Abstract
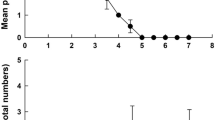
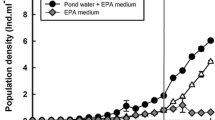
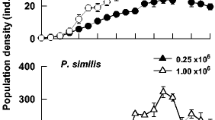
Life history strategies of rotifers vary in relation to their habitats. We compared the demography of Hexarthra jenkinae (de Beauchamp, 1932) from ephemeral pools and the large, permanent Nabor Carrillo Lake, both parts of the saline Lake Texcoco in Mexico. Population growth tests were conducted at salinities of 10, 12.5 and 15 g l−1 (1:1 ratio of NaCl and NaHCO3), at 22 ± 1 °C with two algal food concentrations (0.25 × 106 and 1.0 × 106 cells ml−1 of Nannochloropsis oculata). The population growth experiments were initiated with ten individuals per jar, whereas for the cohort life table study we used ten neonates. Observations were taken once (population growth) or twice (life table) a day; experiments continued until the populations declined or the last individual of each cohort in the life table study died. Survivorship and growth rates increased with increasing food availability. From the population growth studies, we found that the clone established from the ephemeral pools had higher growth rates (0.23–0.55 day−1) than those from the permanent lake (0.12–0.34 day−1). Growth rates were lower in the lifetable experiments than in the population growth studies. Our results are discussed in relation to habitat and life history strategies exhibited by rotifers in shallow saline waterbodies.







Similar content being viewed by others
Data availability
We confirm that our data will be available to anyone interested to re-use them, after the manuscript has been published and with approval of the copyright owner.
References
Afonina, E. & N. Tashlikova, 2019. Plankton of Saline lakes in Southeastern Transbaikalia: Transformation and environmental factors. Contemporary Problems of Ecology 12: 192–209.
Arcifa, M. S., B. B. de Souza, C. S. de Morais-Junior & C. G. C. Bruno, 2020. Functional groups of rotifers and an exotic species in a tropical shallow lake. Scientific Reports 10: 14698.
Berres, T. E., 2000. Climatic change and lacustrine resources at the period of initial Aztec development. Ancient Mesoamerica 11: 27–38.
Borowitzka, M. A. & L. J. Borowitzka, 1988. Micro-algal Biotechnology, Cambridge University Press, London:
Brain, C. K., I. Fouriel & R. J. Shiel, 1995. Rotifers of the Kalahari Gemsbok National Park. South Africa. Hydrobiologia 313(314): 319–324.
Brönmark, C. & L.-A. Hansson, 2017. The Biology of Lakes and Ponds, 3rd ed. Oxford University Press, London.
Burian, A., M. J. Kainz, M. Schagerl & A. Yasindi, 2014. Species-specific separation of lake plankton reveals divergent food assimilation patterns in rotifers. Freshwater Biology 59: 1257–1265.
Carmona Ruiz, J. A., 2015. Análisis de la diversidad y dinámica poblacional del zooplancton en el Lago Nabor Carrillo, Texcoco, Thesis, Universidad Nacional Autónoma de México (UNAM), Mexico, Estado de México. B. Sc.
De la Lanza-Espinoza, G. & J. L. García-Calderon, 2002. Lagos y presas de México, AGT Publishers, Mexico City.
Dieguez, M. & J. J. Gilbert, 2002. Suppression of the rotifer Polyarthra remata by the omnivorous copepod Tropocyclops extensus: predation or competition. Journal of Plankton Research 24: 359–369.
Gilbert, J. J., 2003. Environmental and endogeneous control of sexuality in a rotifer life cycle: developmental and population biology. Evolution and Development 5: 19–24.
Gilbert, J. J., 2017. Resting-egg hatching and early population development in rotifers: a review and a hypothesis for differences between shallow and deep waters. Hydrobiologia 796: 235–243.
Gilbert, J. J., 2020. Variation in the life cycle of monogonont rotifers: commitment to sex and emergence from diapause. Freshwater Biology 65: 786–810.
Grant, W.D., 2006. Alkaline environments and biodiversity. In: Extremophilies (Eds. Charles Gerday, and Nicolas Glansdorff), in Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford, UK.
Hietala, J., M. Reinikainen & M. Walls, 1995. Variation in life history responses of Daphnia to toxic Microcystis aeruginosa. Journal of Plankton Research 17: 2307–2318.
Jaramillo-Londoño, J. C. & R. M. Pinto-Coelho, 2010. Interaction between Hexarthra intermedia (Rotifera) and Bosmina longirostris (Cladocera): a case of opportunistic nutrition or interference competition? Journal of Plankton Research 32: 961–966.
Kak, A. & T. R. Rao, 1998. Does the evasive behavior of Hexarthra influence its competition with cladocerans? Hydrobiologia 387(388): 409–419.
Koste, W., 1978. Rotatoria, die Rädertiere Mitteleuropas: Überordnung Monogononta: ein Bestimmungswerk. Gebrüder Borntraeger, Berlin and Stuttgart. 2 vols.
Krebs, C. J., 1985. Ecology; The Experimental Analysis of Distribution and Abundance, 3rd ed. Harper & Row, New York.
Lavens, P. & P. Sorgeloos (eds), 1996. Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper No. 361. Rome, FAO, 295p.
Li, J., S. Sun, C. Li, Z. Zhang & X. Pu, 2007. Effects of different diets on the reproduction and naupliar development of the copepod Acartia bifilosa. Journal of Experimental Marine Biology and Ecology 355: 95–102.
Nandini, S. & S. S. S. Sarma, 2019. Reproductive strategies of Moina (Cladocera) in relation to their habitat. Limnetica 38: 137–145.
Nandini, S., 2000. Responses of rotifers and cladocerans to Microcystis aeruginosa (Cyanophyceae): A demographic study. Aquatic Ecology 34: 227–242.
Nandini, S. & T. R. Rao, 1998. Somatic and population growth in selected cladoceran and rotifer species offered the cyanobacterium Microcystis aeruginosa as food. Aquatic Ecology 31: 283–298.
Nandini, S., C. A. Zamora-Barrios & S. S. S. Sarma, 2020. A long-term study on the effect of cyanobacterial crude extracts from Lake Chapultepec (Mexico City) on selected zooplankton species. Environmental Toxicology and Chemistry 39: 2409–2419.
Paes, C. R. P. S., G. R. Faria, N. A. B. Tinoco, D. J. F. A. Castro, E. Barbarino & S. O. Lourenço, 2016. Growth, nutrient uptake and chemical composition of Chlorella sp. and Nannochloropsis oculata under nitrogen starvation. Latin American Journal of Aquatic Research 44: 275–292.
Post, D. M., E. P. Palkovacs, E. G. Schielke & S. I. Dodson, 2008. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology 89: 2019–2032.
Pourriot, R. & T. W. Snell, 1983. Resting eggs of rotifers. Hydrobiologia 104: 213–224.
Sanders, R. W., K. G. Porter, S. J. Bennett & A. E. Debiase, 1989. Seasonal pattern of bacterivory by flagellates, ciliates, rotifer and cladocerans in a freshwater planktonic community. Limnology and Oceanography 34: 673–687.
Sarma, S.S.S. & S. Nandini, 2017. Rotíferos Mexicanos (Rotifera). Estado de México. Manual de Enseñanza. Universidad Nacional Autónoma de México, Mexico City / Facultad de Estudios Superiores Iztacala, Tlalnepantla.
Sarma, S. S. S., B. Elguea-Sánchez & S. Nandini, 2002. Effect of salinity on competition between the rotifers Brachionus rotundiformis Tschugunoff and Hexarthra jenkinae (De Beauchamp) (Rotifera). Hydrobiologia 474: 183–188.
Schröder, T., S. Howard, M. L. Arroyo & E. J. Walsh, 2007. Sexual reproduction and diapause of Hexarthra sp. (Rotifera) in short-lived ponds in the Chihuahuan Desert. Freshwater Biology 52: 1033–1042.
Schröder, T. & J. J. Gilbert, 2004. Transgenerational plasticity for sexual reproduction and diapause in the life cycle of monogonont rotifers: intraclonal, intraspecific and interspecific variation in the response to crowding. Functional Ecology 18: 458–466.
Segers, H., 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564: 1–104.
Sigala, I., M. Caballero, A. Correa-Metrio, S. Lozano-García, G. Vázquez, L. Pérez & E. Zawisza, 2017. Basic limnology of 30 continental waterbodies of the Transmexican Volcanic Belt across climatic and environmental gradients. Boletín De La Sociedad Geológica Mexicana 69: 313–370.
Smith, H. A. & T. W. Snell, 2014. Differential evolution of lifespan and fecundity between asexual and sexual females in a benign environment. International Review of Hydrobiology 99: 1–8.
Starkweather, P. L., 2005. Susceptibility of ephemeral pool Hexarthra to predation by the fairyshrimp Branchinecta mackini: can predation drive local extinction? Hydrobiologia 546: 503–508.
Stelzer, C. P., 2005. Evolution of rotifer life histories. Hydrobiologia 546: 335–346.
Storey, R. G. & J. M. Quinn, 2011. Life histories and life history strategies of invertebrates inhabiting intermittent streams in Hawke’s Bay, New Zealand. New Zealand Journal of Marine and Freshwater Research 45: 213–230.
Valenzuela-Encinas, C., I. Neria-González, R. J. Alcántara-Hernández, J. A. Enríquez-Aragón, I. Estrada-Alvarado & C. Hernández-Rodríguez, 2008. Phylogenetic analysis of the archael community in an alkaline-saline soil of the former lake Texcoco (Mexico). Extremophiles 12: 247–254.
Wallace, R. L., T.W. Snell, C. Ricci & T. Nogrady, 2006. Rotifera: Volume 1 Biology, Ecology and Systematics (2nd ed.). Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 23 (Segers, H., ed.). Kenobi Productions, Ghent, and Backhuys Publishers, Leiden.
Wallace, R.L., T.W. Snell, E.J. Walsh, S.S.S. Sarma & H. Segers, 2019. Chapter 8. Phylum Rotifera. Keys to Palaearctic Fauna. In: Thorp and Covich’s Freshwater Invertebrates. Volume IV. Fourth Edition. Academic Press / Elsevier. 219–267.
Walsh, E. J., H. A. Smith & R. L. Wallace, 2014. Rotifers of temporary waters. International Review of Hydrobiology 99: 3–19.
Weber, C.I., 1993. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 4th ed. United States Environmental Protection Agency. EPA/600/4-90/027F, Cincinnati.
Zamora Barrios, C. A., S. Nandini & S. S. S. Sarma, 2017. Effect of crude extracts from cyanobacterial blooms in Lake Texcoco (Mexico) on the population growth of Brachionus calyciflorus (Rotifera). Toxicon 139: 45–53.
Acknowledgements
This study forms part of the M.Sc. thesis of JACR at Posgrado en Ciencias del Mar y Limnología (UNAM). SN and SSSS are grateful to PAPIIT (Grant No. IG 200820) and CONACyT (Grant Nos. 20520, 713345 and 18723) for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that all the co-authors of this manuscript have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: José L. Attayde, Renata F. Panosso, Vanessa Becker, Juliana D. Dias & Erik Jeppesen / Advances in the Ecology of Shallow Lakes
Rights and permissions
About this article
Cite this article
Nandini, S., Carmona-Ruiz, J.A. & Sarma, S.S.S. Demography of Hexarthra jenkinae (de Beauchamp) (Rotifera) from ephemeral and permanent habitats of the shallow waterbody Lake Texcoco (Mexico). Hydrobiologia 849, 4073–4085 (2022). https://doi.org/10.1007/s10750-021-04784-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04784-7