Abstract
This study analysed diversity patterns of sedimentary, littoral and planktic diatoms in 43 mountain lakes in the northern European Alps and identified processes that contribute to these patterns. Linear regression models showed a significant increase of sedimentary α-diversity with lake area and conductivity and a negative trend with increasing elevation, whilst the littoral diatom α-diversity increased significantly with conductivity and lake water temperature. Planktic diatom α-diversity significantly decreased with lake area and depth. August water temperature, total phosphorus, conductivity and lake depth explained a significant part of the variation and were significantly correlated with pairwise β-diversities in the data sets, but spatial and shared effects of space and environment were more important for planktic and littoral diatoms. A null model approach based on assemblages’ dissimilarities revealed that the structure of littoral and planktic assemblages was predominantly stochastic. In contrast, sedimentary diatoms were formed by both deterministic and stochastic processes. Abundant and widespread species contributed a large part to the assemblage β-diversity. The results point to a stronger role of niche assembly in sedimentary than for littoral and planktic diatoms. Dispersal limitation, in turn, is likely to contribute to the spatial patterns and stochastic assembly processes observed for littoral and planktic diatoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important goals of diatom research is to understand the spatial patterns of diatom diversity. The species diversity of a region is composed of α-diversities, i.e. the number and abundance of species in local communities, and β-diversities, i.e. the differences in the composition of local communities (Whittaker, 1972). For diatom α-diversity, especially the role of altitude is controversial. In theory, decreasing α-diversity along the altitudinal gradient can be expected as the number of taxa that can cope with increasingly harsh conditions becomes smaller. However, a study of subarctic ponds along an elevational gradient from 8 to 887 m a.s.l. (metres above sea level) found no influence of elevation on benthic diatom richness (Heikkinen et al., 2022). Another study of Chinese, Spanish and Norwegian streams found either non-significant, decreasing or unimodal patterns of benthic diatoms along the elevational gradient (Wang et al., 2020). In turn, Blanco et al. (2020) and Taxböck et al. (2020) detected increasing species richness of benthic diatoms with increasing altitude in Mediterranean mountain ponds and Swiss springs, respectively. For lakes in the Alps, elevational α-diversity patterns of diatoms remain unclear.
Another important question in diatom ecology is to what extend spatial and environmental factors influence the β-diversity of diatoms (Heino & Soininen, 2005; Soininen et al., 2007). Long environmental gradients may increase the importance of environmental factors, whilst dispersal limitation is promoted by large spatial extent (Keck et al., 2018) or strong topography (Benito et al., 2018). In general, the likelihood of organisms dispersing in more connected systems such as streams (Vilmi et al., 2017) is less limited compared to ponds or lakes (Heino et al., 2015; Shurin et al., 2009). For lakes in the northern European Alps, an influence of dispersal limitation is likely as elevational differences of mountains between lakes can be more than 2000 m. However, different types of assemblages from a lake may be disparately affected by dispersal processes. For example, dispersal effects are more likely for planktic and benthic assemblages, which are easily attached to water birds or waterborne insects than for sedimentary assemblages. In turn, short-term dispersal processes may be less important for sedimentary diatoms because one sample integrates diatoms from several seasons.
The influence of dispersal related factors has important consequences regarding the use of diatoms as paleolimnological proxies or as bioindicators (Leboucher et al., 2020). In this context, it is important to understand how the biological community structure responds to changes of environmental conditions and to which extent spatial constraints influence community patterns. In a 468-lake set in the USA, Winegardner et al. (2015) found that environmental and spatial variables explained a similar part of the variance in sedimentary and planktic assemblages and studies from tropical reservoirs are in line with these findings (Bartozek et al., 2019; Zorzal-Almeida et al., 2017). However, it is less clear, if benthic and planktic diatoms show comparable patterns. Consequently, we aimed to investigate whether α- and β-diversity patterns are congruent amongst sedimentary, littoral and planktic diatoms in the mountain lakes of the northern European Alps using a large altitudinal gradient set (760–2,460 m a.s.l.).
The relative contribution of local environmental variables and spatial predictors was evaluated and the importance of stochastic versus deterministic processes was measured. These complementary approaches were used to disentangle the effects of unmeasured yet important environmental factors and dispersal processes that both may lead to spatial patterns in community structure.
Based on the approaches mentioned, the following hypotheses were tested:
-
(1)
Along the altitudinal gradient, diatom α-diversity decreases as environmental conditions become harsher at higher altitudes, selecting for a few adapted species.
-
(2)
The influence of spatial variables on diatom β-diversity is weaker with sedimentary diatoms than with littoral and planktic diatoms due to the integration of introduced diatoms across several seasons and the short-term dispersal of littoral and planktic diatoms by birds and insects.
-
(3)
Dispersal limitation contributes to spatial patterns in β-diversity amongst littoral and planktic diatoms because most lakes are isolated.
Methods
Study sites
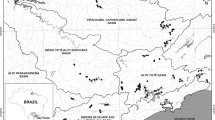
The 43 study lakes are in Bavaria (Germany) and Tyrol (Austria) and span a longitudinal gradient of 220 km and a latitudinal gradient of 50 km (Fig. 1).
Most lakes of this study are located on carbonate bedrock and are well buffered, as reflected by pH values between 8 and 9 (Table S1). The altitudinal gradient comprises the vegetation zones of montane forest (750–1400 m a.s.l.), subalpine forest (1400–1700 m a.s.l.) and alpine meadows (1700–2500 m a.s.l.). In this region, the montane forest is dominated by beech (Fagus sylvatica L.), spruce [Picea abies (L.) H.Karst] and maple (Acer pseudoplatanus L.); the subalpine forest mainly consists of spruce and pine at the ecotone to bare meadows (Pinus mugo ssp. Mugo Turra). In alpine meadows, shrubs (e.g. Rhododendron hirsutum L.), grasslands (e.g. Carex sempervirens Elliott) and fellfields [e.g. Noccaea rotundifolia (L.) Moench] mostly predominate.
Most lakes were formed by cirque glaciers and are typically small (< 3 ha) and shallow (< 10 m) (Table S1) with a single deep zone. Two of the lakes are karstic; they are nearly round and have a higher depth-to-surface ratio than the other lakes. Six of the lakes are polymictic, and macrophytes are found in 26 of the 43 lakes sampled.
Sampling and laboratory procedures
The 43 lakes were sampled twice during the ice-free period: once between June and mid-August and once between August and November. Thirty-six lakes were investigated in 2016 and seven in 2017. On the first sampling date, lake bathymetry was determined with an echo sounder (Lawrence HDS8, Oslo, Norway) and subsequently, a buoy was installed, attached by rope to a stone at the deepest point of each lake. Temperature loggers (Onset Pendant UA-001-64 HOBO, Bourne, USA) were mounted on the rope 0.5 m above the ground and 0.5 m below the water surface. During most of the ice-free period, the loggers in the lakes were exposed to assess the temperature regime and the lake’s mixing type. On both sampling dates, physical variables (temperature, oxygen saturation, pH, and electrical conductivity at 25 °C) were measured with a multiprobe (WTW 350, Weilheim, Germany) in one-metre steps above the deepest point of each lake. After measuring the Secchi depth, 0.5 L of a mixed water sample—comprising the euphotic zone (CEN, 2015)—was collected with a hose sampler. One half of the water sample was filtered (0.45 µm) on-site and stored at 4 °C together with the unfiltered rest until further processing at the laboratory.
Another litre of water was taken from the euphotic zone with the hose sampler and preserved with Lugol’s solution to analyse planktic diatom communities (Throndsen, 1978). Planktic samples were taken from every lake on the first sampling date. Periphytic diatom assemblages were assessed by scraping off the diatom communities of five stones at depths of between 20 and 50 cm in the northern littoral zone of each lake with a single-use toothbrush (DIN, 2014). At nine sites there were no stones present in the littoral zone, so no samples were taken there to maintain consistency of the substratum. On the second sampling date, sediment cores were taken from the deepest point of each lake with a gravity corer (Uwitec, Mondsee, Austria) to assess the sedimentary diatom communities (mostly composed of dead individuals) from all 43 lakes according to Küfner et al. (2020a, b, c). Hence, three diatom assemblages could be sampled: planktic diatoms from the open water, periphytic diatoms from the northern littoral zone and sedimentary diatoms (the uppermost centimetre of each sediment core) from the deepest point of each lake.
All chemical analyses were carried out at the laboratory of the Limnological Research Station Iffeldorf at the Technical University of Munich, Germany. Standard colorimetric methods using a Hitachi 150–200 photometer (Chiyoda, Japan) were applied to determine the concentrations of total phosphorus (Murphy & Riley, 1962), nitrate-N (Navone, 1964), ammonia-N (DIN, 1983) and silica. The concentrations of major ions (calcium, magnesium and sodium) were analysed using a cation chromatograph (Thermo Scientific, ICS-1100, Waltham, USA).
Planktic diatom samples (1 L) were filtered with 0.45-µm syringe filters and the residue on the filters was further processed (Nixdorf et al., 2014). The uppermost centimetre of each sediment core was used to assess the sedimentary diatom assemblages present. The residue on the filters of the planktic samples, the sediment samples and the littoral samples were processed in the same way: preparation of the diatoms was carried out according to van der Werff and Macan (1955). A total of 500 valves were identified in each case using a Leica DNM microscope (Wetzlar, Germany) at ×1000 magnification to analyse the composition of the diatom samples. Eleven of the 43 planktic samples were excluded from the data analysis because they contained an insufficient number of valves. Taxa were counted at the species level and where possible, at the subspecies level. Individuals that could not be identified were given working names. Standard literature was used for identification (Krammer, 2000, 2002; Krammer & Lange-Bertalot, 1991a, b, 1997a, b; Lange-Bertalot, 2001; Lange-Bertalot et al., 2017).
Data analysis
For each sample of the three assemblages, α-diversity was computed as species richness and Shannon diversity. Both measures of α-diversity were included as they are frequently used in the context of conservation (Spellerberg & Fedor, 2003) but may reveal different patterns along environmental gradients (Stirling & Wilsey, 2001). Differences in mean α-diversity amongst the assemblages were assessed with the Wilcoxon Test as diversities were not distributed normally (Wilcoxon, 1945). Relations between environmental variables and α-diversity measures were investigated using linear regression models and R2-values were calculated for significant models. The environmental variables used were selected based on variation inflation factors (see below): altitude (“alt”), lake area (“area”), lake depth (“depth”), nitrate-N (“NO3”), total phosphorous (“TP”), silicate (“Si”), pH, conductivity (“cond”), August bottom temperature (“ABT”) and August surface temperature (“AST”).
The mean and pairwise β-diversity of diatom communities was calculated for sedimentary, littoral and planktic assemblages for abundance and incidence data, based on the method proposed by Baselga and Orme (2012). For the abundance data, the multiple-site Bray–Curtis dissimilarity (βtot) was computed and then partitioned into its “abundance balanced variation” (βbal) and “abundance gradients” (βgra) components (Baselga, 2017). For incidence data, β-diversity was calculated as a Simpson diversity (βsim) and then partitioned into its component for turnover (representing the Sorensen dissimilarity which measures species replacement) (βsor) and nestedness (representing the nestedness component of the Sorensen dissimilarity, reflecting species loss) (βsne) following Baselga (2010). The contribution to abundance and incidence-based β-diversity (SCBD) of each species was assessed according to Legendre and De Caceres (2013) using Hellinger-transformed data. The relationship between SCBD-values and species abundance and occurrence was modelled using polynomial regression models.
The effect of spatial, environmental and altitudinal distance on total β-diversity (βtot, βsim) and its components (βbal, βgra, βsor, βsne) were investigated with partial Mantel tests (Borcard et al., 1992). Significance levels and r-values were calculated for each combination whilst controlling for the other distance matrices to obtain the exclusive effect values of the factor of interest. Moreover, the decay of assemblage similarity with spatial, altitudinal and environmental distance was assessed by fitting negative exponential functions using the “decay.model” function in the “beta part” package (Baselga & Orme, 2012). This method provides additional information compared to Mantel tests through the shape of the regression line (Dray et al., 2020). Distance decay analysis of the abundance and incidence data were complemented with variation partitioning to investigate the proportion of variance explained by environmental and spatial variables using Euclidean distances (Borcard et al., 1992).
To select the environmental variables used in distance decay analysis and variation partitioning, separate redundancy analysis (RDA) models for sedimentary, littoral and planktic diatom assemblages were computed based on Hellinger-transformed assemblage data (Legendre & Gallagher, 2001). Only variables with variance inflation factors less than five for all data sets were included in the RDAs. VIF’s were calculated and variables were selected with the “vifstep” function in the “usdm” R package (Naimi, 2017). The same ten variables were selected for each dataset and used for all further calculations. Pearson correlations were calculated for these variables, and P-values were Bonferroni–Holm corrected. Based on the results, correlation plots were produced using the “corrplot” function (Wei & Simko, 2017). All variables except for altitude and pH were log transformed and the full data set was scaled before RDA using the “scale” function in “vegan”. Only variables that were identified by forward selection using the “ordiR2step” function in “vegan” (P < 0.05) were incorporated into the final environmental distance matrix (Blanchet et al., 2008). The altitudinal distance matrix used in distance decay analysis and variation partitioning was calculated based on Euclidean distances between sites. Distance-based Moran’s eigenvector maps (db MEM) based on the geographical coordinates of the lakes were used in variation partitioning (Dray et al., 2006) to explore spatial effects. Eigenvectors were selected using the “mem.select” function in the “ade4” R package. This function runs a forward selection based on R2-values after a global test of significance (Bauman et al., 2018). The significance of each MEM was assessed with the “moran.randtest” function that computes Moran’s index and calculates P-values using random permutations. To measure the relative strength of stochastic versus deterministic processes, a null model approach was applied using the “tNST” function in the “NST” R package (Ning et al., 2019). Null models were calculated for all three diatom data sets and abundance data using the Bray–Curtis distance and incidence data using the Jaccard index. This function produces results ranging from 0 to 100, with values below 50, indicating a prevalence of deterministic processes and values above 50, denoting stochasticity. The results for the data sets were compared by ANOVA, PANOVA and PERMANOVA with 1000 resampling runs. All the analyses were computed using version 3.6.6 of the “R” free statistics software, except for tNST analysis, which was conducted using version 4.1.3 (R Core Team, 2013). Data curation was facilitated using the “dplyr” package (Wickham & François, 2014).
Results
Environmental variables
Variables with VIFs < 5 were altitude (“alt”), lake area (“area”), lake depth (“depth”), nitrate–N (“NO3”), total phosphorous (“TP”), silicate (“Si”), pH, conductivity (“cond”), August bottom temperature (“ABT”) and August surface temperature (“AST”). A significant negative Pearson correlation was found in all data sets between cond and alt (Psed = 0.007, rsed = − 0.661, Plit = 0.006, rlit = − 0.681, Ppla = 0.013, rpla = − 0.650), cond and pH (Psed = 0.018, rsed = − 0.512, Plit = 0.031, rlit = − 0.495, Ppla = 0.050, rpla = − 0.488) and ABT and depth (Psed = 0.041, rsed = − 0.597, Plit = 0.034, rlit = − 0.625, Ppla = 0.024, rpla = − 0.651, Fig. S1).
α-diversity
The mean species richness and Shannon diversity were significantly higher for sedimentary and littoral diatom assemblages than for planktic assemblages (Wilcoxon Test, P < 0.001; Fig. 2).
Species richness of sedimentary samples was negatively correlated to altitude (P = 0.02, R2 = 0.10) and positively correlated to area (P < 0.001, R2 = 0.21), depth (P = 0.02, R2 = 0.10) and conductivity (P = 0.01, R2 = 0.12). Positive correlations were detected between littoral diatom richness and conductivity (P = 0.04, R2 = 0.10), ABT (P < 0.001, R2 = 0.22) and AST (P = 0.01, R2 = 0.18), whilst a negative correlation was found for planktic diatom richness and depth (P = 0.01, R2 = 0.17, Table 1; Fig. S2). The Shannon diversity of sedimentary diatom assemblages was positively correlated with area (P = 0.04, R2 = 0.08) and positively correlated with conductivity (P = 0.04, R2 = 0.10) for littoral diatoms. The Shannon diversity of planktic diatoms was negatively correlated to area (P < 0.001, R2 = 0.21) and depth (P < 0.001, R2 = 0.24, Table 1; Fig. S3).
β-diversity and community assembly
The β-diversities amongst the lakes were generally high (βtot: 0.925–0.964; βsor: 0.926–0.954), and the component for the balanced variation (βbal) and turnover (βsim) always accounted for the main part of the total β-diversity (Table 2).
Significant variables of the RDA models chosen by forward selection (P < 0.05) were TP, AST and depth for sedimentary and planktic diatom assemblages. Conductivity and AST had a significant effect on littoral diatoms (Table 3).
Mantel tests and negative exponential regression models produced similar results when comparing species dissimilarity with environmental, geographical and altitudinal distance (bold values in Table 4). For five combinations, either Mantel tests or regression models indicated a significant correlation (italics in Table 4). The abundance- and incidence-based dissimilarity of all assemblages increased significantly with increasing environmental distance except for planktic assemblages when using incidence data. In turn, planktic assemblages were significantly less similar with increasing lake distance, whilst for littoral diatoms, this was only true for the components representing Simpson diversity and abundance gradients. Dissimilarity of sedimentary incidence data significantly decreased with altitudinal distance (Table 4; Figs. S4–S9).
The contribution of individual species to β-diversity (SCBD) correlated well with species abundance and the number of lakes (Nlake) for all data sets using species incidence and abundance data, except for sedimentary and littoral species incidence data (Figs. S10, S11). The most abundant species had above-average SCBD-values, indicating that these species do not homogenise diatom metacommunities. Selected eigenvectors based on Hellinger-transformed abundance data were MEM 1 (Sed., P1 = 0.008), MEM 3 and 12 (Lit., P3 = 0.024, P12 = 0.041) and MEM 1, 2, 6 and 7 (Pla., P1,2,6 = 0.006, P7 = 0.042) for sedimentary, littoral and planktic assemblages, respectively. Based on incidence data MEM 3 and 5 (Sed., P3 = 0.020, P5 = 0.009), MEM 1, 2, 3 and 12 (Lit., P1 = 0.49, P2 = 0.007, P3 = 0.022, P12 = 0.009) and MEM 1, 2 and 9 (Pla., P1,9 = 0.005, P2 = 0.002) were selected for sedimentary, littoral and planktic assemblages, respectively.
Results for variation partitioning confirmed the principal patterns detected by distance decay analysis. The forward selected variables explained a significant part of variance for all assemblages. The MEMs selected had a greater explanatory power for littoral and planktic assemblages than for sedimentary diatoms. This also accounted for explained variation through shared effects. Unexplained variation was generally lower when using abundance data (Table 5).
Null models based on abundance and incidence data indicate a strong stochastic influence on littoral and planktic communities. Assembly processes were significantly different for sedimentary diatoms, showing a more balanced deterministic and stochastic influence (Table 6).
Discussion
We compared the patterns of diatom α- and β-diversity for sedimentary, littoral and planktic assemblages within a 43-lake set across the montane, subalpine and alpine region in the northern European Alps. Diatom α-diversity was correlated to altitude, lake depth and area, water temperature and conductivity. The variables explaining a significant portion of community composition were total phosphorus, AST and lake depth for sedimentary and planktic diatoms, as well as AST and conductivity for littoral diatoms. Spatial descriptors and spatial distance were important in explaining littoral and planktic diatom community structure and stochastic community assembly dominated in both groups. In contrast, deterministic and stochastic processes were equally important for sedimentary diatoms and spatial patterns were subordinate.
Niche assembly
Our hypothesis that α-diversity decreases with altitude can only be verified for sedimentary diatom richness, but not for littoral or planktic diatoms. This could be due to the fact that sedimentary diatoms integrate climatic conditions over several years, whilst littoral and planktic diatom richness are rather influenced by short-term environmental fluctuations. Other variables additionally explained the variation in α-diversity for all data sets. This underpins the high variability in local diatom diversity patterns along the elevational gradient. For example, in subarctic mountain ponds (Heikkinen et al., 2022) and streams (Teittinen et al., 2016), no significant richness elevation pattern was found, whilst an unimodal relationship was detected for a different set of subarctic ponds (Teittinen et al., 2017) and a monotonic positive relationship was found for Swiss mountain springs (Taxböck et al., 2020). Combined with these studies, our findings point to the importance of additional mechanisms that act independently of altitude in forming local diatom diversity.
The positive correlation of conductivity with littoral and sedimentary α-diversity probably reflects an influence of alkalinity, which typically correlates with conductivity in mountain lakes (Kamenik et al., 2001). This is in line with the monotonic increase of benthic diatom richness with alkalinity in US streams (Smucker & Vis, 2011), the decrease of diatom richness in fens along a gradient from calcareous to mineral-poor conditions in the western Carpathians (Fránková et al., 2009) and the increase in epilithic diatom richness with conductivity in Swiss mountain springs (Taxböck et al., 2020). Species-specific alkalinity optima (Järvinen et al., 2013) may be coupled to different HCO3− uptake efficiencies (Baattrup-Pedersen et al., 2022). Thus, it is likely that alkalinity has a direct effect on diatom α-diversity. However, we do acknowledge that the correlation of conductivity and elevation may mask a direct effect of elevation on sedimentary diatom richness, as suggested by Teittinen et al. (2017).
For sedimentary diatoms, the positive correlation of lake area and depth with α-diversity likely reflects an influence of increasing habitat diversity, which was correlated to diatom richness in streams and springs (Smucker & Vis, 2011; Taxböck et al., 2020). Sedimentary assemblages integrate diatoms from the whole lake including the pelagic and littoral zone through taphonomic processes and thus reflect the diversity of habitats that is likely to increase with lake size. For planktic diatoms, the negative correlation of α-diversity with lake depth and area probably reflects an effect of lake mixing conditions. This may be through the increased introduction of benthic species under higher mixing frequency in small and shallow lakes. In turn, deep and warm lakes, that tend to be stratified, are frequently dominated by one or only a few taxa (Ossyssek et al., 2020). Our results further show that Shannon diversity and species richness are not interchangeable as a measure of α-diversity, as they are correlated with different environmental variables in some cases. This calls for the application of both measures in diatom studies to assure better comparability. Overall, our results only give a weak indication of the elevational structuring of diatom richness in mountain lakes in the northern European Alps and reveal different processes that control α-diversity amongst sedimentary, littoral and planktic assemblages.
As hypothesised, sedimentary diatom β-diversity was mainly explained by the local environment. The results are in line with previous studies that found environmental filtering effects for sedimentary diatoms in temperate lakes, which may be attributed to the long-term integration of environmental conditions through sedimentary assemblages (Winegardner et al., 2015). The concordance of environmental predictors for sedimentary and planktic assemblage composition reflects the results of previous studies of North American lakes (Winegardner et al., 2015) and tropical reservoirs (Bartozek et al., 2019; Zorzal-Almeida et al., 2017). This indicates that the paleolimnological investigations of diatoms reliably reflect environmental constraints for pelagic diatom assemblages. AST was the sole variable correlating with β-diversity amongst all assemblages, supporting previous paleolimnological studies (Hofmann et al., 2021; Küfner et al., 2020a, b, c; Küfner et al., 2020a, b, c). Influence of AST on diatom composition may be through its influence on diatom silicification (Küfner et al., 2020a, b, c) and due to the coupling of water temperature with lake mixing patterns (Niedrist et al., 2018; Read et al., 2011). In turn, pure environmental effects were low for littoral diatoms and a shared effect of environment and altitude was more important. This suggests an influence of unmeasured variables intercorrelated with altitude and AST. The significant correlation of TP with the sedimentary and planktic diatom β-diversity reveals a sensitive reaction of the assessed assemblages towards nutrient conditions that ranged from ultraoligotrophic to mesotrophic. These findings confirm previous results, suggesting an influence of anthropogenic pressure on the share and abundance of Red List species (Ossyssek et al., 2022).
Spatial patterns
The significant correlation between spatial distance and littoral and planktic β-diversity, as well as the explained variation by the selected eigenvector maps reflect either spatial structures amongst unmeasured variables that influence diatom composition or dispersal effects (Leibold & Chase, 2018). The NTS index indicated a strong influence of stochastic processes for littoral and planktic assemblages. These include stochastic birth–death occurrence, i.e. ecological drift, dispersal processes and historical contingency (Ning et al., 2019). Ecological drift may indeed be relevant for diatoms, but this has not yet been directly proven (Remmer et al., 2019). Historical contingencies are known to influence diatom communities, but they ought to influence sedimentary assemblages even stronger than littoral and planktic diatoms (González-Trujillo et al., 2021). Thus, the NTS results suggest that dispersal processes contribute to the observed stochasticity within littoral and planktic diatoms. Dispersal limitation or mass effects can lead to spatial structures of β-diversity and stochasticity (Leibold & Chase, 2018). Evidence for a prevalence of mass effects in planktic and littoral diatom communities remain scarce and even in regions where high migration rates can be expected, only slight indication of mass effects was found (Cottenie, 2005; Michels et al., 2001). In our data set, the contribution of the most abundant species to β-diversity was above average. If mass effects influenced these species, this fact ought to lead to the homogenisation of assemblages, being associated with a lower contribution of these species to overall β-diversity. However, mass effects amongst adjacent lakes cannot be excluded, and they may also influence less abundant species (Bried & Vilmi, 2022; Leboucher et al., 2020). In our study, three pairs of lakes were within the same water basin, so they are more likely to be influenced by mass effects than the other lakes were. However, it appears unlikely that this would result in a strong stochastic effect across the whole lake set. More generally, the high overall β-diversity of planktic and littoral diatoms and the significance of the component that represents species loss (βSim) when using incidence data, further point to dispersal limitation rather than to mass effects. As a result, different evidence suggest a contribution of dispersal limitation to littoral and planktic diatom β-diversity, confirming our initial hypothesis.
Residual variation
Explained variation of variation partitioning for the sedimentary, littoral and planktic abundance data set was relatively low (9%, 9% and 22%, respectively), and it was even lower for incidence data (5%, 10% and 13%, respectively). Such a low amount of explained variation is not unusual in studies investigating diatom metacommunity structure, and it lies within the range of values reported by other studies for diatoms within Europe (6–27%) (Kernan et al., 2009; Szabó et al., 2019, 2018; Teittinen et al., 2017). The inclusion of further environmental variables may increase the explanatory power of the investigated assemblages (Soininen et al., 2021). For sedimentary and planktic diatoms, further important variables may be total organic and inorganic carbon and dissolved organic carbon (DOC) (Vinebrooke & Leavitt, 1999), which are known to be influenced by catchment variables such as land cover (Kamenik et al., 2001) and which may thus vary within our dataset that spans a variety of vegetation zones. Therefore, adding those environmental variables can potentially enhance the explanatory power of pure environmental effects or of shared effects of environment and altitude. For sedimentary diatoms, this is only likely to reinforce the principal pattern detected, namely that environmental gradients are an important driver of diatom metacommunity structure. For planktic and littoral diatoms, the importance of the environmental component may increase and pure spatial effects may decrease if additional environmental variables are spatially structured. An increase in explanatory power can be expected for littoral diatoms if the microhabitat structure is taken into account (Pla-Rabes & Catalan, 2018). More generally, interspecific interactions do shape diatom community patterns through nutrient competition (Manoylov, 2009), parasitism (Kagami et al., 2007), or grazing (Wigdahl-Perry et al., 2017). Including the composition and density of other biotic groups should also increase the explained variation.
Another factor that may help explain diatom patterns in the study lakes is that of historical legacies (Vyverman et al., 2007). These may lead to priority effects that have already been documented for ostracods (Castillo-Escrivà et al., 2017), aquatic plants (García-Girón et al., 2021) and zooplankton (Leibold et al., 2010; Mergeay et al., 2011). In our dataset, such processes may be caused by the introduction of fish (Chen et al., 2011; Hobbs et al., 2016; Ossyssek et al., 2022) or land-use changes (Martin et al., 2017). Identifying lakes that have been affected by such events and transferring the analytical scheme of this study to downcore assemblages may help interpret historical legacies, yet there are doubts about the completeness and correctness of such information, affecting the ability to track responses of biotic assemblages over time.
A methodological source of residual variation within our study may be that not all samples were collected in the same year. Some seasonal effects such as snow and duration of ice cover or longer-term weather phenomena may have had different effects in the two years of sampling. The impacts on the communities sampled will be greater for littoral and planktic diatoms because sedimentary samples cover several years of sedimentation, levelling out annual patterns. Moreover, previous events may have triggered community-level changes, leading to priority effects (Vass & Langenheder, 2017) that will also increase residual variation.
Conclusion
This study sheds light on the factors controlling diatom metacommunity structure in the mountain lakes of the Northern European Alps. In contrast to our hypothesis, elevation was of minor importance for α-diversity. In turn, alkalinity may be more important for local littoral and sedimentary diatom diversity, whilst habitat diversity and lake stratification may influence sedimentary and planktic diatom α-diversity, respectively. As expected, sedimentary diatom β-diversity was mainly controlled by environmental factors, whilst planktic and littoral diatom communities also revealed spatial constraints. Dispersal limitation likely contributed to the β-diversity patterns of littoral and planktic diatoms, substantiated by the importance of stochastic effects for both groups. In practice, the minimal spatial impact on sedimentary diatoms suggests that they are reliable bioindicators for total phosphorus, surface water temperature and lake depth, whilst the results for littoral and planktic diatoms may be confounded through stochastic assembly processes. In future studies, the inclusion of water chemical measurements of carbon compounds, the assessment of other biotic groups and historical legacies may explain increased variance.
Data availability
The datasets generated or analysed during the current study are available from the corresponding author on reasonable request.
References
Baattrup-Pedersen, A., T. J. Johnsen, S. E. Larsen & T. Riis, 2022. Alkalinity and diatom assemblages in lowland streams: How to separate alkalinity from inorganic phosphorus in ecological assessments? Science of the Total Environment 823: 153829.
Bartozek, E. C. R., A. M. da Silva-Lehmkuhl, I. Gregory-Eaves & D. C. Bicudo, 2019. Environmental and spatial drivers of diatom assemblages in the water column and surface sediment of tropical reservoirs. Journal of Paleolimnology 62(3): 245–257.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19(1): 134–143.
Baselga, A., 2017. Partitioning abundance-based multiple-site dissimilarity into components: balanced variation in abundance and abundance gradients. Methods in Ecology and Evolution 8(7): 799–808.
Baselga, A. & C. D. L. Orme, 2012. betapart: an R package for the study of beta diversity. Methods in Ecology and Evolution 3(5): 808–812.
Bauman, D., T. Drouet, S. Dray & J. Vleminckx, 2018. Disentangling good from bad practices in the selection of spatial or phylogenetic eigenvectors. Ecography 41(10): 1638–1649.
Benito, X., S. C. Fritz, M. Steinitz-Kannan, M. I. Vélez & M. M. McGlue, 2018. Lake regionalization and diatom metacommunity structuring in tropical South America. Ecology and Evolution 8(16): 7865–7878.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89(9): 2623–2632.
Blanco, S., A. Olenici, F. Ortega, F. Jiménez-Gómez & F. Guerrero, 2020. Identifying environmental drivers of benthic diatom diversity: the case of Mediterranean mountain ponds. Peerj 8: 16.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73(3): 1045–1055.
Bried, J. T. & A. Vilmi, 2022. Improved detection of mass effect species assembly for applied metacommunity thinking. Journal of Applied Ecology 59(4): 921–926.
Castillo-Escrivà, A., L. Valls, C. Rochera, A. Camacho & F. Mesquita-Joanes, 2017. Disentangling environmental, spatial, and historical effects on ostracod communities in shallow lakes. Hydrobiologia 787(1): 61–72.
CEN, 2015. Water Quality—Guidance on Quantitative and Qualitative Sampling of Phytoplankton from Inland Waters, European Committee for Standardization, Brussels:
Chen, G. J., E. Saulnier-Talbot, D. T. Selbie, E. Brown, D. E. Schindler, L. Bunting, P. R. Leavitt, B. P. Finney & I. Gregory-Eaves, 2011. Salmon-derived nutrients drive diatom beta-diversity patterns. Freshwater Biology 56(2): 292–301.
Cottenie, K., 2005. Integrating environmental and spatial processes in ecological community dynamics. Ecology Letters 8(11): 1175–1182.
DIN, 1983. Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung; Kationen (Gruppe E); Bestimmung des Ammonium-Stickstoffs (E 5), Beuth Verlag GmbH, Berlin:
DIN, 2014. Water Quality—Guidance for the Routine Sampling and Preparation of Benthic Diatoms from Rivers and Lakes, Beuth Verlag GmbH, Berlin:
Dray, S., P. Legendre & P. R. Peres-Neto, 2006. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecological Modelling 196(3–4): 483–493.
Dray, S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guenard, T. Jombart, G. Larocque, P. Legendre, N. Madi & H. Wagner, 2020. R package adespatial: tools for the multiscale spatial analysis of multivariate data.
Fránková, M., J. Bojková, A. Poulíčková & M. Hájek, 2009. The structure and species richness of the diatom assemblages of the Western Carpathian spring fens along the gradient of mineral richness. Fottea 9(2): 355–368.
García-Girón, J., M. Lindholm, J. Heino, H. Toivonen & J. Alahuhta, 2021. Historical contingency via priority effects counteracts environmental change on metacommunity dynamics across decades. Limnology and Oceanography 67: 38–53.
González-Trujillo, J. D., V. S. Saito, D. K. Petsch, I. Muñoz & S. Sabater, 2021. Historical legacies and contemporary processes shape beta diversity in Neotropical montane streams. Journal of Biogeography 48(1): 101–117.
Heikkinen, J. M., J. Aalto, O. Rantamäki, T. Ruikkala, J. Soininen & V. Pajunen, 2022. Observing diatom diversity and community composition along environmental gradients in subarctic mountain ponds. Freshwater Biology 67(4): 731–741.
Heino, J. & J. Soininen, 2005. Assembly rules and community models for unicellular organisms: patterns in diatoms of boreal streams. Freshwater Biology 50(4): 567–577.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60(5): 845–869.
Hobbs, J. M. R., W. O. Hobbs, M. B. Edlund, K. D. Zimmer, K. M. Theissen, N. Hoidal, L. M. Domine, M. A. Hanson, B. R. Herwig & J. B. Cotner, 2016. The legacy of large regime shifts in shallow lakes. Ecological Applications 26(8): 2660–2674.
Hofmann, A. M., W. Küfner, C. Mayr, N. Dubois, J. Geist & U. Raeder, 2021. Unravelling climate change impacts from other anthropogenic influences in a subalpine lake: a multi-proxy sediment study from Oberer Soiernsee (Northern Alps, Germany). Hydrobiologia 848(18): 4285–4309.
Järvinen, M., S. Drakare, G. Free, A. Lyche-Solheim, G. Phillips, B. Skjelbred, U. Mischke, I. Ott, S. Poikane, M. Søndergaard, A. Pasztaleniec, J. Van Wichelen & R. Portielje, 2013. Phytoplankton indicator taxa for reference conditions in Northern and Central European lowland lakes. Hydrobiologia 704(1): 97–113.
Kagami, M., A. de Bruin, B. W. Ibelings & E. Van Donk, 2007. Parasitic chytrids: their effects on phytoplankton communities and food-web dynamics. Hydrobiologia 578(1): 113–129.
Kamenik, C., R. Schmidt, G. Kum & R. Psenner, 2001. The influence of catchment characteristics on the water chemistry of mountain lakes. Arctic Antarctic and Alpine Research 33(4): 404–409.
Keck, F., A. Franc & M. Kahlert, 2018. Disentangling the processes driving the biogeography of freshwater diatoms: a multiscale approach. Journal of Biogeography 45(7): 1582–1592.
Kernan, M., M. Ventura, P. Bitušík, A. Brancelj, G. Clarke, G. Velle, G. G. Raddum, E. Stuchlík & J. Catalan, 2009. Regionalisation of remote European mountain lake ecosystems according to their biota: environmental versus geographical patterns. Freshwater Biology 54(12): 2470–2493.
Krammer, K., 2000. Diatoms of the European Inland Waters and Comparable Habitats: Pinnularia, G. Gartner Verlag K.G, A.R:
Krammer, K., 2002. Diatoms of the European Inland Waters and Comparable Habitats: Cymbella, G. Gartner Verlag K.G, A.R:
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae, 3, Centrales, Fragilariaceae, Eunotiaceae. Gustav Fischer Verlag, Teil:
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae, 4. Teil: Achnanthaceae, kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gustav Fischer Verlag.
Krammer, K. & H. Lange-Bertalot, 1997a. Bacillariophyceae, 1, Naviculaceae. Gustav Fischer Verlag, Teil:
Krammer, K. & H. Lange-Bertalot, 1997b. Bacillariophyceae, 2, Bacillariaceae, Epithemiaceae, Surirellaceae. Gustav Fischer Verlag, Teil:
Küfner, W., A. Hofmann, S. Ossyssek, N. Dubois, J. Geist & U. Raeder, 2020a. Composition of highly diverse diatom community shifts as response to climate change: a down-core study of 23 central European mountain lakes. Ecological Indicators 117: 106590.
Küfner, W., A. M. Hofmann, J. Geist & U. Raeder, 2020b. Evaluating climate change impacts on mountain lakes by applying the new silicification value to paleolimnological samples. Science of the Total Environment 715: 12.
Küfner, W., S. Ossyssek, J. Geist & U. Raeder, 2020c. The silicification value: a novel diatom-based indicator to assess climate change in freshwater habitats. Diatom Research 35(1): 1–16.
Lange-Bertalot, H., 2001. Diatoms of the european inland waters and comparable habitats: Navicula sensu stricto, 10 genera separated from Navicula sensu stricto, A.R.G. Gartner Verlag K.G, Frustulia:
Lange-Bertalot, H., G. Hofmann, M. Werum & M. Cantonati, 2017. Freshwater benthic diatoms of Central Europe. Over 800 common species used in ecological assessment. Koeltz Botanical Books.
Leboucher, T., J. Tison-Rosebery, W. Budnick, A. Jamoneau, W. Vyverman, J. Soininen, S. Boutry & S. I. Passy, 2020. A metacommunity approach for detecting species influenced by mass effect. Journal of Applied Ecology 57(10): 2031–2040.
Legendre, P. & M. De Caceres, 2013. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16(8): 951–963.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129(2): 271–280.
Leibold, M. A. & J. M. Chase, 2018. Metacommunity ecology, Vol. 59. Princeton University Press:
Leibold, M. A., E. P. Economo & P. Peres-Neto, 2010. Metacommunity phylogenetics: separating the roles of environmental filters and historical biogeography. Ecology Letters 13(10): 1290–1299.
Manoylov, K. M., 2009. Intra- and interspecific competition for nutrients and light in diatom cultures. Journal of Freshwater Ecology 24(1): 145–157.
Martin, S. L., D. B. Hayes, A. D. Kendall & D. W. Hyndman, 2017. The land-use legacy effect: towards a mechanistic understanding of time-lagged water quality responses to land use/cover. Science of the Total Environment 579: 1794–1803.
Mergeay, J., L. De Meester, H. Eggermont & D. Verschuren, 2011. Priority effects and species sorting in a long paleoecological record of repeated community assembly through time. Ecology 92(12): 2267–2275.
Michels, E., K. Cottenie, L. Neys & L. De Meester, 2001. Zooplankton on the move: first results on the quantification of dispersal of zooplankton in a set of interconnected ponds. Hydrobiologia 442(1–3): 117–126.
Murphy, J. & J. P. Riley, 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36.
Naimi, B., 2017. R Package usdm: Uncertainty Analysis for Species Distribution Models.
Navone, R., 1964. Proposed method for nitrate in potable waters. American Water Works Association 56(6): 781–783.
Niedrist, G. H., R. Psenner & R. Sommaruga, 2018. Climate warming increases vertical and seasonal water temperature differences and inter-annual variability in a mountain lake. Climatic Change 151(3–4): 473–490.
Ning, D. L., Y. Deng, J. M. Tiedje & J. Z. Zhou, 2019. A general framework for quantitatively assessing ecological stochasticity. Proceedings of the National Academy of Sciences of the United States of America 116(34): 16892–16898.
Nixdorf, B., E. Hoehn, U. Riedmüller, U. Mischke & I. Schönfelder, 2014. Probenahme und Analyse des Phytoplanktons in Seen und Flüssen zur Ökologischen Bewertung gemäß der EU-WRRL, in Hupfer M. & H. Fischer, eds. Handbuch Angewandte Limnologie: Grundlagen ‐ Gewässerbelastung ‐ Restaurierung ‐ Aquatische Ökotoxikologie ‐ Bewertung ‐ Gewässerschutz. Weinheim:Wiley
Ossyssek, S., J. Geist, P. Werner & U. Raeder, 2020. Identification of the ecological preferences of Cyclotella comensis in mountain lakes of the northern European Alps. Arctic Antarctic and Alpine Research 52(1): 512–523.
Ossyssek, S., A. M. Hofmann, J. Geist & U. Raeder, 2022. Diatom red list species reveal high conservation value and vulnerability of mountain lakes. Diversity 14(5): 389.
Pla-Rabes, S. & J. Catalan, 2018. Diatom species variation between lake habitats: implications for interpretation of paleolimnological records. Journal of Paleolimnology 60(2): 169–187.
R Core Team, 2013. R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna:
Read, J. S., D. P. Hamilton, I. D. Jones, K. Muraoka, L. A. Winslow, R. Kroiss, C. H. Wu & E. Gaiser, 2011. Derivation of lake mixing and stratification indices from high-resolution lake buoy data. Environmental Modelling & Software 26(11): 1325–1336.
Remmer, C. R., C. D. Robichaud, H. Polowyk & R. Rooney, 2019. The role of ecological drift in structuring periphytic diatom communities. Journal of Freshwater Ecology 34(1): 363–377.
Shurin, J. B., K. Cottenie & H. Hillebrand, 2009. Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia 159(1): 151–159.
Smucker, N. J. & M. L. Vis, 2011. Contributions of habitat sampling and alkalinity to diatom diversity and distributional patterns in streams: implications for conservation. Biodiversity and Conservation 20(3): 643–661.
Soininen, J., R. McDonald & H. Hillebrand, 2007. The distance decay of similarity in ecological communities. Ecography 30(1): 3–12.
Soininen, J., V. Tupola, I. Voutilainen, M. Cantonati & A. Teittinen, 2021. Diatom biogeography in freshwaters - new insights from between-region comparisons and the role of unmeasured environmental factors. Diatom Research.
Spellerberg, I. F. & P. J. Fedor, 2003. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the “Shannon-Wiener” Index. Global Ecology and Biogeography 12(3): 177–179.
Stirling, G. & B. Wilsey, 2001. Empirical relationships between species richness, evenness, and proportional diversity. American Naturalist 158(3): 286–299.
Szabó, B., E. Lengyel, J. Padisák, M. Vass & C. Stenger-Kovács, 2018. Structuring forces and β-diversity of benthic diatom metacommunities in soda pans of the Carpathian Basin. European Journal of Phycology 53(2): 219–229.
Szabó, B., E. Lengyel, J. Padisák & C. Stenger-Kovács, 2019. Benthic diatom metacommunity across small freshwater lakes: driving mechanisms, β-diversity and ecological uniqueness. Hydrobiologia 828(1): 183–198.
Taxböck, L., D. N. Karger, M. Kessler, D. Spitale & M. Cantonati, 2020. Diatom species richness in swiss springs increases with habitat complexity and elevation. Water 12(2): 449.
Teittinen, A., L. Kallajoki, S. Meier, T. Stigzelius & J. Soininen, 2016. The roles of elevation and local environmental factors as drivers of diatom diversity in subarctic streams. Freshwater Biology 61(9): 1509–1521.
Teittinen, A., J. J. Wang, S. Strömgård & J. Soininen, 2017. Local and geographical factors jointly drive elevational patterns in three microbial groups across subarctic ponds. Global Ecology and Biogeography 26(8): 973–982.
Throndsen, R., 1978. Monographs on Oceanographic Methodology, Phytoplankton Manual : Preservation and storage, UNESCO, Paris:
van der Werff, A. & T. T. Macan, 1955. A new method of concentrating and cleaning diatoms and other organisms. Proceedings of the International Association of Theoretical and Applied Limnology (SIL) 12(2): 276–277.
Vass, M. & S. Langenheder, 2017. The legacy of the past: effects of historical processes on microbial metacommunities. Aquatic Microbial Ecology 79(1): 13–19.
Vilmi, A., S. M. Karjalainen & J. Heino, 2017. Ecological uniqueness of stream and lake diatom communities shows different macroecological patterns. Diversity and Distributions 23(9): 1042–1053.
Vinebrooke, R. D. & P. R. Leavitt, 1999. Phytobenthos and phytoplankton as potential indicators of climate change in mountain lakes and ponds: a HPLC-based pigment approach. Journal of the North American Benthological Society 18(1): 15–33.
Vyverman, W., E. Verleyen, K. Sabbe, K. Vanhoutte, M. Sterken, D. A. Hodgson, D. G. Mann, S. Juggins, B. Van de Vijver, V. Jones, R. Flower, D. Roberts, V. A. Chepurnov, C. Kilroy, P. Vanormelingen & A. De Wever, 2007. Historical processes constrain patterns in global diatom diversity. Ecology 88(8): 1924–1931.
Wang, J. J., P. Legendre, J. Soininen, C. F. Yeh, E. Graham, J. Stegen, E. O. Casamayor, J. Z. Zhou, J. Shen & F. Y. Pan, 2020. Temperature drives local contributions to beta diversity in mountain streams: Stochastic and deterministic processes. Global Ecology and Biogeography 29(3): 420–432.
Wei, T. & V. R. Simko, 2017. Package “corrplot”: Visualization of a Correlation Matrix.
Whittaker, R. H., 1972. Evolution and Measurement of Species Diversity. Taxon 21(2/3): 213–251.
Wickham, H. & R. François, 2014. dplyr: A Grammar of Data Manipulation.
Wigdahl-Perry, C. R., J. E. Saros, S. C. Fritz & C. T. Hess, 2017. Investigating potential effects of zooplankton grazing on diatom-inferred drought reconstructions. Hydrobiologia 786(1): 149–165.
Wilcoxon, F., 1945. Individual comparisons by ranking methods. Biometrics Bulletin 1(6): 80–83.
Winegardner, A. K., B. E. Beisner, P. Legendre & I. Gregory-Eaves, 2015. Are the landscape-level drivers of water column and surface sediment diatoms different? Freshwater Biology 60(2): 267–281.
Zorzal-Almeida, S., J. Soininen, L. M. Bini & D. C. Bicudo, 2017. Local environment and connectivity are the main drivers of diatom species composition and trait variation in a set of tropical reservoirs. Freshwater Biology 62(9): 1551–1563.
Acknowledgements
We thank our many students who helped with fieldwork, the hydro-chemical analysis of water samples and the microscopical analysis of diatom samples.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a scholarship from the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, DBU) to S.O. (Grant 20016/434).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Judit Padisák
We dedicate this publication to our colleague and friend Dr Wolfgang Kuefner, who died in a fatal accident during a ski tour in the mountains shortly after successfully passing his PhD. As a member of our mountain lake team, he actively supported us in our fieldwork and was a valuable discussion partner whom we miss very much.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10750_2022_5103_MOESM1_ESM.tiff
Fig. S1 Correlation plot of variables with VIF’s < 5 for sedimentary (a), littoral (b) and planktic (c) assemblages. Colour range indicates the correlation coefficient and asterisk indicate P-value: *** ≙ P-value < 0.001; ** ≙ P-value < 0.01; * ≙ P-value < 0.05. Supplementary file1 (TIFF 42187 kb)
10750_2022_5103_MOESM2_ESM.tiff
Fig. S2 Linear regression models for sedimentary (n=43), littoral (n=34) and planktic (n=32) Shannon diversity along environmental gradients. P indicates P-values of linear regression models, and R2 gives the multiple R2-values of these models. For abbreviations, see Table S1. Supplementary file2 (TIFF 31640 kb)
10750_2022_5103_MOESM3_ESM.tiff
Fig. S3 Linear regression models for sedimentary (n=43), littoral (n=34) and planktic (n=32) species richness along environmental gradients. P indicates P-values of linear regression models, and R2 gives the multiple R2-values of these models. For abbreviations, see Table S1. Supplementary file3 (TIFF 12656 kb)
10750_2022_5103_MOESM4_ESM.tiff
Fig. S4 Decay of pairwise β-diversity (βsor) and its components for turnover and nestedness (βsim, βsne) for incidence data for sedimentary, littoral and planktic diatom assemblages along with increasing environmental distance (represented by the significant parameters identified in RDA backward selection for each assemblage). For combinations with significant Mantel statistics (P < 0.05), regression lines are drawn for exponential regression models; model statistics of regression models are given for all combinations. Supplementary file4 (TIFF 85429 kb)
10750_2022_5103_MOESM5_ESM.tiff
Fig. S5 Decay of pairwise β-diversity (βtot) and its components for balanced variation and abundance gradients (βbal, βgrad) for abundance data for sedimentary, littoral and planktic diatom assemblages along with increasing environmental distance. For combinations with significant Mantel statistics (P < 0.05), regression lines are drawn for exponential regression models; model statistics of regression models are given for all combinations. Supplementary file5 (TIFF 85429 kb)
10750_2022_5103_MOESM6_ESM.tiff
Fig. S6 Decay of pairwise β-diversity (βsor) and its components for turnover and nestedness (βsim, βsne) for incidence data for sedimentary, littoral and planktic diatom assemblages along with increasing geographical distance. For combinations with significant Mantel statistics (P < 0.05), regression lines are drawn for exponential regression models; model statistics of regression models are given for all combinations. Supplementary file6 (TIFF 85429 kb)
10750_2022_5103_MOESM7_ESM.tiff
Fig. S7 Decay of pairwise β-diversity (βtot) and its components for balanced variation and abundance gradients (βbal, βgrad) for abundance data for sedimentary, littoral and planktic diatom assemblages along with increasing geographical distance. For combinations with significant Mantel statistics (P < 0.05), regression lines are drawn for exponential regression models; model statistics of regression models are given for all combinations. Supplementary file7 (TIFF 85429 kb)
10750_2022_5103_MOESM8_ESM.tiff
Fig. S8 Decay of pairwise β-diversity (βsor) and its components for turnover and nestedness (βsim, βsne) for incidence data for sedimentary, littoral and planktic diatom assemblages along with increasing altitudinal distance. For combinations with significant Mantel statistics (P < 0.05), regression lines are drawn for exponential regression models; model statistics of regression models are given for all combinations. Supplementary file8 (TIFF 85429 kb)
10750_2022_5103_MOESM9_ESM.tiff
Fig. S9 Decay of pairwise β-diversity (βtot) and its components for balanced variation and abundance gradients (βbal, βgrad) for abundance data for sedimentary, littoral and planktic diatom assemblages along with increasing altitudinal distance. For combinations with significant Mantel statistics (P < 0.05), regression lines are drawn for exponential regression models; model statistics of regression models are given for all combinations. Supplementary file9 (TIFF 85429 kb)
10750_2022_5103_MOESM10_ESM.tiff
Fig. S10 Influence on the values of species contributions to β-diversity (“SCBD”) investigated for species total abundance (“abundance”) and number of occupied sites (“N_lake”) for abundance data. Model statistics for linear models with polynomial terms: k = number of polynomial terms, P = P-value, R2adj = adjusted R2-value. Supplementary file10 (TIFF 37968 kb)
10750_2022_5103_MOESM11_ESM.tiff
Fig. S11 Influence on the values of species contributions to β-diversity (“SCBD”) investigated for species total abundance (“abundance”) and number of occupied sites (“N_lake”) for incidence data. Model statistics for linear models with polynomial terms: k = number of polynomial terms, P = P-value, R2adj = adjusted R2-value. Supplementary file11 (TIFF 37968 kb)
10750_2022_5103_MOESM12_ESM.xlsx
Table S1 Metrics characterising the measured environmental variables of the 43-lake set. Supplementary file12 (XLSX 10 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ossyssek, S., Hofmann, A.M., Geist, J. et al. Sedimentary, littoral and planktic diatoms show different diversity patterns and assembly mechanisms in mountain lakes of the northern European Alps. Hydrobiologia 850, 1941–1954 (2023). https://doi.org/10.1007/s10750-022-05103-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05103-4