Abstract
Recruitment of mussels is a complex process with the successful arrival of individuals hinging on the availability of suitable habitats. We examined the effects of adult mussels as settlement habitat and the degree to which the suitability of habitat they offer is species-specific by comparing the recruitment success of intertidal mussels. We hypothesised that mussel recruitment and early growth are dictated by the quality of habitat offered by conspecifics adults. We used a unique experimental arena on the south coast of South Africa, where Mytilus galloprovincialis and two lineages of Perna perna co-exist. Treatments were based on the translocation of individuals of M. galloprovincialis, western- and eastern lineage of P. perna to a single site, where artificial beds were created and sampled monthly over one year. Recruit’s number, their sizes and growth were greater within beds of the western lineage of Perna than eastern lineage or Mytilus beds. The results clearly demonstrate that the quality of settlement habitat offered by adult beds differs among adult lineages/species and affects rates of settlement and the early growth of recruits. This effect extends to the intraspecific level; we found greater differences in density and growth of recruits between lineages of Perna than between either lineage and M. galloprovincialis.
Similar content being viewed by others
Introduction
Conceptually, the geographic limit of a species’ distribution represents the point where the species cannot balance recruitment and mortality, reflecting the effects of both physical and biological factors (Godsoe et al., 2017; Angert et al., 2020). While understanding the role of physiological limitation in setting distributional limits is often the first consideration for investigations of species distributions (Kroeker & Sanford, 2022), the importance of recruitment success is less well understood. Recruitment is a determinant factor influencing population increase or decrease and depends on a number of biotic and environmental factors (Roughgarden et al., 1988). Populations can be either closed or open in terms of recruitment, and for species with highly dispersive propagules (Cowen et al., 2000), this is strongly regulated by the physical parameters influencing dispersal (Haye et al., 2014; Weidberg et al., 2018). Furthermore, such factors can operate at a wide variety of spatial and temporal scales (Gaines & Roughgarden, 1985; Kinlan et al., 2005). The regulation of recruit dispersal is particularly complex for benthic marine organisms with planktonic propagules and effective dispersal is a function of the duration of planktonic life as well as hydrodynamics (Jenkins et al., 2000; Shanks, 2009). For shallow water and intertidal animals, hydrodynamic effects operate at scales ranging from hundreds of kilometres to centimetres (Keith et al., 2011). In addition to delivery to habitable areas, successful recruitment depends on the presence of suitable settlement habitat (Pinsky et al., 2012), for example soft versus hard substratum (Herbert & Hawkins, 2006). In many cases, larvae are chemically attracted to adult populations and for habitat-forming organisms such as many sedentary filter-feeders, the adults themselves act as a settlement site (Navarrete & Castilla, 1990; Jenkins, 2005; Tamburri et al., 2007).
We examined the degree to which the suitability of settlement habitat offered by the adults of ecological engineers is species-specific by comparing recruitment success among one invasive species of intertidal mussel and two distinct genetic lineages of a native mussel species. The native mussel Perna perna is a warm-temperate to subtropical species that exists as distinct eastern and western genetic lineages (referred to here for convenience as species) along the coast of South Africa (Zardi et al., 2007a, b, 2011; Ntuli et al., 2020). The two lineages have different evolutionary histories and different temperature tolerances. The eastern lineage exhibits better survival at high air temperatures (Nicastro et al., submitted), and western lineage individuals translocated to the subtropical east coast suffer higher mortality than eastern lineage individuals with no difference between lineages when translocated to the west (Zardi et al., 2011). The two lineages do, however, co-exist over approximately 200 km of shoreline (Zardi et al., 2007b). The second species is the temperate invasive Mediterranean mussel, Mytilus galloprovincialis, which dominates mussel populations on the west coast of the country and extends around the Cape of Good Hope as far east as the region of overlap of the two lineages of P. perna (Zardi et al., 2018; Fig. 1). Biogeographic transition zones appear to act as barriers to the spread of M. galloprovincialis and, while small individuals can be found farther east than the overlap zone, they are extremely rare and the distributional limits of the species within South Africa have been stable for decades (Ma et al., 2021). Over much of the south coast, the western lineage of P. perna and M. galloprovincialis co-exist, P. perna dominating the lower mussel zone and M. galloprovincialis the upper, with mixed beds in the middle (Bownes & McQuaid, 2006). This reflects the outcome of complex interactions involving competition and facilitation (Rius & McQuaid, 2006, 2009; McQuaid et al. 2015). Although Bayne (1964) hypothesised that larval mussels settle first onto macroalgae and later undergo detachment and secondary relocation to adult mussel beds, this seems not to be the case for P. perna (Lasiak & Barnard, 1995; Erlandsson & McQuaid, 2004; Reaugh et al., 2007). Rather, this species recruits directly onto adult beds, suffering a high degree of cannibalism by adults in the process (Porri et al., 2008a). There are well known differences in the conditions within beds of different mussel species (Helmuth, 1999; Cole, 2010), and in our case these include the density of byssal threads and the degree to which adults exhibit gaping behaviour (i.e. a periodic closure and opening of the shell), which differ between P. perna and M. galloprovincialis and also between the two lineages of Perna (Nicastro et al., 2010a, b, 2012). Gaping behaviour decreases in the order P. perna eastern lineage > P. perna western lineage > M. galloprovincialis and results in a reduction in body temperature through evaporative cooling, but at the cost of water loss. Gaping also results in higher humidity within mussel beds, influencing the structure and composition of the infaunal community (Nicastro et al., 2020).
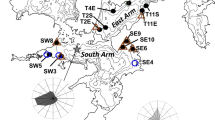
Distributional ranges of Perna lineages (western and eastern) and M. galloprovincialis and sampling sites on the South African coastline. Arrows indicate translocation of individuals of mussels from Nature Valley (NV), i.e. western Perna lineage and M. galloprovincialis; and from Port St Johns (PJ), i.e. eastern Perna lineage, to Old Woman's River (OW; dashed square). Modified figure from Nicastro et al. (2020)
Our aim was to test whether mussel bed identity affects the abundance, size and growth of mussel recruits, in a region where each species approaches the geographic limits of its adult distribution. To do this, we set up artificial mussel beds by translocating individuals from populations at the centre of each species/lineage distribution to a single site at the overlapping range edges. We then destructively sampled artificial beds on a monthly basis to measure the numbers and sizes of recruits. Additionally, this allowed us to test for a preference of settlers for conspecific adults.
Methods
Study site
The study site occurs in an overlap zone where M. galloprovincialis and both lineages of P. perna have co-existed as healthy populations, showing regular recruitment and reproduction at the study site for over 15 years (Robinson et al., 2005; Rius & McQuaid, 2006). Artificial mussel beds were deployed at Old Woman's River (hereafter OW) on the warm-temperate south coast of South Africa (Fig. 1). Shores at OW are mostly flat rock, with gently sloping sandstone platforms and uniform topography (Nicastro et al., 2020). OW is situated within the contact area of the two genetic lineages of P. perna (Zardi et al., 2007b, 2011; Ntuli et al., 2020), where individuals from both eastern and western lineages as well as M. galloprovincialis naturally co-occur as range edges populations (Fig. 1).
Thermal conditions at OW
To characterise intertidal thermal conditions at OW, three temperatures loggers were deployed roughly 10 m apart within the experimental area (≤ 0.1°C precision, ≤ 0.2°C accuracy, and 0.5°C temperature resolution; Envlogger, ElectricBlue CRL, Portugal). Loggers were set to record temperatures hourly and were deployed to capture thermal conditions experienced by translocated mussels (see below) during the study period of June 2019 to September 2020. We calculated the effective shore level (ESL), which is an estimate of the height where loggers were placed (Harley & Helmuth, 2003; Gilman et al., 2006). To calculate the mean ESL and its standard deviation (SD), we identified all sudden drops (≥ 16.5°C/h) in temperature during incoming tides, corresponding to the moment when loggers are immersed with cold sea water, and retrieved corresponding tide levels from published tables (Gilman et al., 2006). We used a threshold of 16.5°C/h, which corresponds to a drop of 5.5°C in 20 min, to avoid false positives (Gilman et al., 2006). Using the estimated ESL along with predicted tide levels (hourly predictions at East London, c. 80 km distant, were obtained from the South African Navy), we defined periods of submergence when tide levels > mean ESL and emergence when tide levels < mean ESL. Note that the ESL can vary daily due to surge and wave exposure and predicted tide levels may not match perfectly realised ones due to the effects of winds, atmospheric pressure, or shore topography (Gilman et al., 2006). To avoid the risk of misidentifying periods of emergence or submergence given these sources of uncertainty, we excluded all temperature data that occurred when tide levels fell within the following buffer zone: mean ESL (− SD) < tide levels < mean ESL (+ SD). After hourly temperatures were attributed to periods of submergence or emergence, we calculated monthly temperature means, SDs, minima and maxima, over the study period.
Translocated mussel beds
A manipulative experiment was set up as described by Nicastro et al. (2020). The experimental design consisted of three treatments: (1) 100% western lineage P. perna (hereafter western Perna bed), (2) 100% eastern lineage P. perna (hereafter eastern Perna bed), and (3) 100% M. galloprovincialis (hereafter Mytilus bed). In late-August 2019, individuals of P. perna were collected from one of two locations outside the overlap zone, as described by Zardi et al. (2007a, b) to ensure pure genetic lineages, i.e. Port St Johns (PJ, eastern lineage; Fig. 1) and Nature’s Valley (NV, western lineage; Fig. 1). Since M. galloprovincialis occurs at low numbers at OWR compared to P. perna abundance (Nicastro et al., 2020; Ma et al., 2021), individuals of the former were collected at the same locations as the western lineage of P. perna, NV (Fig. 1). After collection, mussels were acclimated in tanks in a controlled environment room set at 20°C (± 0.5°C) for 12 h before deployment in the field. Individuals were carefully scrubbed and cleaned of fouling organisms.
Each treatment consisted of mussels (3–5 cm in shell length) placed in 15 × 15 cm metal frames, secured by large washers and screws into rawl plugs in pre-drilled holes. Each quadrat had 100% mussel cover (i.e. n = 30 individuals) representing the natural density of mussel patches at OW (McQuaid & Mostert, 2010). To eliminate possible effects of zonation, each artificial mussel bed was placed in a flat area cleared at the mid-shore levels 60–100 cm from the nearest natural patch, where individuals of P. perna and M. galloprovincialis are mixed in the intertidal zone (Bownes & McQuaid, 2006). To avoid loss of mussels due to wave action or storm events, each quadrat was covered by a strong plastic mesh (16 mm mesh size). The number of mussels was checked every month for one-year period with mesh replacement every 3 months to avoid biofouling effects on all quadrats (e.g. Ulvales growth; first change of meshes made in December 2019). Mortality of translocated adults was < 20% for all treatments with nonsignificant effects among treatments (P > 0.05).
Density and size of recruits on different mussel beds
To evaluate the number and size of recruits of P. perna (hereafter Perna recruits) and M. galloprovincialis (hereafter Mytilus recruits), artificial mussel beds (number of replicates = 5) of each experimental treatment (i.e. western Perna bed, eastern Perna bed and Mytilus bed) were sampled by collecting the whole metal frame monthly for 12-month period starting September 2019 (n = 60 per treatment), with the exception between April and July 2020. This was because of the South African government declaring a National State of Disaster and a rigorous lockdown due to the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic, making it impossible to sample during those months.
Sampled mussel beds were preserved in ethanol, then washed and separated through a 0.5 mm mesh sieve. While the recruits of Mytilus and Perna can be differentiated morphologically (Bownes et al., 2008), it was not possible to separate recruits of the two lineages of Perna. Therefore, recruits were only identified as Perna or Mytilus. All recruits of mussels > 0.5 mm length in each replicate were identified, counted and measured (to the nearest 0.1 mm) under a binocular microscope (× 5 magnification).
Growth patterns of P. perna recruits
Growth estimates were not possible for Mytilus recruits due the low numbers found. Therefore, we focused on estimating growth rates for Perna recruits. Von Bertalanffy growth (VBG) curves were fitted to the length-frequency data for Perna recruits for each experimental translocation, following the methods described in Mildenberger et al. (2017). As we focus on growth patterns of mussels before adulthood in the present study, we excluded all data for animals > 15 mm in maximum length from our length-frequency dataset prior to curve fitting because individuals of P. perna can reach sexual maturity at approximately that size (Kaehler & McQuaid, 1999; McQuaid & Lindsay, 2000). The VBG parameters were estimated from the original equation:
where Lt is the length at time t, L∞ is the theoretical maximum length that species would reach, the K parameter is a growth coefficient estimating how fast the individual approaches L∞ and t0 is the theoretical age at zero length. Note that the parameter t0 is referred to as tanchor and describes the fraction of the year (defined between 0 and 1) where annually repeating growth curves cross length equal to zero (see Mildenberger et al., 2017 for details). In other words, tanchor can be interpreted as the recruitment time because growth is related to time rather than age in this context. For instance, if tanchor = 0.25, it suggests that eggs were theoretically fertilised on the 1st of April.
The TropFishR package was used to estimate von Bertalanffy parameters in the CRAN R project environment (R Core Team 2019, v4.0.1; Mildenberger et al., 2017), which is applicable to any species with length–frequency data for estimations of growth rates (see Schwamborn et al., 2019; Barbosa et al., 2021 for details). Following Mildenberger et al. (2017), we obtained a first estimation of L∞ and its confidence interval using the Powell–Wetherall approach (Wetherall et al., 1987). Then, we used the Electronical LEngth-Frequency ANalysis (ELEFAN) method with a genetic algorithm to refine estimates of tanchor, L∞ and K (Taylor & Mildenberger, 2017). To ensure that the best solution was achieved (after visual examination of the optimisation process), we ran the Genetic Algorithm with the following settings: length-frequency data restructured with moving average = 9, population size = 1,000, maximum number of generations = 1,000, maximum number of generations without improvement = 500, probability of mutation in the parent gene = decreasing over the generations with an initial probability of 0.5 and a limiting probability of 0.1 (Mildenberger et al., 2017; Taylor & Mildenberger, 2017; see also help of the function ELEFAN_GA in the R package for further details on the parameters). As information on L∞ and K is already available from the literature (max L∞ = 117.44 mm and min K = 0.31 from McQuaid & Lindsay, 2000), we restricted the search for L∞ within 5 and 117.5 mm and for K within 0.3 and 3. We obtained confidence intervals for tanchor, L∞ and K using the Jack Knife technique, which is a resampling method with replacement to estimate the variance (Quenouille, 1956; Tukey, 1958), as recommended by Mildenberger et al. (2017).
Data analyses
To determine whether density and size (maximum length) of Perna and Mytilus recruits differed among experimental treatments (i.e. western Perna bed, eastern Perna bed and Mytilus bed), generalised linear model (GLM) analyses were performed for each dependent variable.
The GLM for density of recruits was performed using Gaussian (link ‘identity’) and including two fixed factors: (1) experimental treatment (i.e. the artificial beds), with three levels: western bed, eastern bed and Mytilus bed; and (2) Species, with two levels: Perna and Mytilus. Size frequency distributions of Perna and Mytilus recruits were compared among experimental treatments using pairwise Kolmogorov–Smirnov tests (α < 0.05). An additional GLM was made by including Season (i.e. spring, summer, autumn and winter) as a fixed factor for each dependent variable using Poisson (link ‘log’) family error for density and Gamma (link ‘identity’) family error for size of recruits, respectively.
For model selection, we used backward method whereby interactions and single terms were removed sequentially, one at a time. Then, models were compared and ranked by using the corrected Akaike Information Criterion (AICc), which evaluates the quality of the fit that penalises for overfitting too many parameters, with a correction to account for finite sample size (Akaike, 1974; Burnham & Anderson, 2002). The lowest AICc value was compared with other models using the differences between AICc scores (i.e. ΔAICc). Models with ΔAICc < 2 were considered as competitive and, for the sake of parsimony, the model with the least number of parameters was retained. Diagnostic plots (i.e. histograms of residuals and residuals vs. linear predictor) to evaluate both model fitness and statistical assumptions of residuals were used (Zuur et al., 2010). For each response variable, when interaction terms were significant (α < 0.05), Tukey post hoc tests were carried out for multiple pairwise comparisons. All statistical analyses were made in the CRAN R project environment (R Core Team 2019, v4.0.1). We used the glmmTMB package (i.e. GLMMs using Template Model Builder) to fit both density and size datasets. Tukey’s post hoc tests were carried out using the emmeans package [estimated marginal means (least-squares means)].
Results
Thermal conditions at OW
Maximum and minimum air temperatures were recorded during emergence time and were between 8.3 and 48.2°C (mean = 19.06 ± 5.5). In contrast, seawater temperatures during submergence time exhibited a narrower range (i.e. between 12.5 and 22.8°C, mean = 17.4 ± 1.6). Higher temperatures during emergence time were seen between September 2019 and March 2020, with the highest variability in January and February 2020 (Fig. 2). Conversely, lower and less variables temperatures were recorded between July and August 2019 at the same time before the 1st collection of experimental beds (September 2019; Fig. 2).
Mean (+ SD), minima and maxima of rock temperature at both emergence and submergence times recorded in the experimental area at Old Woman's River between June 2019 and September 2020 (n = 3). Grey and black arrows on the x-axis indicate thermal conditions before the experiment started, and during the sampling period, respectively
Density and size of recruits on different mussel beds
Comparison with earlier studies is difficult because of differences in experimental design, but recruitment of Mytilus was unexpectedly low. Numbers of recruits were an order of magnitude lower for Mytilus than Perna across all months and all treatments (Fig. 1SI). Mytilus beds showed no effect of treatment on recruit densities, while for Perna beds there was a clear treatment effect (Fig. 3); numbers of recruits were greater in beds of the western Perna than in the other two treatments, i.e. eastern Perna bed and Mytilus bed (AICc = 1765.66, GLM output, Treatment × Species: Estimate = 6.82, SE = 1.87, t-value = 3.65, P < 0.05; Tukey post hoc test, P < 0.05).
Boxplot of density (No ind. per 0.026 m2) of recruits per species of mussels (Myt Mytilus and Per Perna) found in three experimental treatments: western bed (a), eastern bed (b) and Mytilus bed (c) between September 2019 and August 2020. Letters indicate significant differences found within and among treatments (Tukey post hoc test; P < 0.05). Lines extending from the boxes (whiskers) indicate minimum and maximum values excluding outliers, which are above the whiskers. Horizontal black lines inside boxes indicate the median values of each group. From those lines, the first and third quartiles on boxes (lower and upper quartiles, respectively) are the median of the lower and upper half of the dataset, respectively
There were no significant effects on recruit densities of either the main factors Treatment, Species and Seasons or their three-way interaction (P > 0.05, Fig. 4). However, there were significant two-way interactions between the factors: Treatment × Species, Season × Species, and Treatment × Season (AICc = 1,523.86; P < 0.05; Table 1S). The significant interaction between Treatment × Species reflects the low numbers of Mytilus recruits across treatments (GLM output: Estimated = 1.15, SE = 0.25, z-value = 4.52, P < 0.05). The interaction between Season × Species was due to differences between species in the seasonality of recruitment (Fig. 4; GLM output: Estimated = − 1.04, SE = 0.35, z-value = − 2.92, P = 0.003). Recruitment of mussel species showed differences between summer and spring (Fig. 4; Table 1S, Tukey’s post hoc test, P < 0.05) with Perna recruits showing the highest peak into the western beds in summer between January and February 2021 (Fig. 4a–c; Fig. 1SI). Density Perna recruits also showed differences between summer and autumn (Table 1S; Tukey’s post hoc test, P < 0.05) that were not identified for Mytilus recruits (Fig. 4). Finally, the interaction between Season and Treatment also indicated differences in density of recruits (GLM output: Estimated = 0.59, SE = 0.24, z-value = 2.42, P = 0.015). Recruitment into the western beds was higher during summer than spring (Fig. 4a; Table 1S, Tukey’s post hoc test, P < 0.05). For eastern beds, higher recruitment was recorded during summer and winter (June–August; Fig. 1SI) than spring and autumn (Fig. 4b, Tukey’s post hoc test, P < 0.05). Density of recruits of mussels did not show a clear seasonal pattern in Mytilus beds (Fig. 4c).
Boxplot of density (No. ind. per 0.026 m2) of Mytilus and Perna recruits recorded in three experimental treatments: western bed (a), eastern bed (b) and Mytilus bed (c) between September 2019 and August 2020 at each season: Spr Spring (September–November), Sum Summer (December–February), Aut Autumn (March–May), Win Winter (June–August). Note April–June 2020 = COVID restrictions. See Table 1S for Tukey post hoc test; P < 0.05
Perna recruits showed right-skewed size distributions across all experimental treatments, while the size distribution of Mytilus recruits showed less clear structure (Fig. 5). Neither species showed an effect of treatment on its size distribution (Table 1). However, Perna and Mytilus recruits showed different size distributions in western and eastern beds, with Perna recruits being generally larger (Fig. 5; Table 1).
Frequency (proportion) of sizes of Mytilus and Perna recruits (maximum length, in 1 mm classes) recorded in three experimental treatments: western bed (a), eastern bed (b) and Mytilus bed (c) between September 2019 and August 2020 (see also Table 1)
There were no significant effects on recruit size of the three factors: Experimental Treatment, Species and Season or their three-way interaction (P > 0.05; Fig. 6a–c). However, there were significant two-way interactions between the factors: Treatment × Season, and Species × Season (AICc = 6,202.11; P < 0.05). The Treatment × Season interaction (GLM output: Estimate = 2.30, SE = 1.15, t-value = 1.98, P = 0.047) indicated that mussel recruits were larger in western bed than eastern bed or Mytilus bed in summer (Fig. 6a–c; Table 2S, Tukey’s post hoc test, P < 0.05), with greater maximum length recorded in February 2020 (Fig. 2SI). The interaction Species × Season interaction (GLM output: Estimated = 8.28, SE = 2.99, t-value = 2.76, P = 0.005) indicated that the size of Perna recruits differed between autumn and winter (Fig. 6a–c; Table 2S, Tukey’s post hoc test, P < 0.05).
Boxplot of sizes (mm) of Mytilus and Perna recruits recorded in three experimental treatments: western bed (a), eastern bed (b) and Mytilus bed (c) between September 2019 and August 2020 at each season: Spr Spring (September–November), Sum Summer (December–February), Aut Autumn (March–May), Win Winter (June–August). Horizontal dotted line in plots for Perna recruits indicates 15 mm in maximum length for those individuals used to build VBG curves (see Fig. 7; Table 2 for details). Note April–June 2020 = COVID restrictions. See also Table 2S for Tukey post hoc test; P < 0.05
Growth of recruits at each experimental bed
VBG curves were fitted to length-frequency data for Perna recruits in all three treatments (Fig. 7). The VBG parameters such L∞ and K (growth coefficient) tended to differ (Table 2), and it was clear that after one year of growth, recruits could be ranked by size according to treatment, i.e. western Perna bed > Mytilus bed > eastern Perna bed, indicating that the growth of Perna recruits differed among treatments (Fig. 7; Table 2).
Von Bertalanffy growth (VBG) curves fitted to length-frequency data for recruits of Perna recorded at three different experimental treatments: western bed (a), eastern bed (b) and Mytilus (c) between September 2019 and August 2020. Horizontal dotted lines are the expected length reached of mussel recruits after 1 year. See Table 2 for parameters of each VBG curve
Discussion
For benthic marine animals with planktonic larvae, the processes regulating settlement from the water column into the benthos are extremely important to population dynamics and can be under strong selective pressure (Roughgarden et al., 1988). It has been suggested that the initial life stages of sessile marine organisms prefer conspecifics as settlement sites (Tamburri et al., 2007), and other studies have found a preference of settling mussel larvae for conspecific adults (Mörtl & Rothhaupt, 2003; Sardiña et al., 2009), though manipulative field experiments of Perna and Mytilus revealed little attraction of larvae to conspecific adults (Porri et al., 2007). Here, we found a preference of Perna settlers for western conspecifics beds, with no such effect for Mytilus settlers. Mytilus and Perna tend to reproduce at different times (Zardi et al., 2007a), and the overall numbers of Mytilus recruits were extremely low, generally an order of magnitude lower than Perna recruits. Nevertheless, this does not affect the interpretation of intraspecific effects of treatment, and we interpret the findings as a clear indication that the quality of settlement habitat offered by adult mussels influences larval recruitment and growth. Beds of adult Mytilus and the two lineages of Perna all differ from one another in terms of the mean numbers of byssal threads and the degree of shell gaping that they exhibit. These two aspects of their biology will influence the quality of habitat they offer to settlers and recruits in terms of both architectural complexity and humidity through evaporative water loss. While we cannot separate the influence on recruit numbers of settlement rate versus post-settlement mortality, the net effect is obvious; recruit abundances, sizes and growth were all greatest in western Perna beds.
The two lineages of Perna have different evolutionary backgrounds, having separated before the closure of the Tethys Sea and migrated independently down the east and the west coast of the African continent, with the study area representing a zone of secondary contact between them (Cunha et al., 2014). The situation is now made more complicated by the eastward expansion of the invasive Mytilus from the west coast of South Africa, reaching an apparently stable eastern limit in the study area (Robinson et al., 2005). All three have co-existed for around 20 years at the study site, where they exhibit healthy populations with regular reproduction and recruitment (Robinson et al., 2005; Rius & McQuaid, 2006). The within-shore and alongshore distributions of the three are set by complex interactions between their biological interactions and abiotic conditions (Mcquaid et al., 2015). Using a completely different experimental design, Porri et al. (2007) found no evidence of conspecific attraction of recruits to conspecific adults under field conditions, and their results indirectly corroborate our interpretation of the present results. Porri et al. (2007) used two approaches, one involving the use of plastic scouring pads as settlement sites placed among adult mussel beds. This tests for coarse attraction of larvae to adults, with larvae potentially settling in the proximity of conspecific adults. The second approach involved placing adults in bags anchored to the shore and tested the possibility of larvae being attracted to individual conspecific adults. Neither approach tests the effects of the structure of habitat formed by adults. In combination with our data, this indicates that chemical attraction to conspecifics is weak or non-existent, and that larvae are attracted to, trapped by, or survive better in the more benign or architecturally complex habitats provided by Perna beds.
Habitat conditions affecting mussel recruitment success
The physical and biological characteristics of a habitat may influence species interactions (Bulleri et al., 2006, 2016), for example, interactions between Perna and Mytilus can be competitive or facilitative depending on position on the shore (Rius & McQuaid, 2009). Our species differ in two aspects likely to affect recruitment success. First, gaping of the shells by mussels reflects the species’ tolerance of anaerobic conditions and results in water loss and evaporative cooling as side effects (Nicastro et al., 2010b). This affects levels of humidity and temperatures inside adult beds (Zardi et al., 2015; Lathlean et al., 2016) with consequences for associated infauna assemblages (Nicastro et al., 2010b, 2020) and potentially the success of mussel recruitment. Mytilus is highly tolerant of anaerobiosis and does not exhibit gaping, while Perna, particularly the eastern lineage, gapes frequently (Nicastro et al., 2010b). The gaping behaviour of Perna has such a marked effect on temperature stress that it can even benefit adjacent Mytilus by leading to reduced body temperatures (Lathlean et al., 2016). Secondly, Perna and Mytilus differ in the approach they take to coping with the stress of wave action (Zardi et al., 2008). For both species, adult mortality through wave action is greater on the open coast than under more sheltered conditions, but Perna essentially adopts a ‘resistance’ strategy, relying on strong attachment through the production of large numbers of byssal threads (Zardi et al., 2006, 2008). In contrast, Mytilus adopts essentially a resilience strategy, exhibiting less strong attachment, with roughly 20% fewer byssal threads than Perna (Zardi et al., 2006), and higher mortality under wave stress, but higher mobility that allows individuals to reposition themselves when neighbours are lost (Nicastro et al., 2008). Thus, the two species differ in the average number of byssal threads produced by adults, but in addition, eastern Perna adults produce more byssal threads than western Perna adults (Zardi et al., 2015), and this will affect the architectural complexity of the habitat as experienced by settlers and recruits. The larvae of Perna produce a mucous thread immediately before settlement (Petrone et al., 2008; Wilkens et al., 2012; Rusk et al., 2017) and in the turbulent environment of the intertidal this seems to result in larvae attaching to substrata in accordance with the likelihood of entanglement, though in the laboratory older recruits (9–10 mm) are more likely to crawl towards adults using the foot (Erlandsson et al., 2008; Porri et al., 2016). In situ comparisons of settlement rates among various potential substrata indicates that mussel larvae settle most commonly on more complex substrata; in that case macroalgae were preferred to adult mussel beds (McQuaid & Lindsay, 2005), highlighting the relevance of architecture to settlement rates. Indeed, the importance of complexity and the provision of more attachment points and spatial refuges for recruits has been highlighted for other mussels species such as Mytilus edulis (McGrath et al., 1988; Christensen et al., 2015).
These points led us to expect higher recruit numbers on beds of Perna east than Perna west, but unexpectedly, we found the reverse. While the key driver for this result is unknown, one other difference between our species may be relevant; as sedentary filter-feeders, adult mussels can exert considerable consumption pressure on larvae attempting to settle into existing beds (Alfaro, 2006). The effect is particularly strong during receding tides, and larviphagy by adults can remove nearly 80% of larvae before they can find refuge among the byssal threads of the adults (Porri et al., 2008a). Porri et al. (2008a) also found that adults of Mytilus can exhibit stronger predation pressure on early mussel stages than adults of Perna and this presumably reflects rates of filtration. While there are no comparisons of filtration rate between Mytilus and P. perna, mussel species are known to differ in their filtering efficiencies and M. galloprovincialis is known to show higher clearance rates than Perna canaliculus (Wright et al., 1982; Gardner, 2002). The study by Porri et al. (2008a) involved only the western adults of Perna and, as the two lineages of Perna have not been compared with one another; differences in the efficiency which they filter out larvae could theoretically help explain our findings.
Growth rates of mussel recruits
Growth of mussels can be estimated using several different approaches and its estimations are highly variable, with local conditions, particularly of water flow, having a strong influence (Gomes et al., 2018; Barbosa et al., 2021). For example, McQuaid & Lindsay (2000) used three complementary techniques to demonstrate faster growth rates on exposed than sheltered shores, but smaller within-shore scales, are also important. McQuaid & Mostert (2010) reduced adult growth rates by roughly 30% by experimentally manipulating water flow at centimetre scales in the field. In both cases, this seems to operate through the supply of food. The models in this study indicate that, after one year of growth, recruits of Perna clearly attain larger sizes in beds of the western lineage of conspecifics than in other beds. Our results differ from those of Bulleri et al. (2006) who experimentally manipulated the habitat-forming macroalga Codium fragile. They found that recruit numbers were greater in areas where the alga was present than in areas where it had been removed, but with no difference in recruit growth rates. Whether our finding of dissimilar growth rates of recruits among different adult mussel beds is related to food or to abiotic conditions is unclear, but one possibility is that differences among species in filtration efficiency or pseudo faeces production by adults may affect the availability of food to recruits, and thus their growth rates.
Conclusions
On scales of 100 m, hydrodynamics in the nearshore environment are particularly powerful and complex, resulting in preferential delivery of more larvae to some sites than others (Porri et al., 2008b). At smaller scales, there is evidence of an ontogenetic shift in how primary and secondary mussel settlers are attracted to biofilm and conspecifics (Von Der Meden et al., 2010). In our study, we could not differentiate between the effects of settlement and post-settlement mortality. Recruits were not only more abundant on beds of western Perna than other treatments; they are also expected to reach larger sizes after recruitment according to the modelled growth, suggesting that western Perna provides a particularly favourable post-settlement environment at the study site.
Beds of adult mussels form critical habitats for the settlement and recruitment of earlier ontogenetic stages. Overall, however, our results indicate that the quality of habitat that adults offer differs among species and, importantly, even between different genetic lineages of the same species. Comparing with earlier studies on chemical attraction of larvae to conspecifics, our data clearly indicate that larval preference for one lineage of Perna reflects differences in the architectural complexity of adult beds and the influence of gaping behaviour on creating more benign conditions within those beds.
Data availability
All data and material generated during the present study are available from the corresponding author upon request.
References
Akaike, H., 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723.
Alfaro, A. C., 2006. Evidence of cannibalism and bentho-pelagic coupling within the life cycle of the mussel, Perna canaliculus. Journal of Experimental Marine Biology and Ecology 329: 206–217.
Angert, A. L., M. G. Bontrager & J. Aringgren, 2020. What do we really know about adaptation at range edges? Annual Review of Ecology, Evolution, and Systematics 51: 341–361.
Barbosa, R. V., C. Bacher, F. Jean & Y. Thomas, 2021. Linking individual and population patterns of rocky-shore mussels. PeerJ 9: e12550.
Bayne, B. L., 1964. Primary and secondary settlement in Mytilus edulis L. (Mollusca). The Journal of Animal Ecology 33: 513–523.
Bownes, S. J. & C. D. McQuaid, 2006. Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. on the south coast of South Africa? Journal of Experimental Marine Biology and Ecology 338: 140–151.
Bownes, S., N. P. Barker & C. D. McQuaid, 2008. Morphological identification of primary settlers and post-larvae of three mussel species from the coast of South Africa. African Journal of Marine Science 30: 233–240.
Bulleri, F., L. Airoldi, G. M. Branca & M. Abbiati, 2006. Positive effects of the introduced green alga, Codium fragile ssp. tomentosoides, on recruitment and survival of mussels. Marine Biology 148: 1213–1220.
Bulleri, F., J. F. Bruno, B. R. Silliman & J. J. Stachowicz, 2016. Facilitation and the niche: implications for coexistence, range shifts and ecosystem functioning. Functional Ecology 30: 70–78.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference. Springer, New York: 488.
Christensen, H. T., P. Dolmer, B. W. Hansen, M. Holmer, L. D. Kristensen, L. K. Poulsen, C. Stenberg, C. M. Albertsen & J. G. Støttrup, 2015. Aggregation and attachment responses of blue mussels, Mytilus edulis – impact of substrate composition, time scale and source of mussel seed. Aquaculture 435: 245–251.
Cole, V. J., 2010. Alteration of the configuration of bioengineers affects associated taxa. Marine Ecology Progress Series 416: 127–136.
Cowen, R. K., K. M. M. Lwiza, S. Sponaugle, C. B. Paris & D. B. Olson, 2000. Connectivity of marine populations: open or closed? Science 287: 857–859.
Cunha, R. L., K. R. Nicastro, J. Costa, C. D. McQuaid, E. A. Serrão & G. I. Zardi, 2014. Wider sampling reveals a non-sister relationship for geographically contiguous lineages of a marine mussel. Ecology and Evolution 4: 2070–2081.
Erlandsson, J. & C. D. McQuaid, 2004. Spatial structure of recruitment in the mussel Perna perna at local scales: effects of adults, algae and recruit size. Marine Ecology Progress Series 267: 173–185.
Erlandsson, J., F. Porri & C. D. McQuaid, 2008. Ontogenetic changes in small-scale movement by recruits of an exploited mussel: implications for the fate of larvae settling on algae. Marine Biology 153: 365–373.
Gaines, S. & J. Roughgarden, 1985. Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proceedings of the National Academy of Sciences of USA 82: 3707–3711.
Gardner, J. P. A., 2002. Effects of seston variability on the clearance rate and absorption efficiency of the mussels Aulacomya maoriana, Mytilus galloprovincialis and Perna canaliculus from New Zealand. Journal of Experimental Marine Biology and Ecology 268: 83–101.
Gilman, S. E., C. D. G. Harley, D. C. Strickland, O. Vanderstraeten, M. J. O’Donnell & B. Helmuth, 2006. Evaluation of effective shore level as a method of characterizing intertidal wave exposure regimes. Limnology and Oceanography: Methods 4: 448–457.
Godsoe, W., J. Jankowski, R. D. Holt & D. Gravel, 2017. Integrating biogeography with contemporary niche theory. Trends in Ecology and Evolution 32: 488–499.
Gomes, I., L. Peteiro, J. Bueno-Pardo, R. Albuquerque, S. Pérez-Jorge, E. R. Oliveira, F. L. Alves & H. Queiroga, 2018. What’s a picture really worth? On the use of drone aerial imagery to estimate intertidal rocky shore mussel demographic parameters. Estuarine, Coastal and Shelf Science 213: 185–198.
Harley, C. D. G. & B. S. T. Helmuth, 2003. Local- and regional-scale effects of wave exposure, thermal stress, and absolute versus effective shore level on patterns of intertidal zonation. Limnology and Oceanography 48: 1498–1508.
Haye, P. A., N. I. Segovia, N. C. Muñoz-Herrera, F. E. Gálvez, A. Martínez, A. Meynard, M. C. Pardo-Gandarillas, E. Poulin & S. Faugeron, 2014. Phylogeographic structure in benthic marine invertebrates of the southeast pacific coast of Chile with differing dispersal potential. PLoS ONE 9: e88613.
Helmuth, B., 1999. Thermal biology of rocky intertidal mussels: quantifying body temperatures using climatological data. Ecology 80: 15–34.
Herbert, R. J. H. & S. J. Hawkins, 2006. Effect of rock type on the recruitment and early mortality of the barnacle Chthamalus montagui. Journal of Experimental Marine Biology and Ecology 334: 96–108.
Jenkins, S. R., 2005. Larval habitat selection, not larval supply, determines settlement patterns and adult distribution in two chthamalid barnacles. Journal of Animal Ecology 74: 893–904.
Jenkins, S. R., P. Åberg, G. Cervin, R. A. Coleman, J. Delany, P. Della Santina, S. J. Hawkins, E. Lacroix, A. A. Myers, M. Lindegarth, A. M. Power, M. F. Roberts & R. G. Hartnoll, 2000. Spatial and temporal variation in settlement and recruitment of the intertidal barnacle Semibalanus balanoides (L.) (Crustacea: Cirripedia) over a European scale. Journal of Experimental Marine Biology and Ecology 243: 209–225.
Kaehler, S. & C. D. Mcquaid, 1999. Use of the fluorochrome calcein as an in situ growth marker in the brown mussel Perna perna. Marine Biology 133: 455–460.
Keith, S. A., R. J. H. Herbert, P. A. Norton, S. J. Hawkins & A. C. Newton, 2011. Individualistic species limitations of climate-induced range expansions generated by meso-scale dispersal barriers. Diversity and Distribution 17: 275–286.
Kinlan, B. P., S. D. Gaines & S. E. Lester, 2005. Propagule dispersal and the scales of marine community process. Diversity and Distribution 11: 139–148.
Kroeker, K. J. & E. Sanford, 2022. Ecological leverage points: species interactions amplify the physiological effects of global environmental change in the ocean. Annual Review of Marine Science 14: 75–103.
Lasiak, T. A. & T. C. Barnard, 1995. Recruitment of the brown mussel Perna perna onto natural substrata: a refutation of the primary/secondary settlement hypothesis. Marine Ecology Progress Series 120: 147–154.
Lathlean, J. A., L. Seuront, C. D. McQuaid, T. P. T. Ng, G. I. Zardi & K. R. Nicastro, 2016. Cheating the locals: invasive mussels steal and benefit from the cooling effect of indigenous mussels. PLoS ONE 11: 1–13.
Ma, K. C. K., M. N. C. Gusha, G. I. Zardi, K. R. Nicastro, J. R. Monsinjon & C. D. McQuaid, 2021. Biogeographic drivers of distribution and abundance in an alien ecosystem engineer: transboundary range expansion, barriers to spread, and spatial structure. Journal of Biogeography 48: 1941–1959.
McGrath, D., P. King & E. Gosling, 1988. Evidence for the direct settlement of Mytilus edulis larvae on adult mussel beds. Marine Ecology Progress Series 47: 103–106.
McQuaid, C. D. & T. L. Lindsay, 2000. Effect of wave exposure on growth and mortality rates of the mussel Perna perna: bottom-up regulation of intertidal populations. Marine Ecology Progress Series 206: 147–154.
McQuaid, C. D. & J. R. Lindsay, 2005. Interacting effects of wave exposure, tidal height and substratum on spatial variation in densities of mussel Perna perna plantigrades. Marine Ecology Progress Series 301: 173–184.
McQuaid, C. D. & B. P. Mostert, 2010. The effects of within-shore water movement on growth of the intertidal mussel Perna perna: an experimental field test of bottom-up control at centimetre scales. Journal of Experimental Marine Biology and Ecology 384: 119–123.
McQuaid, C. D., F. Porri, K. R. Nicastro & G. I. Zardi, 2015. Simple, scale-dependent patterns emerge from very complex effects – an example from the intertidal mussels Mytilus galloprovincialis and Perna perna. Oceanography and Marine Biology 53: 127–156.
Mildenberger, T. K., M. H. Taylor & M. Wolff, 2017. TropFishR: an R package for fisheries analysis with length–frequency data. Methods in Ecology and Evolution 8: 1520–1527.
Mörtl, M. & K. O. Rothhaupt, 2003. Effects of adult Dreissena polymorpha on settling juveniles and associated macroinvertebrates. International Review of Hydrobiology 88: 561–569.
Navarrete, S. A. & J. C. Castilla, 1990. Barnacle walls as mediators of intertidal mussel recruitment: effects of patch size on the utilization of space. Marine Ecology Progress Series 68: 113–119.
Nicastro, K. R., G. I. Zardi & C. D. McQuaid, 2008. Movement behaviour and mortality in invasive and indigenous mussels: resilience and resistance strategies at different spatial scales. Marine Ecology Progress Series 372: 119–126.
Nicastro, K. R., G. I. Zardi & C. D. McQuaid, 2010a. Differential reproductive investment, attachment strength and mortality of invasive and indigenous mussels across heterogeneous environments. Biological Invasions 12: 2165–2177.
Nicastro, K. R., G. I. Zardi, C. D. McQuaid, L. Stephens, S. Radloff & G. L. Blatch, 2010b. The role of gaping behaviour in habitat partitioning between coexisting intertidal mussels. BMC Ecology 10: 17.
Nicastro, K. R., G. I. Zardi, C. D. McQuaid, G. A. Pearson & E. A. Serrão, 2012. Love thy neighbour: group properties of gaping behaviour in mussel aggregations. PLoS ONE 7: e4738.
Nicastro, K. R., C. D. McQuaid, A. Dievart & G. I. Zardi, 2020. Intraspecific diversity in an ecological engineer functionally trumps interspecific diversity in shaping community structure. Science of the Total Environment 743: 140723.
Ntuli, N. N., K. R. Nicastro, G. I. Zardi, J. Assis, C. D. McQuaid & P. R. Teske, 2020. Rejection of the genetic implications of the “Abundant Centre Hypothesis” in marine mussels. Scientific Reports 10: 1–12.
Petrone, L., N. L. C. Ragg & A. J. McQuillan, 2008. In situ infrared spectroscopic investigation of Perna canaliculus mussel larvae primary settlement. Biofouling 24: 405–413.
Pinsky, M. L., S. R. Palumbi, S. Andréfouët, S. J. Purkis, S. E. Applications, N. June, M. L. Pinsky, S. R. Palumbi, S. Andréfouët & S. J. Purkis, 2012. Open and closed seascapes: where does habitat patchiness create populations with high fractions of self-recruitment? Ecological Applications 22: 1257–1267.
Porri, F., G. I. Zardi, C. D. McQuaid & S. Radloff, 2007. Tidal height, rather than habitat selection for conspecifics, controls settlement in mussels. Marine Biology 152: 631–637.
Porri, F., T. Jordaan & C. D. McQuaid, 2008a. Does cannibalism of larvae by adults affect settlement and connectivity of mussel populations? Estuarine, Coastal and Shelf Science 79: 687–693.
Porri, F., C. D. McQuaid, S. M. Lawrie & S. J. Antrobus, 2008b. Fine-scale spatial and temporal variation in settlement of the intertidal mussel Perna perna indicates differential hydrodynamic delivery of larvae to the shore. Journal of Experimental Marine Biology and Ecology 367: 213–218.
Porri, F., C. D. McQuaid & J. Erlandsson, 2016. The role of recruitment and behaviour in the formation of mussel-dominated assemblages: an ontogenetic and taxonomic perspective. Marine Biology 163: 163–157.
Quenouille, M. H., 1956. Notes on bias in estimation. Biometrika 43: 353–360.
R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Reaugh, K. E., J. M. Harris & G. M. Branch, 2007. Further refutation of the primary–secondary settlement hypothesis for the brown mussel Perna perna. African Journal of Marine Science 29: 545–549.
Rius, M. & C. D. McQuaid, 2006. Wave action and competitive interaction between the invasive mussel Mytilus galloprovincialis and the indigenous Perna perna in South Africa. Marine Biology 150: 69–78.
Rius, M. & C. D. McQuaid, 2009. Facilitation and competition between invasive and indigenous mussels over a gradient of physical stress. Basic and Applied Ecology 10: 607–613.
Robinson, T. B., C. L. Griffiths, C. D. McQuaid & M. Rius, 2005. Marine alien species of South Africa – status and impacts. African Journal of Marine Science 27: 297–306.
Roughgarden, J., S. Gaines, H. Possingham, J. Roughgarden, S. Gaines & H. Possingham, 1988. Recruitment dynamics in complex life cycles. Science 241: 1460–1466.
Rusk, A. B., A. C. Alfaro, T. Young, E. Watts & S. L. Adams, 2017. Investigation of early mussel (Perna canaliculus) development using histology, SEM imaging, immunochemistry and confocal microscopy. Marine Biology Research 13: 314–329.
Sardiña, P., D. H. Cataldo & D. Boltovskoy, 2009. Effects of conspecifics on settling juveniles of the invasive golden mussel, Limnoperna fortunei. Aquatic Sciences 71: 479–486.
Schwamborn, R., T. K. Mildenberger & M. H. Taylor, 2019. Assessing sources of uncertainty in length-based estimates of body growth in populations of fishes and macroinvertebrates with bootstrapped ELEFAN. Ecological Modelling 393: 37–51.
Shanks, A. L., 2009. Pelagic larval duration and dispersal distance revisited. Biological Bulletin 216: 373–385.
Tamburri, M. N., R. K. Zimmer & C. A. Zimmer, 2007. Mechanisms reconciling gregarious larval settlement with adult cannibalism. Ecological Monographs 77: 255–268.
Taylor, M. H. & T. K. Mildenberger, 2017. Extending electronic length frequency analysis in R. Fisheries Management and Ecology 24: 330–338.
Tukey, J. W., 1958. Bias and confidence in not-quite large samples. The Annals of Mathematical Statistics 29: 614.
Von Der Meden, C. E. O., F. Porri, C. D. McQuaid, K. Faulkner & J. Robey, 2010. Fine-scale ontogenetic shifts in settlement behaviour of mussels: changing responses to biofilm and conspecific settler presence in Mytilus galloprovincialis and Perna perna. Marine Ecology Progress Series 411: 161–171.
Weidberg, N., B. Bularz, S. López-Rodríguez & S. A. Navarrete, 2018. Wave-modulation of mussel daily settlement at contrasting rocky shores in central Chile: topographic regulation of transport mechanisms in the surf zone. Marine Ecology Progress Series 606: 39–53.
Wetherall, J. A., J. J. Polovina, S. Ralston, D. Pauly & G. R. Morgan, 1987. Estimating growth and mortality in steady-state fish stocks from length–frequency data. ICLARM Conference Proceedings 13: 53–74.
Wilkens, S. L., J. A. Stanley & A. G. Jeffs, 2012. Induction of settlement in mussel (Perna canaliculus) larvae by vessel noise. Biofouling 28: 65–72.
Wright, R. T., R. B. Coffin, C. P. Ersing & D. Pearson, 1982. Field and laboratory measurements of bivalve filtration of natural marine bacterioplankton. Limnology and Oceanography 27: 91–98.
Zardi, G. I., K. R. Nicastro, C. D. McQuaid, M. Rius & F. Porri, 2006. Hydrodynamic stress and habitat partitioning between indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels: constraints of an evolutionary strategy. Marine Biology 150: 79–88.
Zardi, G. I., C. D. McQuaid & K. R. Nicastro, 2007a. Balancing survival and reproduction: seasonality of wave action, attachment strength and reproductive output in indigenous Perna perna and invasive Mytilus galloprovincialis mussels. Marine Ecology Progress Series 334: 155–163.
Zardi, G. I., C. D. McQuaid, P. R. Teske & N. P. Barker, 2007b. Unexpected genetic structure of mussel populations in South Africa: indigenous Perna perna and invasive Mytilus galloprovincialis. Marine Ecology Progress Series 337: 135–144.
Zardi, G. I., K. R. Nicastro, C. D. McQuaid & J. Erlandsson, 2008. Sand and wave induced mortality in invasive (Mytilus galloprovincialis) and indigenous (Perna perna) mussels. Marine Biology 153: 853–858.
Zardi, G. I., K. R. Nicastro, C. D. McQuaid, L. Hancke & B. Helmuth, 2011. The combination of selection and dispersal helps explain genetic structure in intertidal mussels. Oecologia 165: 947–958.
Zardi, G. I., K. R. Nicastro, C. D. McQuaid, R. Castilho, J. Costa, E. A. Serrão & G. A. Pearson, 2015. Intraspecific genetic lineages of a marine mussel show behavioural divergence and spatial segregation over a tropical/subtropical biogeographic transition. BMC Evolutionary Biology 15: 15–100.
Zardi, G. I., C. D. McQuaid, R. Jacinto, C. R. Lourenço, E. A. Serrão & K. R. Nicastro, 2018. Re-assessing the origins of the invasive mussel Mytilus galloprovincialis in southern Africa. Marine and Freshwater Research 69: 607–613.
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14.
Acknowledgements
MHO thanks the Coastal Research (Department of Zoology and Entomology, Rhodes University) Group for help in the field. This work is based upon research supported by the National Research Foundation of South Africa (Grant Number 64801). We thank the Hydrographer Office of the South African Navy for providing tidal predictions.
Funding
This work is based upon research supported by the National Research Foundation of South Africa (Grant Number 64801).
Author information
Authors and Affiliations
Contributions
MHO, AJW, JRM, KRN, GIZ and CDM conceptualised and designed the methodology; MHO, AJW, JRM and CB collected the data; MHO and CDM analysed the data and wrote the original draft; AJW, JRM, KRN, GIZ, CB and CDM reviewed and edited the drafts; CDM provided funding for the research. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Additional information
Handling Editor: Trine Bekkby
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oróstica, M.H., Wyness, A.J., Monsinjon, J.R. et al. Effects of habitat quality on abundance, size and growth of mussel recruits. Hydrobiologia 849, 4341–4356 (2022). https://doi.org/10.1007/s10750-022-04994-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04994-7