Abstract
The timing of entry into freshwater by mature anadromous fishes is an important component of their migration phenology. In iteroparous anadromous species, identifying the migration cues that influence the timing of entry into freshwater, and annual variability in these timings, is important in assessing the extent of individual repeatability. Here, passive acoustic telemetry tracked 71 twaite shad Alosa fallax (‘shad’) returning to the River Severn’s upper estuary over successive spawning seasons, providing individual information on river entry timing and the associated migration cues. The timing of shad returning to the river (defined as their first detection on an acoustic receiver in the upper estuary) was consistent between years and strongly predicted by photoperiod (as increasing day length), whereas water temperatures and flow rates were weak predictors and highly variable between years. Timings of river entry of individual shad were also consistent across consecutive spawning migrations, indicating high individual repeatability. The application of acoustic telemetry in this study thus revealed high repeatability in the timing of annual spawning migrations, which were cued mainly by increasing day length and that, being seasonally predictable, should ensure entry into freshwater with sufficient time to arrive in spawning areas when in-river environmental conditions are favourable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals that live in seasonal environments migrate to maximise their fitness, especially where temporal changes in habitat quality are predictable (Skov et al., 2013; Lok et al., 2015). In anadromous fishes, the timing of entry of adults into freshwater must facilitate their arrival at spawning areas further upstream when conditions are favourable for spawning (Tillotson et al., 2021). The drivers of these spawning migrations can be complex, involving the interaction of factors in both the marine (e.g. abiotic conditions, timing of migration cues, distribution; Bal et al., 2017; Agha et al., 2021) and freshwater environments (e.g. river flows, anthropogenic modifications). The environmental cues that stimulate spawning migrations can differ between species and even between populations of the same species, where some anadromous brown trout Salmo trutta (Linnaeus, 1758) populations are cued primarily by photoperiod (Garcia-Vega et al., 2022) and others by the interaction of river flow and temperature (Goetz et al., 2021). In anadromous fishes that are also iteroparous, the tracking of spawning movements over multiple years suggests that at the individual level, the timing of the annual migration back to riverine spawning grounds is consistent between years, as observed for populations of Arctic charr Salvelinus alpinus (Linnaeus, 1758) (Jensen et al., 2020), brown trout (Eldøy et al., 2019; Jensen et al., 2020; Birnie-Gauvin et al., 2021), and pike Esox lucius (Linnaeus, 1758) (Tibblin et al., 2016).
There are a number of anadromous species within the Clupeidae (herring) family (e.g. European shads, alewife Alosa pseudoharengus (Wilson, 1811)) that have ecological significance through their provision of strong marine-freshwater nutrient fluxes (Walters et al., 2009; West et al., 2010), including being important prey resources for both freshwater (Hossain et al., 2019; Nolan et al., 2019) and marine predators (Dias et al., 2019). Recent declines in many anadromous clupeid populations have been associated with over-exploitation, degraded water quality, and barriers in their spawning rivers that impede their access to spawning grounds (Limburg & Waldman, 2009; Hall et al., 2012). An example is the twaite shad Alosa fallax (Lacépède, 1803), whose population declines have resulted in their listing on Annex II and V of the Habitats Directive and Appendix III of the Bern Convention (Antognazza et al., 2019, 2021). In Britain, the iteroparous twaite shad undertakes annual spawning migrations into four rivers that drain into the Bristol Channel. The timing of their initial entry into the lower Severn Estuary has previously been linked to a water temperature threshold being reached (e.g. fishery by-catch data suggested peak periods of entry occurred between 10.6 and 12.3°C), with peak captures in a static net fishery relating to tidal state (Aprahamian, 1988; Aprahamian et al., 2010). However, there are still considerable knowledge gaps about the timings of entry into the upper reaches of the estuary. Given that spawning migrations can incur substantial energetic costs (Leonard & McCormick, 1999), it is possible that shad may conserve energy by utilising periods of high tidal influence to move through the estuary, as has been observed in other fish species (Beaulaton & Castelnaud, 2005; Kelly et al., 2020). The period of freshwater residency during twaite shad spawning migrations extends from April to June (Aprahamian et al., 2003; Antognazza et al., 2021), but with the relationship between the timing of arrival of fish in the upper estuary and the timing of arrival on upstream spawning grounds being complicated by the presence of several navigation weirs in the lower river; these impede shad upstream migrations through passage over the structures being influenced by factors, including river level (Davies, 2022).
Determining the timing and environmental drivers of the annual return of individual shad to their spawning river has been inhibited by difficulties in tracking fish in large open systems and over successive years. However, recent advances in contemporary tracking technologies, such as acoustic telemetry, provide novel opportunities for the migratory movements of iteroparous shad species to be assessed in finer detail (Davies et al., 2020; Gahagan & Bailey, 2020; Mack et al., 2021). Acoustic telemetry usually involves the internal implantation of long battery life acoustic transmitters (‘tags’) into fish that enables their long-term movements to be measured on arrays of acoustic ‘receivers’ (Matley et al., 2022). For iteroparous twaite shad, the method enables the timing of the return of individuals to their spawning river to be detected over successive years (Bolland et al., 2019).
Here, acoustic telemetry was applied to twaite shad (‘shad’) in the River Severn to identify their timings of entry into the river (downstream of known spawning grounds) for the first time during their annual spawning migrations (where initial detection was in the upper reaches of the estuary) and the influence of abiotic variables on this timing (e.g. water temperature, photoperiod, river flow, lunar tidal cycle). Moreover, with twaite shad being iteroparous, the use of long-life acoustic transmitters enabled these timings of river entry and the factors influencing this to be tested across successive spawning periods for individual fish (i.e. the extent of the repeatability in the timing of river entry). We posit that shad enter the River Severn once warming water temperatures in spring reach a critical value, with the timing of river entry earlier in warmer years and later in colder years, but with the potential for river flow and lunar tidal cycle to also influence the exact timing of river entry. Consequently, we also predict that individuals that return to the river in successive years might also adjust their timing of river entry as a response to inter-annual differences in environmental conditions.
Methods
Study system
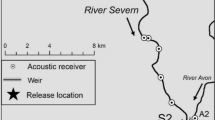
The area of study in the River Severn was the upper estuary and upstream to the first barrier to migration in the river (Fig. 1). The area of the upper estuary used was upstream of where the river is influenced by neap tides (the point during the lunar tidal cycle when tidal amplitude is at its lowest), but is still subject to saline intrusion, especially during spring tides (the point during the lunar tidal cycle when tidal amplitude is at its highest). Consequently, the spatial area of interest commences at ‘Stonebench’ in the upper estuary and then upstream to the normal tidal limit (NTL), at two weirs located on a split channel, at Maisemore Weir (S1a) and Llanthony Weir (S1b) (Fig. 1), which have not been modified to facilitate shad passage but are periodically drowned out during the high spring tides. Upstream of these weirs is the Upper Lode Weir (S2) (Fig. 1) located in the entirely freshwater section of the river but is occasionally drowned out by the highest spring tides and has a ‘notch’ at the upstream end that helps facilitate shad passage.
Map of study area, showing its location in relation to the islands of Great Britain and Ireland (inset a) and the locations of acoustic receivers (red circles) used in this study (inset b), in relation to the River Severn, the neighbouring River Wye, and the Bristol Channel, as well as the locations of the normal tidal limit (NTL) at weirs S1a and S1b (inset b; black rectangles), tagged shad release locations at weirs S1a and S2 (orange circles), and the locations at which environmental data were collected (black circles)
Shad capture, transmitter implantation, and tracking
The period of shad capture and transmitter implantation was in May 2018 and 2019, prior to their period of freshwater residency and spawning (Table 1). Capture methods combined rod and line angling downstream of S1a and S2, where shad migration is often delayed (Davies, 2021), and the use of a manually operated trap positioned at the upstream exit of the notch in weir S2. Shad were captured between Julian days 129–144 (median: 136) in 2018 and 122–136 (median: 133) in 2019. Following their capture, individual shad were anaesthetised (ethyl 3-aminobenzoate methanesulfonate; MS-222), measured (fork length, nearest mm; mass, to 5 g), and scale samples taken (for age analysis). Across both years, 173 shad were internally (peritoneal cavity) tagged with a V9 acoustic transmitter (29 × 9 mm, 4.7 g in weight in air, 69 kHz; www.innovasea.com) using the protocol of Bolland et al. (2019). The transmitters were programmed with a randomised 1-min pulse interval (minimum–maximum interval between signals 30 and 90 s) in their spawning period (April to July) before switching to a 10-min pulse interval until reverting to their randomised 1-min pulse interval the following April. The rationale of this programming was that it extended transmitter battery life to approximately three years, enabling the spawning migrations of these individuals to be measured for up to three consecutive spawning migrations (Davies et al., 2020). At the time of transmitter implantation, a uniquely coded passive integrated transponder (PIT tag) was also inserted to enable recaptured shad to be identified (and so avoid re-tagging), as well as for associated fish pass efficiency studies (unpublished data). Sexing was possible only where gametes were observed incidentally during the tagging process, which allowed for the identification of 35 female and 36 male individuals, with sex undetermined for the remaining 102 fish. All surgical procedures were completed under UK Home Office project licence PD6C17B56.
Once tagged, shad were released when they were able to hold orientation in the water and swim away as normal. Shad were released either up- or downstream of the weir they were captured at (Table 1). Their movements were then tracked on an array of acoustic ‘receivers’ (VR2W; www.innovasea.com). Although there were a wider array of receivers deployed across the lower River Severn basin to track movements further upstream (Davies et al., 2021), for this study only four receivers were used, allowing the timing of entry of shad into this section of river to be identified (Fig. 1). In all cases, where ‘river entry’ is used, it refers to the day/time when a shad (implanted with an acoustic transmitter during a previous spawning migration) was first detected in the upper estuary and thus it was assumed these fish were then motivated to continue moving upstream into freshwater to spawn. Specifically, two acoustic receivers were deployed in the unobstructed reach downstream of the confluence of the channels where S1a and S1b are located and one receiver directly downstream of each of S1a and S1b. River entry for all but one individual in 2019, two in 2020, and one in 2021 was able to be defined as when an individual tagged shad was first detected at the most downstream (Stonebench) receiver and for the remaining individuals, when first detected at one of the three receivers further upstream (Fig. 1).
Environmental data
To enable testing of the environmental cues that influenced the timing of entry into the River Severn by returning shad, river level data at Minsterworth and river temperature data at Gloucester (Fig. 1) were provided as 15-min interval data by the Environment Agency (EA), a publicly funded environmental regulatory agency. Daily sea surface temperature (SST) at Severn Beach (Fig. 1) were obtained from www.seatemperature.info (last accessed 30/09/2021). Daily tidal amplitude was estimated from the Minsterworth river level data, and river temperatures were converted to daily mean river temperature (RT), as the initial entry of shad into the array was analysed at a daily resolution. Sea surface temperatures were converted to 7-day rolling mean sea surface temperature (7-day SST) to control for daily fluctuations and to be more representative of the sea temperatures experienced by shad prior to their entry into the river. Daily river flow data from the EA Haw Bridge gauge (Fig. 1) were obtained from www.nrfa.ceh.ac.uk (last accessed 12/11/2021). Photoperiod was identified in the R package ‘geosphere’ (Hijmans et al., 2021), for the latitude of the most downstream acoustic receiver (51.83471). The point in the lunar tidal cycle was determined through identification of peaks in spring and neap tides using water level data for Portbury (Fig. 1), the closest available location to the River Severn with a > 0m tidal amplitude during neap tides, from www.bodc.ac.uk (last accessed 06/07/2022). Over the course of a full lunar tidal cycle, the day with minimum tidal amplitude (neap tide) was given a value of 0 and 1 (representing the start and end of the lunar tidal cycle, respectively) and the day of peak amplitude (spring tide) given a value of 0.5 (mid-cycle). Due to the circular nature of these data, points in lunar tidal cycle were split into their sine and cosine components for inclusion in linear models. Sine components related to the direction of change in tidal amplitude (positive value = increasing amplitude, negative value = decreasing amplitude), and cosine components related to the proximity to the neap and spring tides (1 = neap, − 1 = spring).
Data and statistical analyses
Testing of inter-annual variation in the timing of river entry, and the environmental conditions influencing this, used non-parametric Wilcoxon rank sum tests with continuity correction that compared Julian day of year, 7-day SST, RT, river flow, and daily tidal amplitude at river entry between years. To avoid issues of pseudo-replication, shad returning for a third tracked spawning season were not compared with shad returning for a second season in the previous year. In order to test the influence of daily variation in river flow and tidal amplitude on river entry, Wilcoxon rank sum tests with continuity correction were also used to compare flow and tidal range on days with and without shad entering the river for the first time within each year. The period over which days were compared consisted of all days from the first to the last Julian day that a shad had been detected entering the river for the first time in that year, so as to encompass the entire period that daily variations in flow and tidal amplitude may have had an influence on river entry. To then test the significance of the lunar tidal cycle on shad river entry, Hermans–Rasson tests were used (R package ‘CircMLE’ (Fitak & Johnsen, 2020)) to analyse how river entries compared to a uniform distribution about the lunar tidal cycle, with each year tested separately due to differences in environmental conditions between years and to avoid pseudo-replication. These tests were used in preference to Rayleigh tests due to their increased power for testing non-uniform data distributions that do not conform with the von Mises distribution (Landler et al., 2019). Point in lunar tidal cycle on the day of first detection in the upper estuary was plotted as a separate rose diagram for each year. Given the high proportion of unsexed shad in the dataset (59%), then the influence of sex on the timing of, and environmental conditions influencing, river entry was not tested.
To determine the influence of each of the environmental variables (photoperiod, 7-day SST, RT, river flow, lunar tidal cycle, daily tidal amplitude) on the probability of river entry of acoustic tagged shad, environmental predictors were entered into a generalised linear mixed model (GLMM; binomial distribution (Rizopoulos, 2022)). The response variable was a binary representation of whether an individual shad had entered the river on each Julian day up to—and including—the day of their river entry in that year. Correspondingly, for each returning fish, a value of 0 was given for each day prior to their detection in the array (taken from day 7 of the year so as to include 7-day SST from Julian day 1 of the year) and then 1 for the day that the fish was first detected.
The environmental variables were all entered into the GLMM as fixed factors, with fish ID as a random factor. The time, location, and method of shad capture varied within and between years, thus time of capture was not used to predict time of river entry in the tagging year. It was thus not possible to control for day of capture when modelling the predictors of river entry. Prior to building the full GLMM, the environmental variables were tested for co-linearity (Pearson’s correlation coefficient); where two variables were highly correlated (r ≥ 0.7), one was removed (Online Resource 1). This resulted in the omission of daily tidal amplitude (keeping the cosine component of lunar tidal cycle, due to the inclusion of the sine component of lunar tidal cycle in the model) and Julian day of year (keeping photoperiod, due to photoperiod being known to influence migration timing in various species (Robart et al., 2018; Garcia-Vega et al., 2022)). However, photoperiod was also highly correlated with 7-day SST and RT, and 7-day SST highly correlated with RT (Online Resource 1). Given these variables all potentially represent important migration cues, then resolving which variable to retain was important.
The method of selection for the co-linear environmental variable(s) to be retained in the full GLMM was based on analysis by Burnside et al. (2021) and was completed by building three separate GLMMs (lme4 package (Bates et al., 2015)), with either photoperiod, 7-day SST or RT at river entry as the response variable, and fish ID used as a random factor. The fixed factor was an annual reference temperature, taken as the 7-day SST on the mean day of river entry across the entire 2019–2021 study period (Julian day 122) for that year. Akaike Information Criterion (AICc) values (corrected for small sample size) were compared between these models and the corresponding models with the fixed factor (annual reference temperature) removed (Online Resource 2a–c) in ‘AICcmodavg’ (Mazerolle, 2020), with predictor effects plots built using the ‘effects’ R package (Fox & Weisberg, 2019) to allow for visual comparisons between each pair of models. Where the model with the fixed factor removed had an AICc value of ≥ 2 higher than the model retaining the fixed factor, the model retaining the fixed factor was the best fitting model and thus annual variation in the response variable (i.e. photoperiod, 7-day SST, or RT at river entry) was predicted by annual reference temperature. Where the model retaining the fixed factor was the best fitting model, the response variable (i.e. photoperiod, 7-day SST, or RT) was omitted as a predictor from the full model predicting river entry. The justification for this selection method was that if annual reference temperature was a significant positive predictor of either 7-day SST or RT at river entry, then annual variation in temperature likely did not influence annual variation in timing of river entry and thus the response variable (i.e. 7-day SST or RT) could be disregarded as a potential migration cue, whereas if annual reference temperature was a significant negative predictor of photoperiod at river entry, annual variation in temperature likely did influence annual variation in timing of river entry and thus photoperiod could be disregarded as a potential migration cue.
The full GLMM predicting the environmental variables responsible for triggering river entry was built using the ‘GLMMadaptive’ package (Rizopoulos, 2022). Model selection was via backward stepwise selection through comparisons of AICc values (Online Resource 3). This model selection dictates that the best fitting model is that with the lowest AICc value; however, a model within 2 AICc can instead be taken as the ‘best fitting model’ should it retain fewer predictors, thus being more parsimonious. Where the selected model retained greater than one predictor, the odds ratios for each predictor were calculated in order to determine their relative influence on river entry, using the ‘sjPlot’ package (Lüdecke, 2022). Predictor effects plots were created to visualise how the probability of an individual shad entering the river in any given year was influenced by the environmental predictors retained in the best fitting model(s) (‘effects’ R package (Fox & Weisberg, 2019)).
To investigate individual repeatability in timing of river entry between years, Julian day of river entry was tested for individual repeatability (R) in ‘rptR’ with a GLMM and parametric bootstrapping of 1000 iterations (Stoffel et al., 2017). The GLMM contained Julian day of river entry as the response variable and the random variable fish ID as the only predictor. Julian day of river entry was considered individually repeatable between years if R differed significantly from 0. Julian day of the year, rather than photoperiod, was used for this analysis, so that our analysis followed the standard methods seen in other studies where photoperiod does not correlate with timing of river entry, due to river entries occurring during periods of both increasing and decreasing photoperiod (Eldøy et al., 2019; Jensen et al., 2020; Birnie-Gauvin et al., 2021).
Results
Inter-annual variability in timing of shad river entry
Of 173 acoustic tagged shad (2018: 73; 2019: 100), 71 ‘returners’ entered the River Severn in their subsequent spawning year (2019: 33; 2020: 38). Of these 71 returners, 21 were then detected returning for a third spawning period (2020: 7; 2021: 14). Of all returning fish, only one entered the neighbouring River Wye (Fig. 1) before entering the River Severn within the same year.
Across the three years, tagged shad entered the river between Julian day 107 and 153. This timing did not vary significantly between years (2019: 109–143 (median = 120); 2020: 107–153 (median = 120); 2021: 111–136 (median = 116); p > 0.05; Table 2b). The environmental conditions on days with shad detected entering the river varied considerably between years (Table 2). On days with at least one shad detected entering the river for the first time, 7-day SST varied between 9.5 and 16.6°C, RT between 10.9 and 20.1°C, and river flow between 24.3 and 152.0 m3 s−1. Shad entered the river at significantly lower 7-day SST and RT in 2021 versus 2019 and 2020 (Table 2b), as both SST and RT were significantly lower in 2021 versus 2019 and 2020 (Online Resource 4). Whilst mean daily river flow was significantly different between all study years (Online Resource 4), the only years where shad entered the river at significantly different flows were 2019 and 2020, with shad entering the river at higher flows in 2019 (Table 2b). However, at a yearly resolution river flow on days with and without a tagged shad entering the river for the first time were not significantly different (Wilcoxon rank sum tests with continuity correction: 2019: P = 0.96; 2020: P = 0.11; 2021: P = 0.22).
Daily tidal amplitude did not differ significantly at river entry between years (Table 2b), nor did tidal amplitude differ significantly between days with and without shad entering the river for the first time (Wilcoxon rank sum tests with continuity correction: 2019: P = 0.86; 2020: P = 0.68; 2021: P = 0.18). The timing of river entries in relation to the lunar tidal cycle differed significantly from a uniform distribution in all years (Hermans–Rasson test: 2019: T = 6.95, P = 0.05; 2020: T = 12.55, P < 0.01; 2021: T = 8.48, P = 0.02), with peaks in river entry associated with different periods of the tidal cycle in each year (Fig. 2).
Rose diagrams illustrating the relationship between the number of tagged shad entering the River Severn and the point in the lunar tidal cycle in a 2019, b 2020, and c 2021. The points of the lunar tidal cycle with the least (neap) and most (spring) influence on water levels are indicated on the plots, with the direction of cycle progression illustrated by the arrows, with increasing (+) and decreasing (−) influence of the lunar tidal cycle on water levels between the neap and spring tides
Environmental influences and individual repeatability of timing of river entry
Photoperiod at river entry was the only one of three co-linear variables determined to be independent of annual reference temperature, with the 7-day SST and RT at river entry being significantly positively correlated with annual reference temperature, whilst the photoperiod at river entry was similar in all years, regardless of yearly variations in annual reference temperature (Fig. 3; Online Resource 2). Thus, photoperiod was deemed to be a candidate environmental cue for shad river entry, whilst 7-day SST and RT were not. Photoperiod was therefore included in the full GLMM over 7-day SST and RT. After backward stepwise selection, the model predicting river entry that had the lowest AICc retained photoperiod and the sine component of the lunar tidal cycle as significant positive predictor variables, with the cosine component retained as a non-significant negative predictor variable (Fig. 4, Table 3b; Online Resource 3). Odds ratios indicated that photoperiod was the main predictor of river entry in this model (Table 3c), which is supported by the most parsimonious model (within 2 AICc of the lowest AICc model) that only retained photoperiod as a predictor, with shad entering the river on days with a photoperiod of 14 h and greater (Fig. 4a, Table 3a; Online Resource 3). For the 21 shad returning to the river for three tracked spawning migrations, timing of river entry had high individual repeatability (R = 0.75, P < 0.001), with Julian day of river entry in the second and third spawning seasons positively correlated (Fig. 5).
Predictor effects plots from GLMMs testing the relationship between the annual reference temperature (fixed predictor) on a photoperiod; b 7-day mean SST; and c mean daily river temperature at river entry (blue lines), compared with GLMMs with the fixed predictor (annual reference temperature) removed (black lines). Labels above points indicate the year for each set of river entries
Discussion
There was relatively high variation in sea surface temperature, river temperature, and flow regime of the River Severn between the three study years, yet the timing of river entry of the returning tagged shad did not differ significantly between years. Tagged shad entered the river’s upper estuary in periods when both sea surface and river temperatures were relatively low (2021), as well as relatively high (2019, 2020). They also tended to enter the river during higher flows in years with higher flows, without adjusting their timing of river entry in relation to daily fluctuations in flow. Although an increasing influence of the lunar tidal cycle on river level had some influence on the timing of river entry, the distribution of shad river entries across the lunar tidal cycle differed between years, and daily tidal amplitude had no significant influence on river entry. Indeed, the most parsimonious model retained only photoperiod as a predictor of river entry, with the critical day length period for shad entering the river being 14 h.
Previous research on twaite shad migration phenology has suggested that the timing of entry into the lower Severn Estuary is associated with water temperature (Aprahamian, 1988). This suggestion resulted from data on shad by-catch in a static net (‘putcher’) fishery between 1979 and 1988, with this fishery located approximately 35-km downstream of the ‘entry’ location of our study (i.e. the Stonebench receiver) and with the putcher rank extending only 107 m from the river bank, thus only partially sampling the river channel (Aprahamian, 1988). Correspondingly, the results of that study and those from the acoustic telemetry data here are not directly comparable, as shad may use different environmental cues at different stages of their migration. However, we are highly confident that our use of passive acoustic telemetry here provided a robust approach for accurately identifying the timing of shad returns to the river (at least at the most downstream receiver in the upper estuary and where it was assumed the returning fish were motivated to enter freshwater to spawn) and provided data that enabled testing against the environmental variables that might influence this timing, where photoperiod was identified as the primary migration cue.
To maximise their fitness, anadromous fish must time their spawning migrations to ensure timely arrival on their upstream spawning grounds that maximises their opportunity for reproductive success (e.g. high mate choice and environmental conditions most appropriate for high egg survival and development). In twaite shad, embryo development is reliant on temperature (Aprahamian et al., 2003), with successful recruitment in the River Severn population related to both high summer temperatures and relatively low flows (Aprahamian & Aprahamian, 2001). High annual variability in the recruitment of many other riverine fish species in England is also related to annual variation in these environmental conditions (Nunn et al., 2007, 2010; Antognazza et al., 2019). However, shad are unaware of the conditions on their spawning grounds when initiating their migration, and high river flows in May have previously been shown to not negatively impact shad recruitment (Aprahamian & Aprahamian, 2001). Correspondingly, that photoperiod was a stronger predictor of the timing of river entry is likely to relate to it being the most reliable predictor of seasonality available to these migrating shad when out at sea and moving through the estuary (given the independence of photoperiod from local weather conditions) (Kudoh, 2019). Thus, the use by shad of photoperiod as a migration cue enables their entry into the river at a relatively consistent time each year, with decisions on the timing of spawning only taken once in the river and in relation to the environmental conditions experienced in their spawning areas. Indeed, this is a migration and spawning strategy already recognised as optimal in the semelparous allis shad Alosa alosa (Linnaeus, 1758), where the fitness costs of arriving late onto the spawning ground and risking arriving after optimal spawning conditions have passed are greater than the fitness costs associated with arriving early onto spawning grounds and potentially having to wait for optimal spawning conditions to occur (Poulet et al., 2021). Probability of river entry may be influenced by photoperiod due to the photoneuroendocrine regulation of gamete production frequently observed in temperate teleost fishes (Migaud et al., 2010), which may result in shad being ready to enter the river once their gametes have reached a critical level of maturation due to increasing day lengths. There is also potential for other environmental variables to act alongside photoperiod to cue shad river entry during these spawning migrations. However, these environmental cues were not detected during the study period, despite relatively high inter-annual variability in environmental conditions across the study. The lack of apparent influence of these environmental conditions might relate to them always being at an acceptable level to stimulate river entry once the critical day length was reached in all study years (i.e. they were ‘silenced cues’), but with the possibility that their influence on timing of river entry could be stronger in years with more extreme environmental conditions.
In this study, photoperiod was the main predictor of river entry and Julian day of arrival was consistent between years for individual twaite shad. Other elements of migration behaviour have previously been identified as individually repeatable in twaite shad, with individuals showing high fidelity for both spawning river (Davies et al., 2020) and home range within their spawning river (Davies, 2021), as well as in barrier passage success between years (Davies, 2021). However, the current study is, to our knowledge, the first to identify individual repeatability in the timing of migration in a shad species either in Europe or North America. Individual repeatability in timing of migration is relatively common in birds (Franklin et al., 2022), with these migrations cued by both temperature (Burnside et al., 2021) and photoperiod (Meunier et al., 2008), although fewer studies have identified comparable behaviour in fish (Bell et al., 2009). Recent studies have, however, suggested that iteroparous anadromous salmonids can exhibit high individual repeatability in migration timings (Eldøy et al., 2019; Jensen et al., 2020; Birnie-Gauvin et al., 2021), and this has also been observed in anadromous non-salmonids (Tibblin et al., 2016). Although in some studies this individual repeatability in timing of river entry is relative to between-year variations in population migration timing, related to variations in environmental conditions (Tibblin et al., 2016; Jensen et al., 2020), we found that for twaite shad, there was no significant variation in their timing of river entry between years, despite high inter-annual variation in environmental conditions.
It has been suggested that male twaite shad tend to begin their spawning migrations earlier than females (Claridge & Gardner, 1978; Aprahamian et al., 2003). In anadromous salmonid fishes, males often also return to the spawning river before females, with this associated with their earlier arrival on spawning grounds to establish dominance over other males, with females then requiring further cues to move upstream (e.g. Esteve, 2017). We were, however, unable to test differences in the timing of, or the environmental variables associated with, river entry between sexes, due to the relatively low proportion of fish that were sexed during transmitter implantation, where it was important that the surgery was completed in a timely fashion to minimise impacts on fish welfare and thus severely limiting the time available for internal examination of gonads (Bolland et al., 2017). Nonetheless, with strong individual repeatability in the timing of river entry being apparent across the dataset relating to photoperiod, then photoperiod is likely an important migration cue regardless of sex. However, it is recommended that in the future work, more emphasis is placed on sexing tagged shad so that the effect of sex on both the timing of river entry and the environmental cues influencing river entry and subsequent arrival onto spawning grounds can be tested rigorously.
Species that rely primarily on photoperiod as a migration cue are predicted to exhibit weaker phenotypic plasticity than those using temperature as a cue, arguably making them less able to adjust the timing of their migration in relation to environmental change and so potentially increasing the potential for mismatches between migration timing and spawning and emergence conditions (Anderson et al., 2013). However, with individually repeatable behaviour having the potential to be heritable or determined by conditions experienced during early life history, using photoperiod as a cue could allow for some intergenerational advancement in migration phenology in twaite shad in response to a warming climate. Evidence of shad species advancing their timing of river entry in response to increasing water temperatures has been found in North America (Nack et al., 2019; Lombardo et al., 2020), although photoperiod may not be the environmental variable stimulating river entry in these cases. As we only had the ability to identify timing of river entry in the second spawning season at the earliest (and potentially only after multiple spawning migrations) then further work is needed to investigate the possible influence of early-life environmental conditions on migration phenology. This could be based on otolith microstructure and chemistry that has already been applied to investigating shad early life history (Lochet et al., 2008).
The Severn Estuary is subject to the second highest tidal range in the world (Binnie, 2016), and with the effort of spawning migrations known to have energetic costs for shad species (Leonard & McCormick, 1999), shad may be able to conserve energy by utilising these tides during their riverward migrations. Here, we found a relatively low influence of the lunar tidal cycle on river entry and thus upstream movements through the upper estuary were seemingly not aligned with spring (rather than neap) tides as a way to conserve energy during their riverward migration. However, it is possible that movements of twaite shad through the lower Severn Estuary were more greatly influenced by the lunar tidal cycle, as was observed in allis shad in the lower rather than upper Gironde Estuary (France) (Rochard, 2001). Some fish species also use selective tidal stream transport (STST) as an energetically efficient strategy to move through estuaries during an individual tidal cycle (Beaulaton & Castelnaud, 2005; Kelly et al., 2020), and a previous study by Aprahamian (1988) found that peaks in by-catch of twaite shad in a static net fishery in the lower Severn Estuary coincided with ebb tides, suggesting that twaite shad may utilise the individual tidal cycle to conserve energy whilst moving through the estuary. Although this was not found to be the driver behind tidally influenced allis shad movements in the lower Gironde Estuary (Rochard, 2001), the acoustic receiver array in the current study did not have the spatial coverage to investigate STST. Thus, whilst we cannot dismiss STST as a potential mechanism used by twaite shad in the Severn, further work would be needed to determine the extent to which individuls use tides in this way. Furthermore, the influence of the lunar and individual tidal cycle on onward migration into freshwater, including at weirs known to be influenced by spring tides (i.e. S1a, S1b, and S2), was not investigated here and so could still be an important driver of the timing of shad arrival at freshwater spawning areas.
Conclusion
In summary, photoperiod was found to be the primary variable predicting the timing of twaite shad entry into the River Severn upper estuary, prior to commencing their upstream migrations to riverine spawning areas, with a small influence of the lunar tidal cycle and no apparent influence of temperature or river flow. High individual repeatability in the timing of river entry was apparent across years, irrespective of variations in environmental conditions. This suggests that individuals follow a specific migration strategy influenced primarily by increasing day length that allows them to be present on their upstream spawning grounds when conditions for reproduction are favourable and mate choice is high.
Data availability
Data from this study will be made available upon reasonable request.
References
Agha, M., J. P. Losee, M. N. C. Litz, C. Smith, J. J. Schaffler, W. S. Patton, A. M. Dufault & G. M. Madel, 2021. Temporal patterns and ecosystem correlates of chum salmon (Oncorhynchus keta) migration phenology in the Pacific Northwest. Canadian Journal of Fisheries and Aquatic Sciences 78: 1565–1575. https://doi.org/10.1139/cjfas-2020-0392.
Anderson, J. J., E. Gurarie, C. Bracis, B. J. Burke & K. L. Laidre, 2013. Modeling climate change impacts on phenology and population dynamics of migratory marine species. Ecological Modelling 264: 83–97. https://doi.org/10.1016/j.ecolmodel.2013.03.009.
Antognazza, C. M., J. R. Britton, C. Potter, E. Franklin, E. A. Hardouin, C. G. Roberts, M. Aprahamian & D. Andreou, 2019. Environmental DNA as a non-invasive sampling tool to detect the spawning distribution of European anadromous shads (Alosa spp.). Aquatic Conservation-Marine and Freshwater Ecosystems 29: 148–152. https://doi.org/10.1002/aqc.3010.
Antognazza, C. M., J. R. Britton, V. De Santis, K. Kolia, O. A. Turunen, P. Davies, L. Allen, E. A. Hardouin, C. Crundwell & D. Andreou, 2021. Environmental DNA reveals the temporal and spatial extent of spawning migrations of European shad in a highly fragmented river basin. Aquatic Conservation-Marine and Freshwater Ecosystems. https://doi.org/10.1002/aqc.3601.
Aprahamian, M. W., 1988. The biology of the twaite shad, Alosa fallax (Lacépède), in the Severn Estuary. Journal of Fish Biology 33: 141–152. https://doi.org/10.1111/j.1095-8649.1988.tb05568.x.
Aprahamian, M. W. & C. D. Aprahamian, 2001. The influence of water temperature and flow on year class strength of twaite shad (Alosa fallax fallax) from the River Severn, England. Bulletin Francais De La Peche Et De La Pisciculture 362–63: 953–972. https://doi.org/10.1051/kmae:2001030.
Aprahamian, N. W., J. L. Bagliniere, M. R. Sabatie, P. Alexandrino, R. Thiel & C. D. Aprahamian, 2003. Biology, status, and conservation of the anadromous Atlantic twaite shad Alosa fallax fallax. Biodiversity, Status, and Conservation of the World’s Shads 35: 103–124.
Aprahamian, M. W., C. D. Aprahamian & A. M. Knights, 2010. Climate change and the green energy paradox: the consequences for twaite shad Alosa fallax from the River Severn, U.K. Journal of Fish Biology 77: 1912–1930. https://doi.org/10.1111/j.1095-8649.2010.02775.x.
Bal, G., L. Montorio, E. Rivot, E. Prevost, J. L. Bagliniere & M. Nevoux, 2017. Evidence for long-term change in length, mass and migration phenology of anadromous spawners in French Atlantic salmon Salmo salar. Journal of Fish Biology 90: 2375–2393. https://doi.org/10.1111/jfb.13314.
Bates, D., M. Mächler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. https://doi.org/10.18637/jss.v067.i01.
Beaulaton, L. & G. Castelnaud, 2005. The efficiency of selective tidal stream transport in glass eel entering the Gironde (France). Bulletin Francais De La Peche Et De La Pisciculture 378–79: 5–21. https://doi.org/10.1051/kmae:2005001.
Bell, A. M., S. J. Hankison & K. L. Laskowski, 2009. The repeatability of behaviour: a meta-analysis. Animal Behaviour 77: 771–783. https://doi.org/10.1016/j.anbehav.2008.12.022.
Binnie, C., 2016. Tidal energy from the Severn estuary, UK. Proceedings of the Institution of Civil Engineers-Energy 169: 3–17. https://doi.org/10.1680/jener.14.00025.
Birnie-Gauvin, K., A. Koed & K. Aarestrup, 2021. Repeatability of migratory behaviour suggests trade-off between size and survival in a wild iteroparous salmonid. Functional Ecology. https://doi.org/10.1111/1365-2435.13917.
Bolland, J. D., A. D. Nunn, N. V. Angelopoulos, J. R. Dodd, P. Davies, C. G. Roberts, J. R. Britton & I. G. Cowx, 2019. Refinement of acoustic-tagging protocol for twaite shad Alosa fallax (Lacepede), a species sensitive to handling and sedation. Fisheries Research 212: 183–187. https://doi.org/10.1016/j.fishres.2018.12.006.
Burnside, R. J., D. Salliss, N. J. Collar & P. M. Dolman, 2021. Birds use individually consistent temperature cues to time their migration departure. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.2026378118.
Claridge, P. N. & D. C. Gardner, 1978. Growth and movements of twaite shad, Alosa fallax (Lacépède), in the Severn Estuary. Journal of Fish Biology 12: 203–211. https://doi.org/10.1111/j.1095-8649.1978.tb04166.x.
Davies, P., R. J. Britton, A. D. Nunn, J. R. Dodd, C. Crundwell, R. Velterop, N. O’Maoileidigh, R. O’Neill, E. V. Sheehan, T. Stamp & J. D. Bolland, 2020. Novel insights into the marine phase and river fidelity of anadromous twaite shad Alosa fallax in the UK and Ireland. Aquatic Conservation-Marine and Freshwater Ecosystems 30: 1291–1298. https://doi.org/10.1002/aqc.3343.
Davies, P., J. R. Britton, A. D. Nunn, J. R. Dodd, C. Bainger, R. Velterop & J. D. Bolland, 2021. Cumulative impacts of habitat fragmentation and the environmental factors affecting upstream migration in the threatened sea lamprey, Petromyzon marinus. Aquatic Conservation-Marine and Freshwater Ecosystems 31: 2560–2574. https://doi.org/10.1002/aqc.3625.
Davies, P., 2021. Movement ecology of two threatened anadromous species, twaite shad Alosa fallax and sealamprey Petromyzon marinus, revealed by acoustic telemetry, PhD thesis. Bournemouth University.
Dias, B. S., M. G. Frisk & A. Jordaan, 2019. Opening the tap: Increased riverine connectivity strengthens marine food web pathways. PLoS ONE. https://doi.org/10.1371/journal.pone.0217008.
Eldøy, S. H., X. Bordeleau, G. T. Crossin & J. G. Davidsen, 2019. Individual repeatability in marine migratory behavior: a multi-population assessment of anadromous brown trout tracked through consecutive feeding migrations. Frontiers in Ecology and Evolution 7: 420. https://doi.org/10.3389/fevo.2019.00420.
Esteve, M., 2017. The velocity of love. The role of female choice in salmonine reproduction. In Lobón-Cerviá, J. & N. Sanz (eds), Brown Trout: Biology, Ecology and Management Chichester, UK: 145–163. https://doi.org/10.1002/9781119268352.ch6.
Fitak, R. and Johnsen, S., 2020. R package 'CircMLE': Maximum Likelihood Analysis of Circular Data.
Fox, J. & S. Weisberg, 2019. An R Companion to Applied Regression, 3rd ed. Sage, Thousand Oaks, CA:
Franklin, K. A., M. A. C. Nicoll, S. J. Butler, K. Norris, N. Ratcliffe, S. Nakagawa & J. A. Gill, 2022. Individual repeatability of avian migration phenology: a systematic review and meta-analysis. Journal of Animal Ecology 91: 1416–1430. https://doi.org/10.1111/1365-2656.13697.
Gahagan, B. I. & M. M. Bailey, 2020. Surgical implantation of acoustic tags in American shad to resolve riverine and marine restoration challenges. Marine and Coastal Fisheries 12: 272–289. https://doi.org/10.1002/mcf2.10108.
Garcia-Vega, A., J. F. Fuentes-Perez, P. M. L. Urretabizkaia, J. A. Ganuza & F. J. Sanz-Ronda, 2022. Upstream migration of anadromous and potamodromous brown trout: patterns and triggers in a 25-year overview. Hydrobiologia 849: 197–213. https://doi.org/10.1007/s10750-021-04720-9.
Goetz, F. A., E. Beamer, E. J. Connor, E. Jeanes, C. Kinsel, J. W. Chamberlin, C. Morello & T. P. Quinn, 2021. The timing of anadromous bull trout migrations in estuarine and marine waters of Puget Sound, Washington. Environmental Biology of Fishes 104: 1073–1088. https://doi.org/10.1007/s10641-021-01136-1.
Hall, C. J., A. Jordaan & M. G. Frisk, 2012. Centuries of anadromous forage fish loss: consequences for ecosystem connectivity and productivity. Bioscience 62: 723–731. https://doi.org/10.1525/bio.2012.62.8.5.
Hijmans, R. J., Karney, C., Williams, E. and Vennes, C., 2021. R package 'geosphere': Spherical Trigonometry.
Hossain, M. S., S. M. Sharifuzzaman, M. A. Rouf, R. S. Pomeroy, M. D. Hossain, S. R. Chowdhury & S. AftabUddin, 2019. Tropical hilsa shad (Tenualosa ilisha): biology, fishery and management. Fish and Fisheries 20: 44–65. https://doi.org/10.1111/faf.12323.
Jensen, A. J., B. Finstad, P. Fiske, O. H. Diserud & E. B. Thorstad, 2020. Repeatable individual variation in migration timing in two anadromous salmonids and ecological consequences. Ecology and Evolution 10: 11727–11738. https://doi.org/10.1002/ece3.6808.
Kelly, J. T., S. Lankford, J. J. Cech & A. P. Klimley, 2020. Estimating the energetic savings for green sturgeon moving by selective tidal stream transport. Environmental Biology of Fishes 103: 455–463. https://doi.org/10.1007/s10641-020-00969-6.
Kudoh, H., 2019. Photoperiod-temperature phase lag: a universal environmental context of seasonal developmental plasticity. Development Growth & Differentiation 61: 5–11. https://doi.org/10.1111/dgd.12579.
Landler, L., G. D. Ruxton & E. P. Malkemper, 2019. The Hermans-Rasson test as a powerful alternative to the Rayleigh test for circular statistics in biology. Bmc Ecology. https://doi.org/10.1186/s12898-019-0246-8.
Leonard, J. B. K. & S. D. McCormick, 1999. The effect of migration distance and timing on metabolic enzyme activity in an anadromous clupeid, the American shad (Alosa sapidissima). Fish Physiology and Biochemistry 20: 163–179. https://doi.org/10.1023/A:1007751701668.
Limburg, K. E. & J. R. Waldman, 2009. Dramatic declines in North Atlantic Diadromous fishes. Bioscience 59: 955–965. https://doi.org/10.1525/bio.2009.59.11.7.
Lochet, A., P. Jatteau, J. Tomas & E. Rochard, 2008. Retrospective approach to investigating the early life history of a Diadromous fish: Allis shad Alosa alosa (L.) in the Gironde-Garonne-Dordogne watershed. Journal of Fish Biology 72: 946–960. https://doi.org/10.1111/j.1095-8649.2007.01776.x.
Lok, T., O. Overdijk & T. Piersma, 2015. The cost of migration: spoonbills suffer higher mortality during trans-Saharan spring migrations only. Biology Letters. https://doi.org/10.1098/rsbl.2014.0944.
Lombardo, S. M., J. A. Buckel, E. F. Hain, E. H. Griffith & H. White, 2020. Evidence for temperature-dependent shifts in spawning times of anadromous alewife (Alosa pseudoharengus) and blueback herring (Alosa aestivalis). Canadian Journal of Fisheries and Aquatic Sciences 77: 741–751. https://doi.org/10.1139/cjfas-2019-0140.
Lüdecke, D., 2022. sjPlot: Data Visualisation for Statistics in Social Science.
Mack, K., H. White & F. C. Rohde, 2021. Use of acoustic telemetry to identify spawning river and spawning migration patterns of American shad in the Albemarle sound, North Carolina. North American Journal of Fisheries Management 41: 242–251. https://doi.org/10.1002/nafm.10555.
Matley, J. K., N. V. Klinard, A. P. B. Martins, K. Aarestrup, E. Aspillaga, S. J. Cooke, P. D. Cowley, M. R. Heupel, C. G. Lowe, S. K. Lowerre-Barbieri, H. Mitamura, J. S. Moore, C. A. Simpfendorfer, M. J. W. Stokesbury, M. D. Taylor, E. B. Thorstad, C. S. Vandergoot & A. T. Fisk, 2022. Global trends in aquatic animal tracking with acoustic telemetry. Trends in Ecology & Evolution 37: 79–94. https://doi.org/10.1016/j.tree.2021.09.001.
Mazerolle, M. J., 2020. AICcmodavg : Model Selection and Multimodel Inference Based on (Q)AIC(c). R package version 2.3-1.
Meunier, J., R. Song, R. S. Lutz, D. E. Andersen, K. E. Doherfy, J. G. Bruggink & E. Oppelt, 2008. Proximate cues for a short-distance migratory species: an application of survival analysis. Journal of Wildlife Management 72: 440–448. https://doi.org/10.2193/2006-521.
Migaud, H., A. Davie & J. F. Taylor, 2010. Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. Journal of Fish Biology 76: 27–68. https://doi.org/10.1111/j.1095-8649.2009.02500.x.
Nack, C. C., D. P. Swaney & K. E. Limburg, 2019. Historical and projected changes in spawning Phenologies of American shad and striped bass in the Hudson river estuary. Marine and Coastal Fisheries 11: 271–284. https://doi.org/10.1002/mcf2.10076.
Nolan, E. T., C. Gutmann Roberts & J. R. Britton, 2019. Predicting the contributions of novel marine prey resources from angling and anadromy to the diet of a freshwater apex predator. Freshwater Biology 64: 1542–1554. https://doi.org/10.1111/fwb.13326.
Nunn, A. D., J. P. Harvey, J. R. Britton, P. A. Frear & I. G. Cowx, 2007. Fish, climate and the Gulf Stream: the influence of abiotic factors on the recruitment success of cyprinid fishes in lowland rivers. Freshwater Biology 52: 1576–1586. https://doi.org/10.1111/j.1365-2427.2007.01789.x.
Nunn, A. D., P. A. Frear, M. Lee & I. G. Cowx, 2010. Is there evidence for a shift in fish growth and recruitment success linked to climate change? Journal of Fish Biology 77: 1780–1792. https://doi.org/10.1111/j.1095-8649.2010.02751.x.
Poulet, C., A. Paumier, G. Lassalle, M. Pierre & P. Lambert, 2021. Simulating upstream migration and spawning timing effects to allis shad reproductive success. Environmental Biology of Fishes. https://doi.org/10.1007/s10641-021-01151-2.
Rizopoulos, D., 2022. R package 'GLMMadaptive': Generalized Linear Mixed Models using Adaptive Guassian Quadrature.
Robart, A. R., M. M. K. McGuire & H. E. Watts, 2018. Increasing photoperiod stimulates the initiation of spring migratory behaviour and physiology in a facultative migrant, the pine siskin. Royal Society Open Science. https://doi.org/10.1098/rsos.180876.
Rochard, E., 2001. Anadromous estuarine migration of adult allis shad Alosa alosa, shape of the phenomenon and influence of the tidal cycle. Bulletin Francais De La Peche Et De La Pisciculture 362–63: 853–867. https://doi.org/10.1051/kmae:2001023.
Skov, C., B. B. Chapman, H. Baktoft, J. Brodersen, C. Bronmark, L. A. Hansson, K. Hulthen & P. A. Nilsson, 2013. Migration confers survival benefits against avian predators for partially migratory freshwater fish. Biology Letters. https://doi.org/10.1098/rsbl.2012.1178.
Stoffel, M. A., S. Nakagawa & H. Schielzeth, 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods in Ecology and Evolution 8: 1639–1644. https://doi.org/10.1111/2041-210X.12797.
Tibblin, P., A. Forsman, T. Borger & P. Larsson, 2016. Causes and consequences of repeatability, flexibility and individual fine-tuning of migratory timing in pike. Journal of Animal Ecology 85: 136–145. https://doi.org/10.1111/1365-2656.12439.
Tillotson, M. D., M. C. Arostegui, C. S. Austin, A. E. Lincoln, W. Matsubu, K. N. McElroy & T. P. Quinn, 2021. Challenges in the identification and interpretation of Phenological shifts: anthropogenic influences on adult migration timing in Salmonids. Reviews in Fisheries Science & Aquaculture 29: 769–790. https://doi.org/10.1080/23308249.2021.1874292.
Walters, A. W., R. T. Barnes & D. M. Post, 2009. Anadromous alewives (Alosa pseudoharengus) contribute marine-derived nutrients to coastal stream food webs. Canadian Journal of Fisheries and Aquatic Sciences 66: 439–448. https://doi.org/10.1139/F09-008.
Wei, T. and Simko, V., 2021. R package 'corrplot': Visualization of a Correlation Matrix.
West, D. C., A. W. Walters, S. Gephard & D. M. Post, 2010. Nutrient loading by anadromous alewife (Alosa pseudoharengus): contemporary patterns and predictions for restoration efforts. Canadian Journal of Fisheries and Aquatic Sciences 67: 1211–1220. https://doi.org/10.1139/F10-059.
Acknowledgements
The authors acknowledge funding for the purchase of acoustic tags from the UK Department of Food and Rural Affairs (DEFRA). MIAY was supported by a match-funded PhD grant from the Fishmongers Company and Bournemouth University, with support from the ‘Unlocking the Severn’ project (Heritage Lottery Fund Grant/Award Number: HG/15/04573, LIFE Nature Programme Grant/Award Number: LIFE15/NAT/UK/000219). We thank staff of the Environment Agency, Natural England, Severn Rivers Trust, and Canal and Rivers Trust for their contributions and permission from the landowner at Maisemore Weir.
Author information
Authors and Affiliations
Contributions
Conceived and designed the field experiments: JDB, ADN, JRD, CC, RV, JRB, and PD. Conducted fieldwork: JDB, ADN, JRD, CC, JRB, PD, and MIAY. Conducted telemetry analysis: MIAY, with advice from PD. Wrote the article: MIAY. Edited the article: JDB, PD, JRB, ADN, JRD, CC, and RV.
Corresponding authors
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Additional information
Handling editor: Louise Chavarie
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeldham, M.I.A., Britton, J.R., Crundwell, C. et al. Individual repeatability in the timing of river entry indicates the strong influence of photoperiod in the spawning migrations of iteroparous twaite shad Alosa fallax. Hydrobiologia 850, 1619–1634 (2023). https://doi.org/10.1007/s10750-023-05168-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05168-9