Abstract
Seed size can have an impact on angiosperm reproductive fitness. Ecological theory predicts plants that will produce larger seeds in stressful environments to increase the chances of seedling survival and numerous small seeds in favourable conditions to increase the number of recruits. We measured seed morphology of the seagrass Heterozostera nigricaulis from four populations under differing environmental conditions in South East Australia. Seed size and mass among sites showed consistent differences over four flowering seasons. Seeds from exposed, ephemeral meadows (Blairgowrie, Edwards Point) were 19%–53% heavier than those from larger, stable meadows at more sheltered sites (Swan Bay, Point Henry). Overall, heavier seeds from exposed sites performed better in germination experiments and persisted (remained viable) longer compared to small seeds from sheltered sites. Seeds from sheltered sites showed contrasting levels of seed performance. Small seeds from Swan Bay had the lowest germination but the proportion of viable seeds after 12 months were much higher (41%) than similar sized seeds from Point Henry (0%). There are clear life history benefits of large seeds that facilitate seed persistence and germination at exposed sites; however, the performance of smaller seeds varied between sites and may be a function of other site-specific advantages.
Similar content being viewed by others
Introduction
The size of seeds produced by angiosperms plays a major role in survival and persistence of plant populations, influencing germination, dispersal and seedling survival (Eriksson, 1999; Fricke et al., 2019). Seeds not only vary in size (dimension and mass) by ten orders of magnitude across species but can also show intra-specific variation (Fenner & Thompson, 2005; Moles et al., 2005). High mortality rates occur at the germination and seedling stage and seed traits such as seed size can have a strong effect on plant fitness (Vaughton & Ramsey, 1998). Large seeds have greater resources for seedling metabolism to utilize during germination and an increased ability to tolerate stress, enabling greater seedling survival and growth (Vaughton & Ramsey, 1998). While larger seeds may provide a competitive advantage, smaller seeds require less energy to produce and disperse further. As a result, theory predicts that plants will tradeoff between seed size and the number of seeds produced, but the evidence is inconsistent with many studies finding no relationship between seed size and the number of seeds produced (Parciak, 2002; Halpern, 2005; Sõber & Ramula, 2013; Brancalion & Rodrigues, 2014).
Variation in seed size across species and individuals within populations are well documented, but intra-specific seed variations across habitats has received less attention (Olejniczak et al., 2018; Fricke et al., 2019). Within a species, seed size can vary at the plant scale, within and across ovules, fruit and individuals, and, at larger scales across habitats, seasons or latitudes (Parciak, 2002; Fenner & Thompson., 2005). Habitat suitability across populations can play a major role in determining seed size as plants adapt to local conditions (Münzbergová & Plačková, 2010). For instance, changing conditions across an elevation gradient determines seed size in species from the Asteracaea and Primulaceae families (Olejniczak et al., 2018). However, seed size is not always adaptive and may be influenced by population parameters including density and heredity (Halpern, 2005).
Advantages of larger seeds include greater maximum germination and faster germination rates, longer dormancy, production of larger more competitive seedlings and increased survival, whereas smaller seeds can disperse further and produce more seedlings relative to reproductive mass (Vaughton & Ramsey, 1998; Fricke et al., 2019). Variation in seed traits is thought to be driven to a large extent by the abiotic and biotic environment the parent plant experiences (Venable & Brown, 1988). In stressful conditions, large seeds are produced, which contain more resources, improving fitness and leading to positive selection (Venable & Brown, 1988; Paz & Martinez-Ramos, 2003; Quero et al., 2007). The advantages of large seeds, however, are diminished in favourable conditions where selection pressures on germination are reduced, and there are sufficient resources for seedling survival from smaller seeds (Venable & Brown, 1988; Paz & Martinez-Ramos, 2003; Hierro et al., 2020).
Seagrasses are globally distributed marine angiosperms and display a range of life history traits ranging from persistent perennials to more ephemeral annual populations (Orth et al., 2006). Historically, asexual growth was thought to be the primary mechanism for inter-annual meadow persistence; however, recent research has shown the importance of seeds in maintaining populations, even in perennial meadows (Kendrick et al., 2012; O'Brien et al., 2017; Johnson et al., 2020). In some seagrass species, the ability of seeds to remain viable in the seed bank and germinate are critical for persistence, resilience and the establishment of new populations (Hughes & Stachowicz, 2011; McMahon et al., 2014; Sherman et al., 2018). Although the impacts of seed size on seed performance is well known in terrestrial plants (Venable & Brown, 1988; Fricke et al., 2019), the effects of seagrass seed size on seed germination, persistence and dispersal have received little attention (Delefosse et al., 2016).
To date, there have been few published studies on seagrass seed size and related ecological implications, with the majority focusing on the widespread species Zostera marina (Linnaeus 1753), which produces small hard seeds (Table 1). For Z. marina, seed size can vary across and within seagrass populations (Wyllie-Echeverria et al., 2003; Delefosse et al., 2016; Jørgensen et al., 2019; Combs et al., 2020). For example, a 14% difference in weight was reported between seagrass meadows in Washington and New York in the United States and a 49% difference across sites in Denmark (Wyllie-Echeverria et al., 2003; Delefosse et al., 2016). While initial viability does not differ across seed size (Combs et al., 2020), larger Z. marina seeds have higher starch reserves relative to smaller seeds and are able to persist longer in the seed bank, produce larger seedlings and emerge from greater burial depths (Delefosse et al., 2016; Jørgensen et al., 2019; Combs et al., 2020). Smaller seeds, on the other hand, are able to germinate more quickly and have lower sinking velocity, which allows for greater dispersal (Delefosse et al., 2016; Jørgensen et al., 2019). The importance of seed size on germination rates and persistence for other seagrass species is unknown and may have important ecological consequences.
The seagrass Heterozostera nigricaulis (Kuo 2005), formerly H. tasmanica (Den Hartog 1970) or Zostera nigricaulis (Jacob and Les 2009), is common throughout southern Australia in nearshore habitats where meadow persistence can be linked to environmental conditions (Hirst et al., 2016, 2017a). It can form extensive meadows under sheltered conditions but is often ephemeral and patchy in exposed locations (Ball et al., 2014). Heterozostera nigricaulis meadows are highly productive, provide important habitats for fish and invertebrates and store large amounts of carbon in their sediment (Bulthuis & Woelkerling, 1983; Edgar & Shaw, 1995; Jenkins et al., 2002; Hutchinson et al., 2014; Macreadie et al., 2014). Reproductive effort of H. nigricaulis varies strongly across both space and time with spathe production ranging from < 10 to < 3000/m2 (Smith et al., 2016a; Cumming et al., 2017) and the limited number of germination studies for this species shows low germination rates of seeds in laboratory trials (Smith et al., 2016a; Cumming et al., 2017). This study aims to (i) quantify differences in H. nigricaulis seed size across four sites in Port Phillip Bay Australia with different environmental, sediment and meadow characteristics and (ii) to determine if greater seed size (length, width, volume) and mass (wet weight) offered ecological benefits such as faster germination rates, higher germination success or greater seed persistence in the seed bank.
Methods
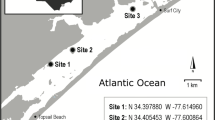
Seagrass seeds from H. nigricaulis were collected from four sites, Blairgowrie, Edwards Point, Point Henry and Swan Bay in Port Phillip Bay, Victoria, Australia to assess variations in seed size across seagrass populations and between years (Fig. 1). Each site has distinct physical and seagrass meadow characteristics (Table 2). Blairgowrie and Edwards Point have similar environmental conditions and are both located 14–15 km from the mouth of Port Philip Bay. They are both exposed to the prevailing wind and wave conditions, have relatively high sediment grain size and low sediment organic matter and nitrogen availability (Jenkins et al., 2015; Thomson et al., 2015; Hirst et al., 2017b). Meadows at Blairgowrie and Edwards Point have high biomass, but the total area of seagrass varies under different climatic conditions (Ball et al., 2014; Smith et al., 2016a). In contrast, seagrass at Point Henry and Swan Bay forms large persistent meadows with fine sediment of high organic matter content that are protected from wind and wave action. While Swan Bay is located closer to the mouth of Port Phillip Bay than Blairgowrie or Edwards Point, there is only a small opening to the larger bay creating calm conditions. The density of reproductive spathes is high but varies annually at Blairgowrie (2,467 ± 578 SE spathes/m2) and moderate at Swan Bay (1,125 ± 340 SE) and Point Henry (1,427 ± 307 SE) (Smith et al., 2016a). Spathe production at Edwards Point (238 ± 93 SE) is much lower than the other sites (Smith, 2016a, b). At Blairgowrie, Swan Bay and Point Henry high levels of sexual reproduction and recruitment have resulted in high genotypic diversity (> 85% unique genotypes), while at Point Edwards, asexual reproduction appears to be more important in maintaining the population with only 55% of the population consisting of unique genotypes (Smith unpublished data).
Flowering shoots with mature spadices were collected by hand from each site during December each year between 2012 and 2015 when seeds reach maturity (Smith et al., 2016a). Samples were stored in separate outdoor mesocosms with flow-through seawater (60 L volume, 60 × 35 × 30 cm) at the Victorian Marine Science Consortium (VMSC) in Queenscliff, Victoria under ambient conditions for 6–8 weeks. Once seeds had dehisced from the reproductive shoots, they were sorted by hand and sieved (710 µm mesh) to separate mature seeds from other material (Marion & Orth, 2010). Seeds were then stored in 1L tanks with light aeration and flow through seawater (~ 21 °C) (Jarvis & Moore, 2010, 2015; Marion & Orth, 2010). Seeds were not collected from Point Edwards in 2013 because of the absence of spathes.
Seed size and mass
Seed mass and size dimension were measured across annual flowering seasons to determine differences across sites. Each year (2012–2015) 25 seeds from each of the four sites, Blairgowrie, Edwards Point, Swan Bay, and Point Henry were weighed to determine any differences in seed mass. Seeds were dabbed dry with paper towel and weighed to the nearest 0.01 mg using an A&D K0001 microbalance. Seed length and width at the widest point were measured for 25 seeds from each site in 2012, 2013 and 2015. Seeds were examined under a microscope (Motic SMZ140), and lengths and widths were calculated from the microscope graticule. Seeds were assumed to be prolate ellipsoids and the volume of each seed calculated using the formula 4/3*π* \(\frac{length}{2}\)* \((\frac{diameter}{2}\))2 (Satterly, 1960).
Seed buoyancy
Seed dispersal distance and quality can be related to seed buoyancy and fall velocity (Marion & Orth, 2010). To assess seed buoyancy, the fall rate for seeds from all sites was quantified in 2013 and 2015 with site being used as a proxy for seed size (Blairgowrie = large, Point Edwards = intermediate, Point Henry, Swan Bay = small). Individual seeds were weighed and fall velocity tests were conducted where seeds were released just under the water surface in water at a salinity of 32 and the time for the seeds to sink 40 cm was recorded. In 2013, the fall velocity of 25 seeds from Blairgowrie, Point Henry and Swan Bay was calculated and in 2015, 15 seeds from all four sites were calculated.
Seed persistence
Changes in seed viability over time were tested to determine if seed size was related to persistence in the seed bank and if this varied between sites. Seeds collected in 2012 were kept in aerated ambient flow through water and after 0, 3, 6 and 12 months, and during each sampling, 4 replicates of 20 seeds for each site were tested for viability. Viability tests were undertaken by removing the seed coat and soaking seeds in 1% tetrazolium chloride solution for 48 h. Seeds were recorded as viable if more than 50% of the cotyledon was pink after 48 h (Conacher et al., 1994). Data were reported as mean (± SE) per cent viability per each time step (N = 4).
Seed germination
Seeds collected in 2012 were germinated under controlled conditions to determine if there was any variation in germination in seeds from different sites. Seeds for each site were placed in 4 replicate petri dishes containing 50 seeds in seawater at a salinity of 30. All replicates were kept under optimal conditions for germination at 14 °C with a 12 h light cycle (Cumming et al., 2017). Prior to the beginning of the experiment, seeds were scarified using a razor and soaked in seawater with a salinity of 20 for 48 h to increase germination levels (Cumming et al., 2017). Throughout the experiment, seeds were monitored for germination every 48 h for 17 weeks. Seeds were deemed to be germinated when the cotyledon had extended 5 mm from the seed (Conacher et al., 1994; Jarvis & Moore, 2010). Maximum per cent germination (mean ± SE) and mean time to germination per site were reported. At the completion of the experiment, all ungerminated seeds were tested for viability. Mean seed viability (± SE) was reported per site (N = 4).
Statistical analysis
All data analysis was performed in R statistical computation software (Team, 2020). Data were examined for outliers, collinearity and variance inflation factors prior to analysis (Zuur et al., 2007). The relationship between seed mass and each of length, width and volume was assessed using a generalized linear model (GLM) using a gamma distribution (link = log) which accounted for the positive only nature of the response variables. Differences in seed mass, length, width and volume across sites and years were also analysed with a gamma distribution GLM with a log link. Seed fall velocity was also analysed with a gamma regression; however, this response was compared across seed mass, date and site. The effect of site and time on viability were analysed using a zero/one-inflated beta binomial (ZOIB) regression with the ‘gamlss’ package (Rigby & Stasinopoulos, 2005). ZOIB regression models are used to model response variables that are bound between or equal to 0 or 1 and contain a non-negligible number of zeros and or ones such as seed viability (Ospina & Ferrari, 2010).
Maximum per cent germination across sites were analysed using a beta regression (‘betareg’, Cribari-Neto & Zeileis, 2010). The use of beta regressions was selected as the variables of interest were continuous and restricted to the interval (0, 1) (Zuur et al., 2007). Due to the potential for a large amount of right-censored data characteristic of germination experiments, Cox models were used to quantify effects of site on mean time to germination (MTG) (Scott et al., 1984; McNair et al., 2012). Non-germinated seeds were censored if germination did not occur and were flagged prior to analysis. Each seed was analysed independently using the ‘survival’ package (Therneau & Grambsch, 2000) to assess the distribution of germination times at each site. Violations of the proportional hazards function were explored graphically by plotting separate non-parametric Kaplan–Meier survivorship functions for each site (McNair et al., 2012), KM surv (Klein & Moeschberger, 2003). Cox models were then used to calculate the effects of site on time to germination (survival, Therneau & Grambsch, 2000). The effect of site and time on viability at the completion of the germination experiment were analysed using a ZOIB regression (Rigby & Stasinopoulos, 2005).
After running the global model, all non-relevant covariates were removed, and the model was re-run until only relevant covariates remained (Zuur et al., 2007). Residuals were also inspected visually for patterns by plotting the fitted versus response variables. The best-fit model was considered to be the simplest model with the lowest Akaike information criterion (AIC) score (Zuur et al., 2007) which was calculated using the ‘MuMIn’ package (Barton, 2020). Overall effects of categorical variables were quantified via analysis of deviance for all model parameters using a type II ANOVA for models with no interaction terms and a type III ANOVA when interactions were present (‘car’ package; Fox & Weisberg 2019). During post hoc analysis, all pairwise comparisons were computed from the contrasts between factors with a ‘tukey’ adjustment using ‘lsmeans’ package (Lenth et al. 2021).
Results
Seed size and mass
Seed mass was positively related to seed length, width and volume (Fig. S1). There was a strong relationship between seed mass and both seed width (F1 = 620.14, P < 0.001, r2 = 0.72) and volume (F1 = 738.31, P < 0.001, r2 = 0.70). Seed mass and length had a significant relationship, but it was not as strong as seed width or volume (F1 = 82.86, P < 0.001, r2 = 0.20).
Seed mass, length, width and volume showed significant interactions between sites and the year seeds were sampled (Table 3). Seeds from exposed sites with ephemeral meadows were consistently larger and heavier than seeds from sheltered sites with larger and more stable meadows. Blairgowrie, an exposed ephemeral meadow site, had heavier, wider and larger volume seeds than all other sites in all years sampled (Tukey’s Test P < 0.001). Overall Blairgowrie seeds were 53% heavier than seeds from Point Henry and Swan Bay (Fig. 2, 3). Seed lengths at Blairgowrie were longer than Point Henry and Swan Bay in 2012 (P < 0.001) and 2015 (Point Henry P = 0.041; Swan Bay P < 0.001), but there was no significant difference in 2013. Blairgowrie seeds were longer than seeds from Point Edwards in 2012 (P = 0.015) but not in 2015. Seeds from Point Edwards were heavier, wider and had greater volume than those from Point Henry and Swan Bay (the protected and persistent meadow sites) in all years (P < 0.001), but there was no difference in length in any year except in 2015 when they were longer than Swan Bay (P < 0.001). Seeds from Point Henry were longer than Swan Bay in 2013 (P = 0.006) and 2015 (P < 0.001), but there was no differences in 2012 or mass, width and volume in any years sampled.
Boxplot of Heterozostera nigricaulis a seed mass (mg), b length (mm), c width (mm) to and d volume (mm3) from each site in Port Phillip Bay each year from 2012 to 2015. No seeds were collected from Point Edwards in 2013. Lower and upper box boundaries are the 25th and 75th percentiles, respectively, line inside the box is the median and the lower and upper error bars represent the 10th and 90th percentiles
The best model comparing seed fall velocity across sites, months and mass included mass and year (AIC = 241.5, R2 = 0.59). Seed mass had a strong positive relationship with fall rate (Wald Chi Square = 109.43, df = 1, P < 0.001) and year (Wald Chi Square = 35.44, df = 1, P < 0.001). Seeds from 2015 sank faster than those from 2013, but both years had a similar positive relationship (Fig. 4).
Seed persistence
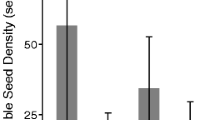
Seed viability varied significantly across sites and time (Table 4, Fig. 5). Viability of seeds from stable/protected meadows in Swan Bay (mean 84 ± 1.2%), the smallest seeds by mass, was greater than the other sites after 3 months and only decreased slightly after 6 months (82 ± 3.2%). There was no difference across the other sites after three months (64 – 75% viability), but after 6 months, viability was lower at Point Henry (24 ± 3.5%) than Blairgowrie (46 ± 7.5%) and Edwards Point (44 ± 5.3%). After 12 months, viability at Swan Bay decreased to 40% and was not significantly different to seeds from Blairgowrie but was higher than those at Edwards Point and Point Henry which had no viable seeds.
Seed germination
Germination of seeds from all sites was low (mean 16 ± 8.9%); however, there were significant differences across sites (t3 = − 9.61, P < 0.001, Table 5, Fig. 6). Seeds from exposed meadows at Edwards Point (23 ± 4.1%) and Blairgowrie (21 ± 4.3%) had the highest germination, followed by seeds from protected meadows at Point Henry (15 ± 6.7%), which all had similar mean germination. Seeds from protected meadows at Swan Bay had only 3 ± 1.7% germination, significantly lower than the other sites (P < 0.001). Mean time to germination (MTG) was significantly different across sites (Wald Chi Square3 = 25.36, P < 0.001) ranging from 32 to 55 days. Survival analysis found MTG at Blairgowrie (mean 47 days), Point Edwards (46 days) and Point Henry (32 days) had shorter MTG than Swan Bay (55 days, P < 0.001, Fig. 7). Seed viability at the end of the experiment (119 days) was twice as high at Blairgowrie (13 ± 3.6%) compared to Point Edwards (4 ± 3.3%) and Point Henry (3 ± 1.8%) (Table 4, Fig. 8). No seeds remained viable from Swan Bay at the end of the experimental period.
Boxplot of proportional number of seeds germinated in each replicate (n = 3) from each site. Lower and upper box boundaries are the 25th and 75th percentiles, respectively, line inside the box is the median and the lower and upper error bars represent the 10th and 90th percentiles. Note the y-axis only goes to 0.3
Boxplot of the proportion of viable seeds at the end of the germination experiment (119 days) at exposed/variable meadows at Blairgowrie and Edwards Point and protected/stable sites at Point Henry and Swan Bay. Lower and upper box boundaries are the 25th and 75th percentiles, respectively, line inside the box is the median and the lower and upper error bars represent the 10th and 90th percentilesa
Discussion
The size and mass of seeds produced by angiosperms play a major role in survival and persistence of plant populations, influencing germination, dispersal and seedling survival (Eriksson, 1999; Fricke et al., 2019). Seeds of the seagrass H. nigricaulis in Port Phillip Bay showed consistent differences in size and mass between sites and years. This is the first time consistent inter-annual patterns in seed size and mass variation at relatively local spatial scales that have been shown for seagrass, with only a few studies previously investigating seagrass seed size or mass (Combs et al., 2020; Delefosse et al., 2016; Jørgensen et al., 2019; Wyllie-Echeverria et al., 2003). Seeds from the exposed sites, Blairgowrie & Edwards Point, were 53 and 19% heavier, respectively, than those from the most sheltered sites, Point Henry and Swan Bay, regardless of year. Differences in seed size across sites may underpin variations in life-history strategies as populations adapt to local conditions.
Environmental conditions can be a major contributor to seed size and mass variation (Muller-Landau, 2010; Qi et al., 2014; Bergholz et al., 2015). The fitness advantages of larger seeds: higher germination rates, greater seedling growth and survival, are manifested in unfavourable conditions when parental provisions can substitute environmental provisions (Venable & Brown, 1988; Bergholz et al., 2015). Poor maternal conditions can lead to either the production of larger seeds that provide an advantage during germination and seedling establishment, or smaller seeds due to resource limitations (Qi et al., 2014). Populations exposed to unfavourable light, water and nutrient conditions produce larger seeds than those in favourable conditions (Parciak, 2002; Quero et al., 2007; Lázaro & Traveset, 2009; Muller-Landau, 2010; Bergholz et al., 2015) although the results are often mixed including for seagrass where unfavourable conditions can result in the production of many small seeds (Halpern, 2005; Qi et al., 2014; Suárez-Vidal et al., 2017; Combs et al., 2020). Populations with larger seeds are, therefore, more able to establish and colonize new or disturbed habitats, a strategy often used by invasive plant species which have been shown to produce larger seeds outside their natural range (Hierro et al., 2013, 2020; Sõber & Ramula, 2013).
In contrast, the benefits of large seeds are less important when the required resources can be readily acquired from the local environment (Venable & Brown, 1988; Hierro et al., 2020). Consistent differences in seed size and mass across sites in Port Phillip Bay suggest beneficial reproductive strategies that have evolved to ensure seedling recruitment and survival under the conditions experienced at each site leading to different reproductive strategies. Seagrass species increase reproductive output when stressed (Alexandre et al., 2005; Diaz-Almela et al., 2007; Cabaço & Santos, 2012) and in fragmented habitats (Livernois et al., 2017; Marín-Guirao et al., 2019; Stubler et al., 2017) which may be an important factor causing the greater seed mass observed at exposed sites (Blairgowrie and Edwards Point) where patches are smaller relative to the larger, more sheltered continuous meadows of Point Henry and Swan Bay.
Sites with large seeds, Blairgowrie and Edwards Point, had the highest rate of germination, germinated fastest and remained viable the longest showing clear benefits of large seeds in unfavourable conditions. Seagrass area and reproductive output at these sites vary temporally and are exposed to relatively high wave action, high current, low nitrogen availability and coarse sediment (Ball et al., 2014; Hirst & Jenkins, 2017; Smith et al., 2016a; Tran et al., 2021). High sediment transport via waves and longshore currents at these sites have led to a constant flux in seagrass cover as meadows are smothered and eroded as sediment moves along the shore reducing meadow persistence (Ball et al., 2014). Furthermore, nutrient availability can be limited at Blairgowrie reducing seagrass growth (Hirst & Jenkins, 2017). Greater seed germination and provision of more resources in larger seeds will provide a fitness advantage under these conditions. Larger seeds also have greater germination success at deeper burial depths which may be important at these sites that experience high wind and wave energy that resuspend sandy sediment and bury seeds more easily (Jørgensen et al., 2019). Larger seeds also sink faster, reducing dispersal but allowing seeds to stay within the meadow where conditions are more favourable (Delefosse et al., 2016) which may be beneficial at patchy sites like Blairgowrie and Edwards Point. Additionally, seeds from Blairgowrie remained viable longer than other sites providing an additional fitness advantage. Longer seed persistence allows seeds to remain dormant throughout stressful conditions and germinate when conditions improve or when opportunities arise within the meadow.
Production of small seeds has several fitness benefits including producing a larger number of propagules per plant and greater dispersal potential (Fricke et al., 2019). Small seeds were sampled at two sites, Point Henry and Swan Bay that have similar environmental conditions and generally have greater seed production than other sites (Smith et al., 2016a). Smaller, lighter seeds sank slower than larger heavier seeds, increasing the ability to disperse which may be important in large continuous meadows where competition for space is a key factor for seedling success (Delefosse et al., 2016). Seeds from Point Henry and Swan Bay (which were the smallest and lightest seeds), however, showed contrasting results in the germination and storage experiments indicating that there may be specific adaptive pressures at each site (Lázaro & Traveset, 2009). Seeds from Point Henry germinated quickly, similar to the larger seeds but had poor capacity to persist over time whereas seeds from Swan Bay had very low germination but had a high proportion of viable seeds after 12 months in storage similar to the large seeds from Blairgowrie. The ability of seeds from Swan Bay to remain viable for extended periods suggests some level of dormancy that requires specific cues to instigate germination that were not met in the germination experiment. Nitrogen levels in Swan Bay are low relative to the other sites and nitrogen uptake and provision by seagrass occurs via microbial pathways in the sediment, whereas at the other sites, nitrogen is catchment derived (Cook et al., 2015; Hirst & Jenkins, 2017); (Wang et al., 2017). Germination cues required to break dormancy can be site specific (Barga et al., 2017), and the microbial and/or nutrient conditions may be required to instigate germination in seeds from Swan Bay. Nutrients can play a key role in seagrass germination, increasing germination rates and reducing MGT (Wang et al., 2017). Differences in nitrogen levels at Swan Bay and Point Henry may help explain differences in seed performance as seeds from Point Henry can germinate rapidly and have access to nutrients allowing seedling to grow rapidly whereas, at Swan Bay, seeds can remain dormant until nutrient or microbial conditions are favourable. The contrasting performance of small seeds under similar, favourable conditions suggests that local conditions play a key role in determining life-history traits within a species as local population adapt to specific environmental conditions.
Ecological theory predicts resources are allocated to producing few large seeds that improves germination rates, seedling success and survival or to producing many small seeds, increasing the probability that some will survive and increasing dispersal abilities (Venable & Brown, 1988; Fricke et al., 2019). There is, however, increasing evidence that this is not always the case, and the tradeoff between seed size and number can be influenced by temporal and spatial conditions (Sõber & Ramula, 2013; Brancalion & Rodrigues, 2014). In Port Phillip Bay, the tradeoff between seed production and size is complex. Sites that produced small seeds (Point Henry and Swan Bay) consistently produce high densities of reproductive spathes and had large seed banks and Edwards Point, which had intermediate sized seeds, produced few spathes and had a small seed bank (Smith et al., 2016a, b) in line with ecological theory. Blairgowrie, on the other hand, where larger seeds were produced, has strong annual variation in spathe and seeds density but maintains large seed sizes regardless of year (Smith et al., 2016a, b). Variation in seed density but not size suggests that the benefits of producing large seeds (higher germination, longer persistence) by seagrass at Blairgowrie outweigh the benefits of producing larger amounts of seed, but when conditions are favourable, they are able to avoid risk and take advantage to produce high volumes of larger, heavier seeds.
Conclusion
This study highlights the complex nature of seagrass life history strategies and the need to assess multiple performance traits in the context of local environmental conditions. Larger seeds can provide multiple fitness benefits (e.g. greater germination and persistence) but smaller seeds performed well at only single-contrasting traits, either germination or persistence. These results are consistent with traditional ecological theory where plants trade off between the benefits of seed size on germination producing larger seeds in stressful conditions (Blairgowrie) but provided conflicting results where small seeds have high germination (Point Henry) or long persistence (Swan Bay) at different sites. The fitness benefits of producing large or small seeds at each site will depend on local selection pressures and tradeoffs between the benefits of seed size on seedling success, dispersal, dormancy and fecundity (Venable & Brown, 1988; Lázaro & Traveset, 2009). Our understanding of seagrass reproductive ecology is still in its infancy for many species and requires further research to improve our understanding of seagrass life history strategies (York et al., 2017). Greater understanding of the benefits of large seeds on germination success and local adaption of reproductive strategies will improve management strategies and restoration programmes of this keystone species.
Data availability
The datasets used and/or analysed during the current study will be made available in DRYAD on the acceptance of this manuscript.
References
Alexandre, A., R. Santos & E. Serrao, 2005. Effects of clam harvesting on sexual reproduction of the seagrass Zostera noltii. Marine Ecology Progress Series 298: 115–122.
Artika, S. R., R. Ambo-Rappe, M. Teichberg, A. Moreira-Saporiti & I. G. Viana, 2020. Morphological and physiological responses of Enhalus acoroides seedlings under varying temperature and nutrient treatment. Frontiers in Marine Science 7: 325.
Balestri, E., S. Gobert, G. Lepoint & C. Lardicci, 2009. Seed nutrient content and nutritional status of Posidonia oceanica seedlings in the northwestern Mediterranean Sea. Marine Ecology Progress Series 388: 99–109.
Ball, D., M. Soto-Berelov & P. Young, 2014. Historical seagrass mapping in Port Phillip Bay, Australia. Journal of Coastal Conservation 18(3): 257–272.
Barga, S., T. E. Dilts & E. A. Leger, 2017. Climate variability affects the germination strategies exhibited by arid land plants. Oecologia 185(3): 437–452.
Barton, K., 2020. MuMIn: Multi-Model Inference. 1.43.17. edn. R package.
Bergholz, K., F. Jeltsch, L. Weiss, J. Pottek, K. Geißler & M. Ristow, 2015. Fertilization affects the establishment ability of species differing in seed mass via direct nutrient addition and indirect competition effects. Oikos 124(11): 1547–1554.
Brancalion, P. H. S. & R. R. Rodrigues, 2014. Seed size-number trade-off in Euterpe edulis in plant communities of the Atlantic Forest. Scientia Agricola 71: 226–231.
Bulthuis, D. A. & W. J. Woelkerling, 1983. Seasonal variation in standing crop, density and leaf growth rate of the seagrass, Heterozostera tasmanica, in western Port and Port Phillip Bay, Victoria, Australia. Aquatic Botany 16(2): 111–136.
Cabaço, S. & R. Santos, 2012. Seagrass reproductive effort as an ecological indicator of disturbance. Ecological Indicators 23: 116–122.
Combs, A. R., J. C. Jarvis & W. J. Kenworthy, 2020. Quantifying variation in Zostera marina seed size and composition at the species’ southern limit in the Western Atlantic: implications for eelgrass population resilience. Estuaries and Coasts 44(2): 367–382.
Conacher, C. A., I. R. Poiner, J. Butler, S. Pun & D. J. Tree, 1994. Germination, storage and viability testing of seeds of Zostera capricorni Aschers. from a tropical bay in Australia. Aquatic Botany 49(1): 47–58.
Cook, P. L. M., V. Evrard & R. J. Woodland, 2015. Factors controlling nitrogen fixation in temperate seagrass beds. Marine Ecology Progress Series 525: 41–51.
Cribari-Neto, F., & A. Zeileis, 2010. Beta regression in R. Journal of Statistical Software 34: 1–24.
Cumming, E., J. C. Jarvis, C. D. Sherman, P. H. York & T. M. Smith, 2017. Seed germination in a southern Australian temperate seagrass. PeerJ 5: e3114.
Darnell, K. M., D. M. Booth, E. W. Koch & K. H. Dunton, 2015. The interactive effects of water flow and reproductive strategies on seed and seedling dispersal along the substrate in two sub-tropical seagrass species. Journal of Experimental Marine Biology and Ecology 471: 30–40.
Delefosse, M., K. Povidisa, D. Poncet, E. Kristensen & B. Olesen, 2016. Variation in size and chemical composition of seeds from the seagrass Zostera marina– Ecological implications. Aquatic Botany 131: 7–14.
Diaz-Almela, E., N. MarbÀ & C. M. Duarte, 2007. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Global Change Biology 13(1): 224–235.
Edgar, G. J. & C. Shaw, 1995. The production and trophic ecology of shallow-water fish assemblages in southern Australia 1. Species richness, size structure and production of fishes in Western Port, Victoria. Journal of Experimental Marine Biology and Ecology 194: 53–81.
Eriksson, O., 1999. Seed size variation and its effect on germination and seedling performance in the clonal herb Convallaria majalis. Acta Oecologica 20(1): 61–66.
Fenner, M. & K. Thompson, 2005. The ecology of seeds, Cambridge University Press:
Fox, J., & S. Weisberg. 2019. An R companion to applied regression, Third edition. Sage, Thousand Oaks CA. https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
Fricke, E. C., J. J. Tewksbury & H. S. Rogers, 2019. Linking intra-specific trait variation and plant function: seed size mediates performance tradeoffs within species. Oikos 128(12): 1716–1725.
Halpern, S. L., 2005. Sources and consequences of seed size variation in Lupinus perennis (Fabaceae): adaptive and non-adaptive hypotheses. American Journal of Botany 92(2): 205–213.
Hierro, J. L., Ö. Eren, D. Montesinos, K. Andonian, L. Kethsuriani, R. Özcan, A. Diaconu, K. Török, L. Cavieres & K. French, 2020. Increments in weed seed size track global range expansion and contribute to colonization in a non-native region. Biological Invasions 22(3): 969–982.
Hierro, J. L., Ö. Eren, D. Villarreal & M. C. Chiuffo, 2013. Non-native conditions favor non-native populations of invasive plant: demographic consequences of seed size variation? Oikos 122(4): 583–590.
Hirst, A., A. Longmore, D. Ball, P. Cook & G. Jenkins, 2016. Linking nitrogen sources utilised by seagrass in a temperate marine embayment to patterns of seagrass change during drought. Marine Ecology Progress Series 549: 79–88.
Hirst, A. J., K. Giri, D. Ball & R. S. Lee, 2017a. Determination of the physical drivers of Zostera seagrass distribution using a spatial autoregressive lag model. Marine and Freshwater Research 68: 1752–1763.
Hirst, A. J. & G. P. Jenkins, 2017. Experimental test of N-limitation for Zostera nigricaulis seagrass at three sites reliant upon very different sources of N. Journal of Experimental Marine Biology and Ecology 486: 204–213.
Hirst, A. J., S. McGain & G. P. Jenkins, 2017b. The impact of burial on the survival and recovery of the subtidal seagrass Zostera nigricaulis. Aquatic Botany 142: 10–15.
Hughes, A. R. & J. J. Stachowicz, 2011. Seagrass genotypic diversity increases disturbance response via complementarity and dominance. Journal of Ecology 99(2): 445–453.
Hutchinson, N., G. P. Jenkins, A. Brown & T. M. Smith, 2014. Variation with depth in temperate seagrass-associated fish assemblages in southern Victoria, Australia. Estuaries and Coasts 37(4): 801–814.
Infantes, E. & P.-O. Moksnes, 2018. Eelgrass seed harvesting: flowering shoots development and restoration on the Swedish west coast. Aquatic Botany 144: 9–19.
Jarvis, J. & K. Moore, 2015. Effects of seed source, sediment type, and burial depth on mixed-annual and perennial Zostera marina L. seed germination and seedling establishment. Estuaries and Coasts 38(3): 964–978.
Jarvis, J. C. & K. A. Moore, 2010. The role of seedlings and seed bank viability in the recovery of Chesapeake Bay, USA, Zostera marina populations following a large-scale decline. Hydrobiologia 649: 55–68.
Jenkins, G. P., M. J. Keough, D. Ball, P. L. M. Cook, A. Ferguson, J. Gay, A. J. Hirst, R. Lee, A. D. Longmore, P. I. Macreadie, S. Nayar, C. D. H. Sherman, T. M. Smith & P. H. York, 2015. Seagrass resilience in Port Phillip Bay, University of Melbourne, Melbourne:
Jenkins, G. P., G. K. Walker-Smith & P. A. Hamer, 2002. Elements of habitat complexity that influence harpacticoid copepods associated with seagrass beds in a temperate bay. Oecologia 131: 598–605.
Johnson, A. J., R. J. Orth & K. A. Moore, 2020. The role of sexual reproduction in the maintenance of established Zostera marina meadows. Journal of Ecology 108(3): 945–957.
Jørgensen, M. S., R. Labouriau & B. Olesen, 2019. Seed size and burial depth influence Zostera marina L. (eelgrass) seed survival, seedling emergence and initial seedling biomass development. PLoS ONE 14(4): e02151570.
Kendrick, G. A., M. Waycott, T. J. B. Carruthers, M. L. Cambridge, R. Hovey, S. L. Krauss, P. S. Lavery, D. H. Les, R. J. Lowe, O. I. Vidal, J. L. S. Ooi, R. J. Orth, D. O. Rivers, L. Ruiz-Monyoya, E. A. Sinclair, J. Statton, J. K. van Dijk & J. J. Verduin, 2012. The central role of dispersal in the maintenance and persistence of seagrass populations. Biosience 62(1): 56–65.
Klein, J. P. & M. L. Moeschberger, 2003. Survival analysis: techniques for censored and truncated data. Berlin: Springer
Lázaro, A. & A. Traveset, 2009. Does the spatial variation in selective pressures explain among-site differences in seed mass? A test with Buxus balearica. Evolutionary Ecology 23(6): 847.
Lenth, R. V., P. Buerkner, M. Herve, J. Love, H. Riebl, & H. Singmann. 2021. emmeans: Estimated Marginal Means, aka Least-Squares Means (Version 1.6.3).
Livernois, M. C., J. H. Grabowski, A. K. Poray, T. C. Gouhier, A. R. Hughes, K. F. O’Brien, L. A. Yeager & F. J. Fodrie, 2017. Effects of habitat fragmentation on Zostera marina seed distribution. Aquatic Botany 142: 1–9.
Macreadie, P. I., P. H. York, C. D. H. Sherman, M. J. Keough, D. J. Ross, A. M. Ricart & T. M. Smith, 2014. No detectable impact of small-scale disturbances on ‘blue carbon’ within seagrass beds. Marine Biology 161(12): 2939–2944.
Marín-Guirao, L., L. Entrambasaguas, J. M. Ruiz & G. Procaccini, 2019. Heat-stress induced flowering can be a potential adaptive response to ocean warming for the iconic seagrass Posidonia oceanica. Molecular Ecology.
Marion, S. R. & R. J. Orth, 2010. Innovative techniques for large-scale seagrass restoration using Zostera marina (eelgrass) seeds. Restoration Ecology 18(4): 514–526.
McMahon, K. M., K.-J. van Dijk, L. Ruiz-Montoya, G. A. Kendrick, S. L. Krauss, M. Waycott, J. Verduin, R. Lowe, J. Statton, E. Brown & C. Duarte, 2014. The movement ecology of seagrasses. Proceedings of the Royal Society B: Biological Sciences 281: 1795.
McNair, J. N., A. Sunkara & D. Frobish, 2012. How to analyse seed germination data using statistical time-to-event analysis: non-parametric and semi-parametric methods. Seed Science Research 22(02): 77–95.
Moles, A. T., D. D. Ackerly, C. O. Webb, J. C. Tweddle, J. B. Dickie & M. Westoby, 2005. A brief history of seed size. Science 307(5709): 576–580.
Muller-Landau, H. C., 2010. The tolerance–fecundity trade-off and the maintenance of diversity in seed size. Proceedings of the National Academy of Sciences 107(9): 4242–4247.
Münzbergová, Z. & I. Plačková, 2010. Seed mass and population characteristics interact to determine performance of Scorzonera hispanica under common garden conditions. Flora - Morphology, Distribution, Functional Ecology of Plants 205(8): 552–559.
O’Brien, K. R., M. Waycott, P. Maxwell, G. A. Kendrick, J. W. Udy, A. J. Ferguson, K. Kilminster, P. Scanes, L. J. McKenzie & K. McMahon, 2017. Seagrass ecosystem trajectory depends on the relative timescales of resistance, recovery and disturbance. Marine Pollution Bulletin 134: 166.
Olejniczak, P., M. Czarnoleski, A. Delimat, B. M. Majcher & K. Szczepka, 2018. Seed size in mountain herbaceous plants changes with elevation in a species-specific manner. PLoS ONE 13(6): e0199224.
Orth, R. J., T. J. B. Carruthers, W. C. Dennison, C. M. Duarte, J. W. Fourqurean, K. L. Heck, A. R. Hughes, G. A. Kendrick, W. J. Kenworthy, S. Olyarnik, F. T. Short, M. Waycott & S. L. Williams, 2006. A global crisis for seagrass ecosystems. Bioscience 56(12): 987–996.
Orth, R. J., M. Luckenbach & K. A. Moore, 1994. Seed dispersal in a marine macrophyte: implications for colonization and restoration. Ecology 75(7): 1927–1939.
Ospina, R. & S. L. Ferrari, 2010. Inflated beta distributions. Statistical Papers 51(1): 111–126.
Parciak, W., 2002. Environmental variation in seed number, size, and dispersal of a fleshy-fruited plant. Ecology 83(3): 780–793.
Paz, H. & M. Martinez-Ramos, 2003. Seed mass and seedling performance within eight species of Psychotria (Rubiaceae). Ecology 84(2): 439–450.
Qi, W., S. Guo, X. Chen, J. H. C. Cornelissen, H. Bu, G. Du, X. Cui, W. Li & K. Liu, 2014. Disentangling ecological, allometric and evolutionary determinants of the relationship between seed mass and elevation: insights from multiple analyses of 1355 angiosperm species on the eastern Tibetan Plateau. Oikos 123(1): 23–32.
Quero, J. L., R. Villar, T. Marañón, R. Zamora & L. Poorter, 2007. Seed-mass effects in four Mediterranean Quercus species (Fagaceae) growing in contrasting light environments. American Journal of Botany 94(11): 1795–1803.
Rigby, R. A. & D. M. Stasinopoulos, 2005. Generalized additive models for location, scale and shape. Journal of the Royal Statistical Society: Series C (applied Statistics) 54(3): 507–554.
Ruiz-Montoya, L., R. J. Lowe, K. P. Van Niel & G. A. Kendrick, 2012. The role of hydrodynamics on seed dispersal in seagrasses. Limnology and Oceanography 57(5): 1257–1265.
Satterly, J., 1960. Formulae for volumes, surface areas and radii of gyration of spheres, elliposids and spheroids. The Mathematical Gazette 44(347): 15–19.
Scott, S. J., R. A. Jones & W. A. Williams, 1984. Review of data analysis methods for seed germination. Crop Science 24(6): 1192–1199.
Sherman, C. D. H., T. M. Smith, P. H. York, J. C. Jarvis, L. Ruiz-Montoya & G. A. Kendrick, 2018. Reproductive, dispersal and recruitment strategies in Australian seagrasses. In Larkum, A. W. D., G. A. Kendrick & P. J. Ralph (eds), Seagrasses of Australia: structure, ecology and conservation. Cham: Springer. 213–256.
Smith, T. M., P. H. York, M. J. Keough, P. I. Macreadie, D. J. Ross & C. D. H. Sherman, 2016a. Spatial variation in reproductive effort of a Southern Australian seagrass. Marine Environmental Research 120: 214–224.
Smith, T. M., P. H. York, P. I. Macreadie, M. J. Keough, D. J. Ross & C. D. H. Sherman, 2016b. Recovery pathways from small-scale disturbance in a temperate Australian seagrass. Marine Ecology Progress Series 542: 97–108.
Sõber, V. & S. Ramula, 2013. Seed number and environmental conditions do not explain seed size variability for the invasive herb Lupinus polyphyllus. Plant Ecology 214(6): 883–892.
Stubler, A. D., L. J. Jackson, B. T. Furman & B. J. Peterson, 2017. Seed Production Patterns in Zostera marina: Effects of patch size and landscape configuration. Estuaries and Coasts 40(2): 564–572.
Suárez-Vidal, E., L. Sampedro & R. Zas, 2017. Is the benefit of larger seed provisioning on seedling performance greater under abiotic stress? Environmental and Experimental Botany 134: 45–53.
Sullivan, B., 2019. Heterozostera resilience, University of Melbourne, Melbourne:
Team, R. C., 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing.
Therneau, T. M. & P. M. Grambsch, 2000. Modeling survival data: extending the Cox model. Berlin: Springer
Thomson, A. C. G., P. H. York, T. M. Smith, C. D. H. Sherman, D. J. Booth, M. J. Keough, D. J. Ross & P. I. Macreadie, 2015. Seagrass viviparous propagules as a potential long-distance dispersal mechanism. Estuaries and Coasts 38(3): 927–940.
Tran, H. Q., D. Provis & A. V. Babanin, 2021. Hydrodynamic climate of Port Phillip Bay. Journal of Marine Science and Engineering 9(8): 898.
Vaughton, G. & M. Ramsey, 1998. Sources and consequences of seed mass variation in Banksia marginata (Proteaceae). Journal of Ecology 86(4): 563–573.
Venable, D. L. & J. S. Brown, 1988. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. The American Naturalist 131(3): 360–384.
Wang, M., X. Tang, H. Zhang & B. Zhou, 2017. Nutrient enrichment outweighs effects of light quality in Zostera marina (eelgrass) seed germination. Journal of Experimental Marine Biology and Ecology 490: 23–28.
Wyllie-Echeverria, S., P. Cox, A. Churchill, J. Brotherson & T. Wyllie-Echeverria, 2003. Seed size variation within Zostera marina L. (Zosteraceae). Botanical Journal of the Linnean Society 142(3): 281–288.
York, P. H., T. M. Smith, R. G. Coles, S. A. McKenna, R. M. Connolly, A. D. Irving, E. L. Jackson, K. McMahon, J. W. Runcie, C. D. H. Sherman, B. K. Sullivan, S. M. Trevathan-Tackett, K. E. Brodersen, A. B. Carter, C. J. Ewers, P. S. Lavery, C. M. Roelfsema, E. A. Sinclair, S. Strydom, J. E. Tanner, K.-J. van Dijk, F. Y. Warry, M. Waycott & S. Whitehead, 2017. Identifying knowledge gaps in seagrass research and management: an Australian perspective. Marine Environmental Research 127: 163–172.
Zuur, A., E. N. Ieno & G. M. Smith, 2007. Analyzing ecological data. Berlin: Springer.
Acknowledgements
All research was undertaken at the Victorian Marine Science Consortium and funded by the Department of Environment, Land, Water and Planning Victoria. We would like to thank Rod Watson and Liz McGrath for field and laboratory assistance. All research was undertake in accordance with relevant approvals and permits.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project was funded by the Department of Environment, Land, Water and Planning Victoria.
Author information
Authors and Affiliations
Contributions
TS, CS, EC, PY and JJ conceived and designed the experiments. TS, EC and PY performed the experiments. TS, EC and JJ analysed the data. TS, CS, EC, PY and JJ wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Ethics approval was not required for this study according to Victorian law.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Handling Editor: Daniele Nizzoli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smith, T.M., Sherman, C.D.H., Cumming, E.E. et al. Size matters: variations in seagrass seed size at local scales affects seed performance. Hydrobiologia 849, 2335–2352 (2022). https://doi.org/10.1007/s10750-022-04873-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04873-1