal) tissue layer (Davy et al., 2012). Following symbiont uptake, symbionts replicate and are thought to re-establish symbiosis with neighbouring host cells, and are maintained within the host-derived symbiosome membrane (Muscatine et al., 1998). While we cannot precisely identify the location of the bacteria that are enriched Symbiodiniaceans (future work should conduct ultrastructure studies on the resulting portion that we isolate with our washing steps), we suggest they could occur within several key locations in which we illustrate in a conceptual diagram (Fig. 5) to show the key attributes within the coral-Symbiodiniaceae-bacteria system to explain processes otherwise not visible to the naked eye. The location of bacteria can include; (1) within vacuoles of the gastroderm; (2) within the dinoflagellate cell or vacuoles of the dinoflagellate; (3) within the peri-algal space; (4) within the host cell; (5) within vacuoles of the peri-algal space; (6) surface of the dinoflagellate cell, outside of symbiosome; and (7) on the gut-gastrodermal lining (Fig. 5a). These interactions could be established and altered through key cellular processes occurring both during the maintenance of normal symbiosis and the breakdown of symbiosis under stress. The establishment of symbiosis through endocytosis could result in bacteria normally on the dinoflagellate surface to also be endocytosed (Fig. 5b-1); during symbiont division bacteria, and/or growing bacteria could be removed (Fig. 5b-2, b-3). Similarly established endosymbioses may propagate bacterial interactions within the host cell, allow the establishment of pathogens in compromised hosts, or trigger symbiont removal and re-uptake (Fig. 5b, 3–6). Likewise, bacterial DNA isolated from the gastrodermal tissue layer, peri-algal space, or dinoflagellate may represent bacteria taken up and digested through endocytosis. Given the presence of bacteria in a range of hosts and environments we acknowledge the bacteria isolated from Symbiodiniaceae extracted from the coral host could be susceptible to contamination from a range of sources including the coral host (Ainsworth et al., 2015), coral skeleton (Pollock et al., 2018; Pernice et al., 2019; Ricci et al., 2019), and seawater (Glasl et al., 2019) as well as from DNA extraction kits (Salter et al., 2014). It is also not possible within the current study to determine whether the interactions reported here reflect a response of the bacteria to changing health of (and resource provision by) the Symbiodiniaceae under stress; a fundamental change in Symbiodiniaceae fitness as a result of heat stress impacts on the bacteria community; or even an independent temperature effect on both, or equally on some or many, members of the microbiome. We suggest that various microhabitat drivers influencing the host, such as health status, age, and life history traits; the symbiont type and/or symbiont growth phase; and the bacterial type may also influence the type of interaction (co-occurrence, competition or infectivity) occurring (Fig. 5c). The establishment, maintenance and role of coral-dinoflagellate-bacteria interactions requires further investigation to determine if these interactions are stable over the life of a coral and if changes to these interactions influence coral health, disease, and response to environmental stress.
Schematic model showing the mode of bacterial uptake within the coral holobiont showing the possible locations of bacteria in the various components of the coral holobiont (a) and possible processes of symbiosis establishment between bacteria, Symbiodiniaceans and the coral host (b) and factors affecting regulation and drivers of Symbiodiniacean-bacterial associations (c)
Conclusion
Our study provides the first description of bacteria associated with Symbiodiniaceans isolated directly from a thermally stressed coral host. Given the metabolic capabilities of the symbiotic bacteria identified in this study, their global association with corals, and their localisation with endosymbiotic Symbiodiniaceans, it is possible that some of these bacteria play an important role in the physiological and energy requirements of the coral host. Our results suggest that Symbiodiniaceans have core associations with specific bacteria, however, further work investigating these interactions is required to fully understand the dynamics occurring between this symbiosis towards the end of a bleaching event. Our work also provides compelling evidence that cumulative temperature stress leads to destabilisation of the Symbiodiniacean-associated microbiome. Hence, considering the importance of microbes to reef health and function, further development of sensitive molecular approaches is necessary to accurately improve detection of microbial responses to environmental stressors and better resolve the functional contributions of bacteria to Symbiodiniacean thermal tolerance in the context of coral holobiont health under future predicted climate scenarios.
Data availability
The raw sequencing data used in this study is available in the NCBI Sequence Read Archive under BioProject number PRJNA689859. Scripts to reproduce these analyses are available on GitHub (https://github.com/StephGardner/Symbiodiniaceae-associated-bacteria). Additional information is available in the supplementary files.
References
Agostini, S., Y. Suzuki, B. E. Casareto, Y. Nakano, M. Hidaka & N. Badrun, 2009. Coral symbiotic complex: hypothesis through vitamin B12 for a new evaluation. Galaxea, Journal of Coral Reef Studies 11(1): 1–11. https://doi.org/10.3755/galaxea.11.1.
Ainsworth, T. D., L. Krause, T. Bridge, G. Torda, J.-B. Raina, M. Zakrzewski, R. D. Gates, J. L. Padilla-Gamiño, H. L. Spalding, C. Smith, E. S. Woolsey, D. G. Bourne, P. Bongaerts, O. Hoegh-Guldberg & W. Leggat, 2015. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. The ISME Journal 9(10): 2261–2274. https://doi.org/10.1038/ismej.2015.39.
Ainsworth, T. D., S. F. Heron, J. C. Ortiz, P. J. Mumby, A. Grech, D. Ogawa, M. C. Eakin & W. Leggat, 2016a. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352(6283): 338–342. https://doi.org/10.1126/science.aac7125.
Ainsworth, T. D., W. Leggat, J. C. Ortiz & S. F. Heron, 2016b. Great Barrier Reef A. aspera data files for physiology, photophysiology, and SST trajectories/offsets. Figshare.
Amin, S. A., L. R. Hmelo, H. M. van Tol, B. P. Durham, L. T. Carlson, K. R. Heal, R. L. Morales, C. T. Berthiaume, M. S. Parker, B. Djunaedi, A. E. Ingalls, M. R. Parsek, M. A. Moran & E. V. Armbrust, 2015. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 522(7554): 98–101. https://doi.org/10.1038/nature14488.
Bayer, T., M. J. Neave, A. Alsheikh-Hussain, M. Aranda, L. K. Yum, T. Mincer, K. Hughen, A. Apprill & C. R. Voolstra, 2013. The microbiome of the Red Sea coral Stylophora pistillata is dominated by tissue-associated Endozoicomonas bacteria. Applied and Environmental Microbiology 79(15): 4759–4762. https://doi.org/10.1128/aem.00695-13.
Berleman, J. E. & J. R. Kirby, 2007. Multicellular development in Myxococcus xanthus is stimulated by predator-prey interactions. Journal of Bacteriology Research 189(15): 5675–5682. https://doi.org/10.1128/jb.00544-07.
Bernasconi, R., M. Stat, A. Koenders & M. J. Huggett, 2019. Global networks of Symbiodinium-bacteria within the coral holobiont. Microbial Ecology 77(3): 794–807. https://doi.org/10.1007/s00248-018-1255-4.
Bosch, T. C. G. & M. J. McFall-Ngai, 2011. Metaorganisms as the new frontier. Zoology (Jena, Germany) 114(4): 185–190. https://doi.org/10.1016/j.zool.2011.04.001.
Bourne, D. G. & C. B. Munn, 2005. Diversity of bacteria associated with the coral Pocillopora damicornis from the Great Barrier Reef. Environmental Microbiology 7(8): 1162–1174. https://doi.org/10.1111/j.1462-2920.2005.00793.x.
Bourne, D., Y. Iida, S. Uthicke & C. Smith-Keune, 2008. Changes in coral-associated microbial communities during a bleaching event. The ISME Journal 2(4): 350–363.
Callahan, B. J., P. J. McMurdie, M. J. Rosen, A. W. Han, A. J. A. Johnson & S. P. Holmes, 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods 13: 581. https://doi.org/10.1038/nmeth.3869.
Callahan, B. J., P. J. McMurdie & S. P. Holmes, 2017. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. The ISME Journal 11(12): 2639–2643. https://doi.org/10.1038/ismej.2017.119.
Camp, E. F., T. Kahlke, M. R. Nitschke, D. Varkey, N. L. Fisher, L. Fujise, S. Goyen, D. J. Hughes, C. A. Lawson, M. Ros, S. Woodcock, K. Xiao, W. Leggat & D. J. Suggett, 2020. Revealing changes in the microbiome of Symbiodiniaceae under thermal stress. Environmental Microbiology 22(4): 1294–1309. https://doi.org/10.1111/1462-2920.14935.
Cárdenas, A., L. M. Rodriguez-R, V. Pizarro, L. F. Cadavid & C. Arévalo-Ferro, 2012. Shifts in bacterial communities of two Caribbean reef-building coral species affected by white plague disease. The ISME Journal 6(3): 502–512. https://doi.org/10.1038/ismej.2011.123.
Cárdenas, A., M. J. Neave, M. F. Haroon, C. Pogoreutz, N. Rädecker, C. Wild, A. Gärdes & C. R. Voolstra, 2017. Excess labile carbon promotes the expression of virulence factors in coral reef bacterioplankton. The ISME Journal 12: 59. https://doi.org/10.1038/ismej.2017.142.
Carvalho, F. M., R. C. Souza, F. G. Barcellos, M. Hungria & A. T. R. Vasconcelos, 2010. Genomic and evolutionary comparisons of diazotrophic and pathogenic bacteria of the order Rhizobiales. BMC Microbiology 10(1): 37. https://doi.org/10.1186/1471-2180-10-37.
Croft, M. T., A. D. Lawrence, E. Raux-Deery, M. J. Warren & A. G. Smith, 2005. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438(7064): 90–93. https://doi.org/10.1038/nature04056.
Cruz-Lopez, R. & H. Maske, 2016. The Vitamin B1 and B12 required by the marine dinoflagellate Lingulodinium polyedrum can be provided by its associated bacterial community in culture. Frontiers in Microbiology 7: 560. https://doi.org/10.3389/fmicb.2016.00560.
Dana, J. D., 1846. United States exploring expedition during the years 1838–1842. Zoophytes 7: 1–740.
Davy, S. K., D. Allemand & V. M. Weis, 2012. Cell biology of cnidarian–dinoflagellate symbiosis. Microbiology and Molecular Biology Reviews 76(2): 229–261. https://doi.org/10.1128/MMBR.05014-11.
Dunphy, C. M., T. C. Gouhier, N. D. Chu & S. V. Vollmer, 2019. Structure and stability of the coral microbiome in space and time. Scientific Reports 9(1): 6785. https://doi.org/10.1038/s41598-019-43268-6.
Fiore, C. L., J. K. Jarett, N. D. Olson & M. P. Lesser, 2010. Nitrogen fixation and nitrogen transformations in marine symbioses. Trends in Microbiology 18(10): 455–463. https://doi.org/10.1016/j.tim.2010.07.001.
Frommlet, J. C., M. L. Sousa, A. Alves, S. I. Vieira, D. J. Suggett & J. Serôdio, 2015. Coral symbiotic algae calcify ex hospite in partnership with bacteria. PNAS 112(19): 6158–6163. https://doi.org/10.1073/pnas.1420991112.
Garcia, G. D., G. B. Gregoracci, E. O. Santos, P. M. Meirelles, G. G. Z. Silva, R. Edwards, T. Sawabe, K. Gotoh, S. Nakamura, T. Iida, R. L. de Moura & F. L. Thompson, 2013. Metagenomic analysis of healthy and white plague-affected Mussismilia braziliensis corals. Microbial Ecology 65(4): 1076–1086. https://doi.org/10.1007/s00248-012-0161-4.
Gardner, S. G., J.-B. Raina, M. R. Nitschke, D. A. Nielsen, M. Stat, C. A. Motti, P. J. Ralph & K. Petrou, 2017. A multi-trait systems approach reveals a response cascade to bleaching in corals. BMC Biology 15(1): 117. https://doi.org/10.1186/s12915-017-0459-2.
Gardner, S. G., E. F. Camp, D. J. Smith, T. Kahlke, E. O. Osman, G. Gendron, B. C. C. Hume, C. Pogoreutz, C. R. Voolstra & D. J. Suggett, 2019. Coral microbiome diversity reflects mass coral bleaching susceptibility during the 2016 El Niño heat wave. Ecology and Evolution 9(3): 938–956. https://doi.org/10.1002/ece3.4662.
Garren, M., K. Son, J. Tout, J. R. Seymour & R. Stocker, 2015. Temperature-induced behavioral switches in a bacterial coral pathogen. The ISME Journal 10: 1363. https://doi.org/10.1038/ismej.2015.216.
Glasl, B., G. J. Herndl & P. R. Frade, 2016. The microbiome of coral surface mucus has a key role in mediating holobiont health and survival upon disturbance. The ISME Journal 10(9): 2280–2292. https://doi.org/10.1038/ismej.2016.9.
Glasl, B., D. G. Bourne, P. R. Frade, T. Thomas, B. Schaffelke & N. S. Webster, 2019. Microbial indicators of environmental perturbations in coral reef ecosystems. Microbiome 7(1): 94. https://doi.org/10.1186/s40168-019-0705-7.
Guest, J. R., J. Low, K. Tun, B. Wilson, C. Ng, D. Raingeard, K. E. Ulstrup, J. T. I. Tanzil, P. A. Todd, T. C. Toh, D. McDougald, L. M. Chou & P. D. Steinberg, 2016. Coral community response to bleaching on a highly disturbed reef. Scientific Reports 6: 20717. https://doi.org/10.1038/srep20717.
Hadaidi, G., T. Rothig, L. K. Yum, M. Ziegler, C. Arif, C. Roder, J. Burt & C. R. Voolstra, 2017. Stable mucus-associated bacterial communities in bleached and healthy corals of Porites lobata from the Arabian Seas. Scientific Reports 7: 45362. https://doi.org/10.1038/srep45362.
Heberle, H., G. V. Meirelles, F. R. da Silva, G. P. Telles & R. Minghim, 2015. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16(1): 169. https://doi.org/10.1186/s12859-015-0611-3.
Hernandez-Agreda, A., W. Leggat, P. Bongaerts & T. D. Ainsworth, 2016. The microbial signature provides insight into the mechanistic basis of coral success across reef habitats. Mbio 7(4): e00560-e616. https://doi.org/10.1128/mBio.00560-16.
Hernandez-Agreda, A., R. D. Gates & T. D. Ainsworth, 2017. Defining the core microbiome in corals’ microbial soup. Trends in Microbiology 25: 125–140. https://doi.org/10.1016/j.tim.2016.11.003.
Hernandez-Agreda, A., W. Leggat & T. D. Ainsworth, 2018. A comparative analysis of microbial DNA preparation methods for use with massive and branching coral growth forms. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2018.02146.
Hester, E. R., K. L. Barott, J. Nulton, M. J. A. Vermeij & F. L. Rohwer, 2016. Stable and sporadic symbiotic communities of coral and algal holobionts. The ISME Journal 10(5): 1157–1169. https://doi.org/10.1038/ismej.2015.190.
Hughes, T. P., J. T. Kerry, M. Álvarez-Noriega, J. G. Álvarez-Romero, K. D. Anderson, A. H. Baird, R. C. Babcock, M. Beger, D. R. Bellwood, R. Berkelmans, T. C. Bridge, I. R. Butler, M. Byrne, N. E. Cantin, S. Comeau, S. R. Connolly, G. S. Cumming, S. J. Dalton, G. Diaz-Pulido, C. M. Eakin, W. F. Figueira, J. P. Gilmour, H. B. Harrison, S. F. Heron, A. S. Hoey, J.-P.A. Hobbs, M. O. Hoogenboom, E. V. Kennedy, C.-Y. Kuo, J. M. Lough, R. J. Lowe, G. Liu, M. T. McCulloch, H. A. Malcolm, M. J. McWilliam, J. M. Pandolfi, R. J. Pears, M. S. Pratchett, V. Schoepf, T. Simpson, W. J. Skirving, B. Sommer, G. Torda, D. R. Wachenfeld, B. L. Willis & S. K. Wilson, 2017. Global warming and recurrent mass bleaching of corals. Nature 543(7645): 373–377. https://doi.org/10.1038/nature21707.
Hughes, T. P., K. D. Anderson, S. R. Connolly, S. F. Heron, J. T. Kerry, J. M. Lough, A. H. Baird, J. K. Baum, M. L. Berumen, T. C. Bridge, D. C. Claar, C. M. Eakin, J. P. Gilmour, N. A. J. Graham, H. Harrison, J.-P.A. Hobbs, A. S. Hoey, M. Hoogenboom, R. J. Lowe, M. T. McCulloch, J. M. Pandolfi, M. Pratchett, V. Schoepf, G. Torda & S. K. Wilson, 2018. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359(6371): 80–83. https://doi.org/10.1126/science.aan8048.
Jessen, C., J. F. Villa Lizcano, T. Bayer, C. Roder, M. Aranda, C. Wild & C. R. Voolstra, 2013. In-situ effects of eutrophication and overfishing on physiology and bacterial diversity of the Red Sea coral Acropora hemprichii. PLoS ONE 8(4):e62091. https://doi.org/10.1371/journal.pone.0062091.
Jones, K. M., H. Kobayashi, B. W. Davies, M. E. Taga & G. C. Walker, 2007. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nature Reviews Microbiology 5(8): 619–633. https://doi.org/10.1038/nrmicro1705.
Kassambara, A., 2021. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 0.7.0 Version.
Keane, R. & J. Berleman, 2016. The predatory life cycle of Myxococcus xanthus. Microbiology 162(1): 1–11. https://doi.org/10.1099/mic.0.000208.
Kellogg, C. A., 2019. Microbiomes of stony and soft deep-sea corals share rare core bacteria. Microbiome 7(1): 90. https://doi.org/10.1186/s40168-019-0697-3.
Kellogg, C. A., Y. M. Piceno, L. M. Tom, T. Z. DeSantis, M. A. Gray, D. G. Zawada & G. L. Andersen, 2013. comparing bacterial community composition between healthy and white plague-like disease states in orbicella annularis using PhyloChip™ G3 Microarrays. PLoS ONE 8(11):e79801. https://doi.org/10.1371/journal.pone.0079801.
Klaus, J. S., I. Janse, J. M. Heikoop, R. A. Sanford & B. W. Fouke, 2007. Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environmental Microbiology 9(5): 1291–1305. https://doi.org/10.1111/j.1462-2920.2007.01249.x.
Klebs, G., 1884. Ein kleiner Beitrag zur Kenntnis der Peridineen. Bot Zeit 42: 721–745.
Krediet, C. J., K. B. Ritchie, V. J. Paul & M. Teplitski, 2013. Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proceedings of the Royal Society B: Biological Sciences 280: 1755. https://doi.org/10.1098/rspb.2012.2328.
Lawson, C. A., J.-B. Raina, T. Kahlke, J. R. Seymour & D. J. Suggett, 2018. Defining the core microbiome of the symbiotic dinoflagellate, Symbiodinium. Env Micro Rep 10(1): 7–11. https://doi.org/10.1111/1758-2229.12599.
Leggat, W. P., E. F. Camp, D. J. Suggett, S. F. Heron, A. J. Fordyce, S. Gardner, L. Deakin, M. Turner, L. J. Beeching, U. Kuzhiumparambil, C. M. Eakin & T. D. Ainsworth, 2019. Rapid coral decay is associated with marine heatwave mortality events on reefs. Curr Biol 29(16): 2723-2730.e4. https://doi.org/10.1016/j.cub.2019.06.077.
Lema, K. A., B. L. Willis & D. G. Bourne, 2012. Corals form characteristic associations with symbiotic nitrogen-fixing bacteria. Appl Environ Microbiol 78(9): 3136–3144. https://doi.org/10.1128/aem.07800-11.
Lema, K. A., B. L. Willis & D. G. Bourne, 2014. Amplicon pyrosequencing reveals spatial and temporal consistency in diazotroph assemblages of the Acropora millepora microbiome. Environmental Microbiology 16(10): 3345–3359. https://doi.org/10.1111/1462-2920.12366.
Lesser, M. P., K. M. Morrow, S. M. Pankey & S. H. C. Noonan, 2018. Diazotroph diversity and nitrogen fixation in the coral Stylophora pistillata from the Great Barrier Reef. The ISME Journal 12(3): 813–824. https://doi.org/10.1038/s41396-017-0008-6.
Li, J., Q. Chen, L.-J. Long, J.-D. Dong, J. Yang & S. Zhang, 2014. Bacterial dynamics within the mucus, tissue and skeleton of the coral Porites lutea during different seasons. Scientific Reports 4: 7320. https://doi.org/10.1038/srep07320.
Love, M. I., W. Huber & S. Anders, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15(12): 550. https://doi.org/10.1186/s13059-014-0550-8.
Lozupone, C., M. E. Lladser, D. Knights, J. Stombaugh & R. Knight, 2011. UniFrac: an effective distance metric for microbial community comparison. ISME Journal: Multidisciplinary Journal of Microbial Ecology 5(2): 169–172. https://doi.org/10.1038/ismej.2010.133.
Lueders, T., R. Kindler, A. Miltner, M. W. Friedrich & M. Kaestner, 2006. Identification of bacterial micropredators distinctively active in a soil microbial food web. Applied and Environmental Microbiology 72(8): 5342–5348. https://doi.org/10.1128/aem.00400-06.
Lundberg, D. S., S. L. Lebeis, S. H. Paredes, S. Yourstone, J. Gehring, S. Malfatti, J. Tremblay, A. Engelbrektson, V. Kunin, T. G. d. Rio, R. C. Edgar, T. Eickhorst, R. E. Ley, P. Hugenholtz, S. G. Tringe & J. L. Dangl, 2012. Defining the core Arabidopsis thaliana root microbiome. Nature 488(7409):86–90. http://www.nature.com/nature/journal/v488/n7409/abs/nature11237.html#supplementary-information.
Maire, J., S. K. Girvan, S. E. Barkla, A. Perez-Gonzalez, D. J. Suggett, L. L. Blackall & M. J. H. van Oppen, 2021. Intracellular bacteria are common and taxonomically diverse in cultured and in hospite algal endosymbionts of coral reefs. The ISME Journal 15: 2028–2042. https://doi.org/10.1038/s41396-021-00902-4.
Matthews, J. L., J.-B. Raina, T. Kahlke, J. R. Seymour, M. J. H. van Oppen & D. J. Suggett, 2020. Symbiodiniaceae-bacteria interactions: rethinking metabolite exchange in reef-building corals as multi-partner metabolic networks. Environmental Microbiology 22: 1675–1687. https://doi.org/10.1111/1462-2920.14918.
McDevitt-Irwin, J. M., J. K. Baum, M. Garren & R. L. Vega Thurber, 2017. Responses of coral-associated bacterial communities to local and global stressors. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2017.00262.
McMurdie, P. J. & S. Holmes, 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4):e61217. https://doi.org/10.1371/journal.pone.0061217.
Miller, M. A., W. Pfeiffer & T. Schwartz, Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), 2010, New Orleans, Los Angeles, pp. 1–8, 14 November 2010.
Motone, K., T. Takagi, S. Aburaya, N. Miura, W. Aoki & M. Ueda, 2020. A zeaxanthin-producing bacterium isolated from the algal phycosphere protects coral endosymbionts from environmental stress. mBio 11(1):e01019. https://doi.org/10.1128/mBio.01019-19.
Muscatine, L., C. Ferrier-Pagès, A. Blackburn, R. D. Gates, G. Baghdasarian & D. Allemand, 1998. Cell-specific density of symbiotic dinoflagellates in tropical anthozoans. Coral Reefs 17(4): 329–337. https://doi.org/10.1007/s003380050133.
Nitschke, M. R., C. Fidalgo, J. Simões, C. Brandão, A. Alves, J. Serôdio & J. C. Frommlet, 2020. Symbiolite formation: a powerful in vitro model to untangle the role of bacterial communities in the photosynthesis-induced formation of microbialites. The ISME Journal 14: 1533–1546. https://doi.org/10.1038/s41396-020-0629-z.
Ogawa, D., T. Bobeszko, T. Ainsworth & W. Leggat, 2013. The combined effects of temperature and CO2 lead to altered gene expression in Acropora aspera. Coral Reefs 32(4): 895–907. https://doi.org/10.1007/s00338-013-1046-9.
Oksanen, J., R. Kindt, P. Legendre & R. B. O’Hara, 2007. Vegan: community ecology package.
Oldroyd, G. E. D., 2013. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nature Reviews Microbiology 11(4): 252–263. https://doi.org/10.1038/nrmicro2990.
Ortiz, J.-C., N. H. Wolff, K. R. N. Anthony, M. Devlin, S. Lewis & P. J. Mumby, 2018. Impaired recovery of the Great Barrier Reef under cumulative stress. Science Advances 4(7):eaar6127. https://doi.org/10.1126/sciadv.aar6127.
Pernice, M., J.-B. Raina, N. Rädecker, A. Cárdenas, C. Pogoreutz & C. R. Voolstra, 2019. Down to the bone: the role of overlooked endolithic microbiomes in reef coral health. The ISME Journal 14: 325–334. https://doi.org/10.1038/s41396-019-0548-z.
Philippot, L., J. M. Raaijmakers, P. Lemanceau & W. H. van der Putten, 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nature Reviews Microbiology 11(11): 789–799. https://doi.org/10.1038/nrmicro3109.
Pogoreutz, C., N. Rädecker, A. Cárdenas, A. Gärdes, C. Wild & C. R. Voolstra, 2018. Dominance of Endozoicomonas bacteria throughout coral bleaching and mortality suggests structural inflexibility of the Pocillopora verrucosa microbiome. Ecology and Evolution 8(4): 2240–2252. https://doi.org/10.1002/ece3.3830.
Pollock, F. J., R. McMinds, S. Smith, D. G. Bourne, B. L. Willis, M. Medina, R. V. Thurber & J. R. Zaneveld, 2018. Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny. Nature Communications 9(1): 4921. https://doi.org/10.1038/s41467-018-07275-x.
Price, M. N., P. S. Dehal & A. P. Arkin, 2009. FastTree: computing large minimum-evolution trees with profiles instead of a distance matrix. Molecular Biology and Evolution 26: 1641–1650. https://doi.org/10.1093/molbev/msp077.
Rädecker, N., C. Pogoreutz, C. R. Voolstra, J. Wiedenmann & C. Wild, 2015. Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends in Microbiology 23(8): 490–497. https://doi.org/10.1016/j.tim.2015.03.008.
Raina, J. B., D. Tapiolas, B. L. Willis & D. G. Bourne, 2009. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Applied in Environmental Microbiology 75(11): 3492–3501. https://doi.org/10.1128/aem.02567-08.
Reichenbach, H., 1999. The ecology of the myxobacteria. Environmental Microbiology 1(1): 15–21. https://doi.org/10.1046/j.1462-2920.1999.00016.x.
Reshef, L., O. Koren, Y. Loya, I. Zilber-Rosenberg & E. Rosenberg, 2006. The coral probiotic hypothesis. Environmental Microbiology 8(12): 2068–2073.
Ricci, F., V. Rossetto Marcelino, L. L. Blackall, M. Kühl, M. Medina & H. Verbruggen, 2019. Beneath the surface: community assembly and functions of the coral skeleton microbiome. Microbiome 7(1): 159. https://doi.org/10.1186/s40168-019-0762-y.
Ritchie, K. B., 2006. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Marine Ecology – Progress Series 322: 1–14.
Ritchie, K. B. & G. W. Smith, 2004. Microbial communities of coral surface mucopolysaccharide layers. In Rosenberg, E. & Y. Loya (eds), Coral Health and Disease Springer, Berlin: 259–264.
Robbins, S. J., C. M. Singleton, C. X. Chan, L. F. Messer, A. U. Geers, H. Ying, A. Baker, S. C. Bell, K. M. Morrow, M. A. Ragan, D. J. Miller, S. Forêt, E. Ball, R. Beeden, M. Berumen, M. Aranda, T. Ravasi, P. Bongaerts, O. Hoegh-Guldberg, I. Cooke, B. Leggat, S. Sprungala, A. Fitzgerald, C. Shang, P. Lundgren, T. Fyffe, F. Rubino, M. van Oppen, K. Weynberg, S. J. Robbins, C. M. Singleton, C. Xin Chan, L. F. Messer, A. U. Geers, H. Ying, A. Baker, S. C. Bell, K. M. Morrow, M. A. Ragan, D. J. Miller, S. Foret, C. R. Voolstra, G. W. Tyson, D. G. Bourne, C. R. Voolstra, G. W. Tyson, D. G. Bourne & C. ReFuGe, 2019. A genomic view of the reef-building coral Porites lutea and its microbial symbionts. Nature Microbiology 4(12): 2090–2100. https://doi.org/10.1038/s41564-019-0532-4.
Rohwer, F., M. Breitbart, J. Jara, F. Azam & N. Knowlton, 2001. Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs 20(1): 85–91. https://doi.org/10.1007/s003380100138.
Rohwer, F., V. Seguritan, F. Azam & N. Knowlton, 2002. Diversity and distribution of coral-associated bacteria. MEPS 243: 1–10.
Rosales, S. M., M. W. Miller, D. E. Williams, N. Traylor-Knowles, B. Young & X. M. Serrano, 2019. Microbiome differences in disease-resistant vs. susceptible Acropora corals subjected to disease challenge assays. Scientific Reports 9(1):18279. https://doi.org/10.1038/s41598-019-54855-y.
Rosenberg, E., O. Koren, L. Reshef, R. Efrony & I. Zilber-Rosenberg, 2007. The role of microorganisms in coral health, disease and evolution. Nature Reviews Microbiology 5: 355. https://doi.org/10.1038/nrmicro1635.
Rosales, S. M., A. S. Clark, L. K. Huebner, R. R. Ruzicka & E. M. Muller, 2020. Rhodobacterales and Rhizobiales are associated with stony coral tissue loss disease and its suspected sources of transmission. Frontiers in Microbiology 11: 681. https://doi.org/10.3389/fmicb.2020.00681.
Röthig, T., M. A. Ochsenkühn, A. Roik, R. van der Merwe & C. R. Voolstra, 2016. Long-term salinity tolerance is accompanied by major restructuring of the coral bacterial microbiome. Molecular Ecology 25(6): 1308–1323. https://doi.org/10.1111/mec.13567.
Safaie, A., N. J. Silbiger, T. R. McClanahan, G. Pawlak, D. J. Barshis, J. L. Hench, J. S. Rogers, G. J. Williams & K. A. Davis, 2018. High frequency temperature variability reduces the risk of coral bleaching. Nature Communications 9(1): 1671. https://doi.org/10.1038/s41467-018-04074-2.
Salter, S. J., M. J. Cox, E. M. Turek, S. T. Calus, W. O. Cookson, M. F. Moffatt, P. Turner, J. Parkhill, N. J. Loman & A. W. Walker, 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biology 12(1): 87. https://doi.org/10.1186/s12915-014-0087-z.
Schleheck, D., M. Weiss, S. Pitluck, D. Bruce, M. L. Land, S. Han, E. Saunders, R. Tapia, C. Detter, T. Brettin, J. Han, T. Woyke, L. Goodwin, L. Pennacchio, M. Nolan, A. M. Cook, S. Kjelleberg & T. Thomas, 2011. Complete genome sequence of Parvibaculum lavamentivorans type strain (DS-1(T)). Standards in Genomic Sciences 5(3): 298–310. https://doi.org/10.4056/sigs.2215005.
Seymour, J. R., S. A. Amin, J.-B. Raina & R. Stocker, 2017. Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nature Microbiology 2: 17065. https://doi.org/10.1038/nmicrobiol.2017.65.
Shoguchi, E., C. Shinzato, T. Kawashima, F. Gyoja, S. Mungpakdee, R. Koyanagi, T. Takeuchi, K. Hisata, M. Tanaka, M. Fujiwara, M. Hamada, A. Seidi, M. Fujie, T. Usami, H. Goto, S. Yamasaki, N. Arakaki, Y. Suzuki, S. Sugano, A. Toyoda, Y. Kuroki, A. Fujiyama, M. Medina, Mary A. Coffroth, D. Bhattacharya & N. Satoh, 2013. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Current Biology 23(15): 1399–1408. https://doi.org/10.1016/j.cub.2013.05.062.
Stamatakis, A., 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9): 1312–1313. https://doi.org/10.1093/bioinformatics/btu033.
Steinke, M., P. Brading, P. Kerrison, M. E. Warner & J. D. Suggett, 2011. Concentrations of dimethylsulfoniopropionate and dimethyl sulfide are strain-specific in symbiotic dinoflagellates (Symbiodinium sp. Dinophyceae). Journal of Phycology 47: 775–783. https://doi.org/10.1111/j.1529-8817.2011.01011.x.
Sunda, W., D. J. Kieber, R. P. Kiene & S. Huntsman, 2002. An antioxidant function for DMS and DMSP in marine algae. Nature 418: 317–320.
Sweet, M. J., A. Croquer & J. C. Bythell, 2011. Bacterial assemblages differ between compartments within the coral holobiont. Coral Reefs 30(1): 39–52. https://doi.org/10.1007/s00338-010-0695-1.
Thompson, A. W., R. A. Foster, A. Krupke, B. J. Carter, N. Musat, D. Vaulot, M. M. M. Kuypers & J. P. Zehr, 2012. Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 337(6101): 1546–1550. https://doi.org/10.1126/science.1222700.
Torda, G., J. M. Donelson, M. Aranda, D. J. Barshis, L. Bay, M. L. Berumen, D. G. Bourne, N. Cantin, S. Foret, M. Matz, D. J. Miller, A. Moya, H. M. Putnam, T. Ravasi, M. J. H. van Oppen, R. V. Thurber, J. Vidal-Dupiol, C. R. Voolstra, S.-A. Watson, E. Whitelaw, B. L. Willis & P. L. Munday, 2017. Rapid adaptive responses to climate change in corals. Nature Climate Change 7: 627. https://doi.org/10.1038/nclimate3374.
van Oppen, M. J. H. & L. L. Blackall, 2019. Coral microbiome dynamics, functions and design in a changing world. Nature Reviews Microbiology 17(9): 557–567. https://doi.org/10.1038/s41579-019-0223-4.
Vega Thurber, R., D. Willner-Hall, B. Rodriguez-Mueller, C. Desnues, R. A. Edwards, F. Angly, E. Dinsdale, L. Kelly & F. Rohwer, 2009. Metagenomic analysis of stressed coral holobionts. Environmental Microbiology 11(8): 2148–2163. https://doi.org/10.1111/j.1462-2920.2009.01935.x.
Vega Thurber, R. L., D. E. Burkepile, C. Fuchs, A. A. Shantz, R. McMinds & J. R. Zaneveld, 2014. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global Change Biology 20(2): 544–554. https://doi.org/10.1111/gcb.12450.
Webster, N. S. & T. B. H. Reusch, 2017. Microbial contributions to the persistence of coral reefs. The ISME Journal 11(10): 2167–2174. https://doi.org/10.1038/ismej.2017.66.
Wegley, L., R. Edwards, B. Rodriguez-Brito, H. Liu & F. Rohwer, 2007. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environmental Microbiology 9(11): 2707–2719. https://doi.org/10.1111/j.1462-2920.2007.01383.x.
Weiler, B. A., J. T. P. Verhoeven & S. C. Dufour, 2018. Bacterial communities in tissues and surficial mucus of the cold-water coral Paragorgia arborea. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2018.00378.
Weinbauer, M. G., J. Ogier & C. Maier, 2012. Microbial abundance in the coelenteron and mucus of the cold-water coral Lophelia pertusa and in bottom water of the reef environment. Aquatic Biology 16(3): 209–216.
Xiang, T., E. Lehnert, R. E. Jinkerson, S. Clowez, R. G. Kim, J. C. DeNofrio, J. R. Pringle & A. R. Grossman, 2020. Symbiont population control by host-symbiont metabolic interaction in Symbiodiniaceae–cnidarian associations. Nature Communications 11(1): 108. https://doi.org/10.1038/s41467-019-13963-z.
Zaneveld, J. R., R. McMinds & R. Vega Thurber, 2017. Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nature Microbiology 2(9): 17121. https://doi.org/10.1038/nmicrobiol.2017.121.
Zhou, X.-W., S.-G. Li, W. Li, D.-M. Jiang, K. Han, Z.-H. Wu & Y.-Z. Li, 2014. Myxobacterial community is a predominant and highly diverse bacterial group in soil niches. Environmental Microbiology Reports 6(1): 45–56. https://doi.org/10.1111/1758-2229.12107.
Ziegler, M., F. O. Seneca, L. K. Yum, S. R. Palumbi & C. R. Voolstra, 2017. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nature Communications 8: 14213. https://doi.org/10.1038/ncomms14213.
Acknowledgements
The authors would like to thank Dr. Martina de Freitas Prazeres for assisting with sample collection and analysis including cell counts and DNA extraction.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by the Australian Research Council Discovery Projects DP130101421 and DP180103199 (to TA, WL).
Author information
Authors and Affiliations
Contributions
TA and WL designed and conducted the experiment, SG and WL analysed the data, SG and TA led the writing of the manuscript and all authors contributed to the final manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Isa Schön, Diego Fontaneto & Elena L. Peredo / Aquatic Microbiomes
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gardner, S.G., Leggat, W. & Ainsworth, T.D. The microbiome of the endosymbiotic Symbiodiniaceae in corals exposed to thermal stress. Hydrobiologia 850, 3685–3704 (2023). https://doi.org/10.1007/s10750-023-05221-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05221-7