Abstract
Ecological impacts caused by invasive alien species can be severe but may vary depending upon environmental conditions. Many European populations of the native mussel, Mytilus edulis, have been invaded by the Pacific oyster, Crassostrea (Magallana) gigas. Although widespread invasions have occurred, interactions between M. edulis and C. gigas have largely been investigated with regards to competition for space and food as well as effects on species assemblages. Experimental investigation of competitive interactions on physiological responses of the two species requires further exploration. To this end, we used a 12-month field manipulation experiment to examine growth rates, mortality and condition indices of the two species occurring in monospecific and heterospecific groups. Growth rates and mortality of both species were similar in monospecific and heterospecific groups, whereas condition indices were significantly reduced for both species in heterospecific groups. Growth rates and condition indices also differed amongst experimental sites, potentially due to differing water motion. Shell weight-length relationships did not explain the observed differences in condition for either species. We show that coexistence between the two species may occur but could be detrimental for both species. We also provide a preliminary viewpoint that water motion can mediate competitive interactions between these species.
Similar content being viewed by others
Introduction
The rate of introductions of invasive alien species (IAS) is ever increasing (Seebens et al., 2017, 2018), potentially threatening biodiversity and ecosystem functioning (Bax et al., 2003; Molnar et al., 2008; Simberloff et al., 2013). Interconnectivity between regions is one of the largest sources of marine IAS across the globe (Molnar et al., 2008; Ricciardi et al., 2017), which may become exacerbated with faster ships and climate change revealing inter-ocean shortcuts (Miller & Ruiz, 2014). Introductions of IAS can induce competition with taxonomically and trophically analogous native species for resources such as space (Connell, 1961) and food (Bergstrom & Mensinger, 2009). Success of IAS is often related to their superior competitive ability to exploit resources, which in turn can reduce the fitness of inferior competitors and eventually result in competitive exclusion (Byers, 2000; Carlton et al., 1999). However, asymmetries in competitive ability as well as susceptibility to biotic interactions can lead to the coexistence of species with similar niches (Heard & Sax, 2013). Quantifying IAS success and impacts has shown success through the use of methodologies such as comparative functional responses (Dick et al., 2017), but such methods do not measure effects of invaders on native species physiology and health. Identifying such physiological effects requires manipulative experiments (Zwerschke et al., 2018), however, these can be challenging and require a number of variables to be measured to fully understand invader impacts (Kumschick et al., 2014).
The Pacific oyster, Crassostrea (Magallana) gigas (Thunberg, 1793) (Salvi & Mariottini, 2017, but see Bayne et al., 2017), after introductions through aquaculture activities, has become one of the most globalised marine invertebrates (Herbert et al., 2016). Successful range expansion of C. gigas has increased the threats to native bivalve species. The Wadden Sea, for example, has seen mass colonisation onto beds of the native blue mussel, Mytilus edulis Linnaeus, 1758, causing shifts in assemblages of reef associated fauna (Kochmann et al., 2008). As mussels provide an important and abundant food resource for a range of intertidal and subtidal predators including birds, sea stars and crabs (Ebling et al., 1964; Paine, 1974; Nehls et al., 1997), declines in numbers may have significant consequences for higher trophic levels. On the other hand, oyster reefs can provide refuge for mussels from predation threats potentially allowing increased survival (Eschweiler & Christensen, 2011). In addition, multiple cases of oyster invasion on mussel beds has led to coexisting populations (Diederich, 2005; Holm et al., 2016; Reise et al., 2017), yet, the lack of competitive exclusion of mussels has postulated further questions regarding interactions between the two.
Invasions by C. gigas have been shown to alter benthic species assemblages (Guy et al., 2018), sediment chemistry and ecosystem functioning (Green et al., 2012, 2013) for a range of native habitats including mussel beds, soft sediment beds, and native oyster reefs. Although several negative impacts of C. gigas have been documented, they are suggested to be context dependent (Padilla, 2010). That is, a myriad of biotic and abiotic factors are likely to alter the magnitude of the invasion success and ecological impacts exerted by invaders (Alexander et al., 2012; Laverty et al., 2015; Joyce et al., 2019a; 2020). For example, Green & Crowe (2014) showed that C. gigas increased biodiversity compared to mudflats, whereas Walles et al. (2015) demonstrated that C. gigas reef formation increased coastal protection of tidal flats from erosion owing to their ecosystem engineering effects.
Although a range of ecological impacts of C. gigas are apparent, investigations of competitive interactions and effects on the physiological state of trophically similar native species are lacking. For many sessile species, investigations of competition largely observe space as the most important factor and focus on growth rates or density (Connell, 1961; Konar & Iken, 2005). Although space is a primary resource for such organisms, their ability to grow relies on their ability to ingest food from the surrounding environment. Plankton resources are thought to be plentiful in coastal environments, nonetheless, seston depletion can occur in close proximity to bivalve beds (Dolmer, 2000; Vismann et al., 2016). This, in turn, may result in interspecific competition for resources and thus, competitive interactions are more complex than identifying the ability of an organism to occupy space. Further, as bivalve shell and flesh growth are not directly related (Hilbish, 1986), quantifying growth rates alone may achieve a poor assessment of population health, therefore, documenting condition indices is essential to fully understand competitive interactions.
Observations in Europe show that M. edulis and C. gigas colonise similar areas and patterns of coexistence are emerging (Diederich, 2005; Reise et al., 2017). Although resource segregation between many filter feeding species has been found (Dubois et al., 2007), some competition between M. edulis and C. gigas for plankton resources may occur due to overlapping particle retention size (Ward & Shumway, 2004) and similar retention efficiency for particular phytoplankton species (Bougrier et al., 1997). Further competition can occur through intraguild predation. Troost et al. (2008) showed that both species filter M. edulis larvae more efficiently from the water column compared to larvae of C. gigas. Experimental investigations have shown differential effects of the two species on ecosystem functioning and how various environmental factors may affect biological responses of both species in isolation (Diederich, 2006; Kochmann et al., 2008; Kochmann & Crowe, 2014). Interactions and impacts of invading C. gigas with native species have been investigated with regards to feeding (Joyce et al., 2019b), parasite loading (Goedknegt et al., 2017), competition for space (Padilla, 2010) and have been shown to be largely context dependent (Krassoi et al., 2008; Zwerschke et al., 2018). Although such interactions of C. gigas with native mussels have been investigated, direct competitive interactions on their physiological responses regarding co-occurrence are not fully understood (but see Eschweiler & Christensen, 2011).
Here, we thus conducted a field manipulation experiment to identify competitive interactions between the native blue mussel, Mytilus edulis, and the invasive Pacific oyster, Crassostrea gigas, by comparing their growth rates, mortality and condition indices when occurring in monospecific cultures compared to heterospecific cultures. Key environmental conditions including seawater particulate matter and temperature were measured throughout the experimental period to identify whether key variables differed amongst the experimental replicate sites. Knowledge of the competitive interactions between M. edulis and C. gigas, as well as how they can change with environmental conditions, will facilitate the quantification and potential prediction of ecological impacts of invasions.
Methods
Study area and experimental setup
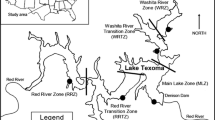
This experiment was conducted on the intertidal rocky shores of Strangford Lough, Northern Ireland, and the surrounding area. One site was located within the main body of the Lough (Killyleagh; KL; 54° 24′ 03′′ N, 5° 38′ 25′′ W), one site was within the narrow inlet to the Lough (Walter Shore; WS; 54° 23′ 05′′ N, 5° 33′ 25′′ W) and one site was outside the Lough on the Irish Sea coast (Ballyhornan; BH; 54° 18′ 24′′ N, 5° 32′ 36′′ W). Occurrence of M. edulis at the sites is common, however, extensive beds such as those found on soft sediment shores do not occur on these rocky shores. Occurrence of C. gigas is rare at the selected sites, but frequently occurs in the northern basin of Strangford Lough (Zwerschke et al., 2017). The experiment was conducted at the selected sites to incorporate environmental variability allowing for greater generalisation of the results. The use of a field experiment setting allows the organisms to be subject to more realistic environmental conditions than in a laboratory setting, however, the precision of the laboratory is somewhat lost in this trade-off.
Blue mussels, M. edulis (mean shell length ± SD: 22.8 ± 1.6 mm), were collected from a rocky shore within Strangford Lough (54o 28′ 11.2″ N, 5o 32′ 25.4″ W) and Pacific oysters, C. gigas (mean shell length ± SD: 18.4 ± 1.0 mm), were obtained from Guernsey Sea Farms Ltd (July 2017). Note that as the experiment was designed to examine the effects of species presence on performance, and not to compare absolute differences in growth and condition between the two species, it was not necessary to use animals of the same size and age. In the laboratory, individuals were dabbed dry, weighed and shell length was measured to the nearest 0.1 mm with digital calipers. To identify individuals throughout the experiment, shells were marked randomly with either a coloured plastic stone attached to the shell with cyanoacrylate glue or with nail varnish covered with a layer of cyanoacrylate.
Perspex plates (25 × 25 cm) had 26 individuals of either mussels only, oysters only, or a mixture of 13 mussels and 13 oysters attached for placement in the field. This density is lower than long-established beds (e.g. Vismann et al., 2016; Reise et al., 2017) but is not uncommon (Dolmer, 2000) and similar densities have been used in previous experimental manipulations (Kochmann & Crowe, 2014; Zwerschke et al., 2018). Mussels were placed on Perspex plates and kept submerged in running seawater to allow natural attachment with byssus threads. Oysters were attached cupped valve down using Milliput© (Dolgellau, UK) 2-part epoxy and were attached in random positions on the plate and oriented randomly (i.e. not all facing the same way). The natural attachment of mussels to the experimental plates led to some clumping and a less uniform distribution than for oysters, however, plate density remained the same. The manipulation of species treatments using experimental plates rather than manipulation on natural substrates was unavoidable as the likelihood of recapturing the organisms in the highly dynamic environment is very low. Experimental plates were covered with a rigid plastic cage (5 cm height × 25 cm × 25 cm, mesh size 1 cm) lined with a fine plastic mesh (mesh size ~ 0.3 cm) to prevent loss of any detached animals. The combination of mesh materials was designed to have minimal effects on internal water motion. Cages were secured to the Perspex plates and animals were kept for < 1 week in the laboratory on the plates before placement at respective sites in August 2017. Cattle tags were attached to plates to identify each plate. Here, uncaged controls were not used as previous studies have shown high mortality in uncaged treatments (Kochmann & Crowe, 2014; Zwerschke et al., 2018) and therefore would not be informative for this study. At each site, 3 replicate plates of each species combination were attached to large rocks during spring low tides (n = 9 plates per site), totalling 27 experimental plates. Experimental plates were placed at the top end of the Laminaria digitata intertidal distribution zone at the respective sites and were spaced at least 1 m apart. This zone within the intertidal is common for occurrence of these species. Each month, cages were cleared of any fouling organisms to ensure sufficient water flow through the cages. Further, every three months fouling from the experimental plates as well as cages were also cleared. No noticeable settlement of potentially competing filter feeders such as barnacles or mussel spat occurred. Although predators (crabs and whelks) were excluded from direct contact with the study organisms, they were present at all experimental sites, but densities were not quantified.
After 12 months, animal lengths were measured using digital calipers to calculate relative growth rates. The flesh and shell of all remaining live animals were separated and dried at 60°C for > 48 h, until weights remained stable and condition indices were calculated according to Walne (1976) as:
to be compared between experimental species composition type for mussels and oysters, separately. Mortality was also quantified for each experimental plate.
Due to storm events, only 1 replicate plate per species composition remained at the BH site but these plates were included in the analyses. At both the WS and KL sites, all replicates remained after 1 year of field exposure.
Environmental variables
As a proxy for food availability, monthly water samples were collected at each site to assess average yearly seston quality including total particulate matter (TPM), organic particulate matter (OPM) and the organic percentage of the total particulate matter (OPM/TPM). Water samples were filtered through pre-combusted, pre-weighed glass fibre GF-F filters, rinsed with two 10 ml samples of ammonium formate to remove salts from the filter, and dried at 60°C for 48 h. Filters were left to cool for 1 h in a desiccator at room temperature and re-weighed for TPM content. Filters were then combusted at 450°C for 6 h, left to cool at 60°C over-night, cooled for 1 h in a desiccator and re-weighed for OPM content.
During the experiment, Odyssey temperature loggers (Dataflow Systems Pty Ltd., Christchurch, New Zealand) were placed alongside growth plates at the respective sites which recorded temperature every 10 min.
Data analyses
Growth rates, mortality and condition indices were assessed after 1 year of growth. Data for each species were analysed separately due to large morphological differences between the species. Growth rates of mussels are lower than those of oysters and the focus of the study was to investigate the effects of interspecific competition on the growth rates of mussels and oysters rather than determine absolute differences in their growth rates.
Growth rates and condition indices were analysed using random intercept linear mixed-effects models with regards to the fixed factor ‘species composition’ (i.e. monospecific or heterospecific). Mortality for each species was assessed using a generalised linear mixed-effects model with a binomial distribution with ‘species composition’ included as a fixed factor. Shell weights were analysed using mixed-effects models with regards to the fixed factors ‘shell length’ (continuous) and ‘species composition’. All mixed-effects models included the random factor of ‘experimental plate’ nested within ‘site’ to account for the ecological variability between plates and sites. Models were fit and the estimates of regression coefficients were obtained using the R package ‘lme4’ (Bates et al., 2015). Likelihood ratio tests were used to compare models with and without the fixed factor to determine its statistical significance (Zuur et al., 2009). Statistical significance of the random effects (i.e. site) was assessed using likelihood ratio tests via the ‘ranova’ function in the package ‘lmerTest’ (Kuznetsova et al., 2017). Visual inspection of Q–Q plots and residual versus fitted values confirmed assumptions were met for all models (Zuur et al., 2009).
Seawater total particulate matter (TPM) and organic particulate matter (OPM) and seston quality (i.e. organic percentage of TPM) between sites were examined using linear models. Normality and homoscedasticity assumptions were assessed visually using Q–Q plots and residual plots, respectively, and data were log transformed where necessary to meet assumptions.
Temperature data were not included in analyses of growth, condition indices or mortality due to logger malfunctioning and data loss. Instead, median and quantile temperatures were plotted to visualise differences amongst sites and seasons.
All analyses were undertaken in R v3.4.2 (R Development Core Team, 2017).
Results
Growth rates
Growth rates of both M. edulis (p = 0.31, Table 1) and C. gigas (p = 0.846, Table 1) were not significantly affected by species composition. For C. gigas, growth rates differed significantly amongst experimental sites (Table 1) with growth rates at KL ~ 25% and ~ 16% higher than at WS and BH, respectively (Fig. 1).
Mortality
Species composition had no effect on final mortality of either M. edulis or C. gigas (Table 2); however, mortality of M. edulis (average ~ 60%) was greater than C. gigas (average ~ 15%) in all treatments.
Condition indices and shell weights
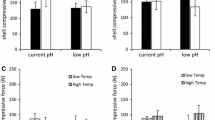
Both species showed significantly reduced condition indices when occurring in heterospecific plates compared to monospecific plates (Table 1). Monospecific cultures had condition indices ~ 15% and ~ 13% greater than those in heterospecific cultures for M. edulis and C. gigas, respectively (Fig. 2). Both species also showed significant differences in condition indices amongst experimental sites. For M. edulis, condition indices at BH were ~ 20% and ~ 22% lower than at WS and KL, respectively (Fig. 3). For C. gigas, condition indices at the BH were ~ 25% lower than at WS and ~ 35% lower than at KL (Fig. 2).
Shell weight-length relationships for both species were unaffected by species composition (Table 1). Experimental site was found to have no effect on shell weight-length relationships for M. edulis, however, C. gigas weight-length relationships differed amongst sites. For C. gigas, shell weights for individuals of similar lengths were greater at KL compared to both BH and WS (Fig. 3; Table 1).
Environmental variables
Average total particulate matter (TPM) and organic particulate matter (OPM) measured ~ 5 mg L−1 and ~ 1.3 mg L−1, respectively. Neither differed amongst sites (TPM - F2,96 = 2.19, p > 0.05; OPM – F2,96 = 0.64, p > 0.05) but both were most variable at WS (Fig. 4). Seston quality (i.e. organic percentage of TPM) was on average 27% and was similar amongst sites (F2,96 = 2.46, p > 0.05).
Where temperature data were returned, daily average temperature quantiles overlapped with median values between sites during all seasons other than summer (Fig. 5). During summer, quantiles of temperatures did not overlap between sites but were within 1°C of each other (15.6°C and 14.6°C, respectively).
Discussion
Introductions of invasive alien species (IAS) have the potential to induce detrimental effects on recipient ecosystems (Bax et al., 2003; Molnar et al., 2008; Simberloff et al., 2013). New introductions can lead to competition between native species and IAS for resources such as food and space, thus reducing the health of native species. Here, a field manipulation experiment identified that co-occurrence of the native Mytilus edulis and invasive Crassostrea gigas bivalves did not affect their growth rates or mortality, but significantly reduced their body condition in comparison to monocultures. Growth rates of C. gigas, and condition indices of both species, also differed significantly amongst experimental sites, with both species showing reductions in these responses on the open coast compared to more protected areas. These results suggest that coexistence may indeed be possible due to a lack of competitive effects on growth rates, however, reductions in the condition indices of both species could lead to reduced health of bivalve populations and community wide ecological impacts.
Although growth rates and mortality were unaffected by species composition, competitive interactions were observed through measured condition indices. Similar growth and mortality of both species between the tested species compositions concurs with recent documented patterns of coexistence (Holm et al., 2016; Reise et al., 2017). However, growth rates alone do not reflect the health of bivalve populations as shell and tissue growth are not consistent with each other (Fréchette & Bourget, 1985; Hilbish, 1986; Borrero & Hilbish, 1988). Bivalve condition indices relates the amount of flesh to the amount of shell giving an important indication of their allocation of energy to tissue or shell production (Seed & Suchanek, 1992). Reduced condition indices for both species when occurring in heterospecific groups may be due to competition for plankton resources which can occur when beds become food limited (e.g. Dolmer, 2000; Vismann et al., 2016) and is more likely in areas with low currents as it is the bulk water flow that replenishes plankton. Although it has been suggested that the two species do not compete for food resources (Dubois et al. 2007), even though their particle retention sizes overlap (Bougrier et al., 1997), we show here that coexistence between the two species may be detrimental for their health.
Condition indices were also observed to differ amongst the experimental sites. Previous studies around Strangford Lough have shown that differences in water motion occur within a relatively small area (Kregting & Elsäber, 2014; Kregting et al., 2016; Millar et al., 2020), which can play an important role in species morphology (Steffani & Branch, 2003; Millar et al., 2020). Our results show that the condition of M. edulis was greatest at sites with restricted water motion or unidirectional currents (KL & WS, respectively), compared to areas where waves dominate (BH; see Millar et al., 2020 for hydrodynamic characterisation of sites). Condition of C. gigas was also greatest at KL compared to both other sites, suggesting a preference for areas where little water motion occurs. A possible mechanism driving the difference in condition indices for both species amongst sites relates water motion to feeding ability, as feeding of the two species has been shown to respond differently to changes in flow velocity (Joyce et al., 2019b). Increased flow velocity can reduce the feeding ability of filter feeders (Jørgensen et al., 1986; Wildish et al., 1987), which may drive the observed reduction in growth and condition of both species in waves. In currents, however, the semi-sessile M. edulis can direct their siphons away from the incoming flow to allow continuous feeding (Newell et al., 2001), supporting the species-specific responses observed in high currents. Although this experiment was not replicated across multiple sites within each type of water motion, we believe that this is a strong starting point in determining the differential effects of water motion type (i.e. waves vs currents) on physiological responses of coastal bivalves.
Whilst we provide an indication of the effects of water motion on the physiological responses of these organisms, other drivers such as food availability should not be ruled out. Our results suggest that food availability was similar amongst experimental sites, however, daily fluctuations in seston and chlorophyll concentrations were not captured by the snapshot sampling events. To further clarify the effects of water motion and food availability on bivalve physiological responses, continuous sampling and measurement of hydrodynamics and plankton availability should be conducted alongside experimental trials.
Bivalves may allocate more energy to shell production/thickening for protection in response to predator occurrence and chemical cues (Scherer & Smee, 2017) or in highly energetic environments (Akester & Martel, 2000) leading to a reduced condition index. Here, predators were present at all experimental sites (personal observation), but their abundances were not quantified and could have influenced the results through defensive responses to chemical cues (Scherer & Smee, 2017). Although shell production for C. gigas differed amongst sites, the similar shell production by M. edulis amongst sites suggests that predator cues did not induce behavioural or morphological defences and are unlikely the driver of the differences in condition indices found here. Also, the lack of difference in shell weight-length relationships for both species with regards to species composition, suggests that shell production as a form of competitive response did not occur. Additionally, C. gigas showed a greater shell weight-length relationship at KL compared to the other sites, which would reduce the calculated condition indices. Yet, it is at KL where the greatest condition indices were observed, thus, the difference in the observed condition indices amongst the experimental sites is due to differences in tissue production rather than shell thickening.
The environmental repercussions of coexistence and competition between native and invasive species has been shown to be a powerful driver behind ecological change from both a community and ecosystem perspective (Grosholz, 2002). Beds of M. edulis are important biogenic habitats considered under the EU habitats directive (EU Commission, 1992) and provide an abundant food source for a range of coastal predators (Ebling et al., 1964; Paine, 1974; Nehls et al., 2006). Native invertebrate predators select native M. edulis over invasive C. gigas (Joyce et al., 2019a), therefore, reductions in condition may lead to reduced energy transfer to higher trophic levels. Further, flesh weights to calculate condition indices measured here included gonadal tissue of the animals which indirectly provides information regarding reproductive fitness (Seed & Suchanek, 1992). As larval supply is key for recruitment and persistence of biogenic reefs (Knights & Walters, 2010), the inferred reduction in reproductive fitness of both species under coexistence could have implications for both species. With regards to M. edulis, reduced reproductive output may affect their ability to persist in some areas, whereas reduced output from C. gigas may eventually reduce their invasion potential in these habitats. Although C. gigas also showed reduced condition when occurring alongside M. edulis, the implications for such effects are not as pressing owing to the greater reproductive superiority of C. gigas (Troost, 2010).
The differences in condition index between species compositions does not only have implications for the dominance of one species over another in an invasion scenario (Nehls et al., 2006), but could have significant consequences for aquaculture. Decreased fish landings has increased the pressure on the aquaculture sector to meet the shortfall (Granada et al., 2016). As available licensed spaces for cultures decrease, an increasing number of growers are likely to combine shore lays of M. edulis with trestle grown C. gigas (Abate et al., 2018). The results presented here suggest that the combined culture of these species may not be the best fit for successful production.
Overall, we have shown that coexistence between M. edulis and C. gigas is indeed possible but potentially costly for both species, with possible ecological ramifications. We also show that different environmental contexts (e.g. type of hydrodynamic motion) may induce different physiological responses both within and between species. Such investigations are gathering momentum regarding marine macroalgae (Bekkby et al., 2014; Kregting et al., 2015; Millar et al., 2020), however, the effects of different water motion type on marine bivalve growth, fitness and physiology remains understudied. Thus, we suggest that physiological responses and species interactions should be further investigated under such environmental contexts.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abate, T. G., R. Nielsen & M. Nielsen, 2018. Agency rivalry in a shared regulatory space and its impact on social welfare: the case of aquaculture regulation. Aquaculture Economics and Management 22(1): 27–48.
Akester, R. J. & A. L. Martel, 2000. Shell shape, dysodont tooth morphology, and hinge-ligament thickness in the bay mussel Mytilus trossulus correlate with wave exposure. Canadian Journal of Zoology 78(2): 240–253.
Alexander, M. M. E., J. Dick, N. O’Connor, N. Haddaway & K. Farnsworth, 2012. Functional responses of the intertidal amphipod Echinogammarus marinus: effects of prey supply, model selection and habitat complexity. Marine Ecology Progress Series 468: 191–202.
Bates, D., M. Mächler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1): 1–48.
Bax, N., A. Williamson, M. Aguero, E. Gonzalez & W. Geeves, 2003. Marine invasive alien species: a threat to global biodiversity. Marine Policy 27(4): 313–323.
Bayne, B. L., M. Ahrens, S. K. Allen, et al., 2017. The proposed dropping of the genus Crassostrea for all pacific cupped oysters and its replacement by a new genus Magallana: a dissenting view. Journal of Shellfish Research 36(3): 545–547.
Bekkby, T., E. Rinde, H. Gundersen, K. Norderhaug, J. Gitmark & H. Christie, 2014. Length, strength and water flow: relative importance of wave and current exposure on morphology in kelp Laminaria hyperborea. Marine Ecology Progress Series 506: 61–70.
Bergstrom, M. A. & A. F. Mensinger, 2009. Interspecific Resource Competition between the Invasive Round Goby and Three Native Species: logperch, Slimy Sculpin, and Spoonhead Sculpin. Transactions of the American Fisheries Society 138(5): 1009–1017.
Borrero, F. J. & T. J. Hilbish, 1988. Temporal variation in shell and soft tissue growth of the mussel Geukensia demissa*. Marine Ecology Progress Series 42: 9–15.
Bougrier, S., A. J. S. Hawkins & M. Héral, 1997. Preingestive selection of different microalgal mixtures in Crassostrea gigas and Mytilus edulis, analysed by flow cytometry. Aquaculture 150(1–2): 123–134.
Byers, J. E., 2000. Competition between two estuarine snails: implications for invasions of exotic species. Ecology 81(5): 1225–1239.
Carlton, J. T., J. B. Geller, M. L. Reaka-Kudla & E. A. Norse, 1999. Historical extinctions in the sea. Annual Review of Ecology and Systematics 30: 515–538.
Council of the European Commission, 1992. Council directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal of the European Communities. Series L 206: 7–49.
Connell, J. H., 1961. The Influence of Interspecific Competition and Other Factors on the Distribution of the Barnacle Chthamalus Stellatus. Ecology 42(4): 710–723.
Dick, J. T. A., M. E. Alexander, A. Ricciardi, C. Laverty, P. O. Downey, M. Xu, J. M. Jeschke, W.-C. Saul, M. P. Hill, R. Wasserman, D. Barrios-O’Neill, O. L. F. Weyl & R. H. Shaw, 2017. Functional responses can unify invasion ecology. Biological Invasions 19(5): 1667–1672.
Diederich, S., 2005. Differential recruitment of introduced Pacific oysters and native mussels at the North Sea coast: coexistence possible? Journal of Sea Research 53(4): 269–281.
Diederich, S., 2006. High survival and growth rates of introduced Pacific oysters may cause restrictions on habitat use by native mussels in the Wadden Sea. Journal of Experimental Marine Biology and Ecology 328(2): 211–227.
Dolmer, P., 2000. Algal concentration profiles above mussel beds. Journal of Sea Research 43(2): 113–119.
Dubois, S., F. Orvain, J. C. Marin-Leal, M. Ropert & S. Lefebvre, 2007. Small-scale spatial variability of food partitioning between cultivated oysters and associated suspension-feeding species, as revealed by stable isotopes. Marine Ecology Progress Series 336: 151–160.
Ebling, F. J., J. A. Kitching, L. Muntz & C. M. Taylor, 1964. The Ecology of Lough Ine. The Journal of Animal Ecology 33(1): 73.
Eschweiler, N. & H. T. Christensen, 2011. Trade-off between increased survival and reduced growth for blue mussels living on Pacific oyster reefs. Journal of Experimental Marine Biology and Ecology 403: 90–95.
Fréchette, M., & Bourget, E. (1985). Food-Limited Growth of Mytilus edulis L. in Relation to the Benthic Boundary Layer. Canadian Journal of Fisheries and Aquatic Sciences, 42: 1166–1170.
Goedknegt, M. A., A.-K. Schuster, C. Buschbaum, R. Gergs, A. S. Jung, P. C. Luttikhuizen, J. van der Meer, K. Troost, K. M. Wegner & D. W. Thieltges, 2017. Spillover but no spillback of two invasive parasitic copepods from invasive Pacific oysters (Crassostrea gigas) to native bivalve hosts. Biological Invasions 19(1): 365–379.
Granada, L., N. Sousa, S. Lopes & M. F. L. Lemos, 2016. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges? – A review. Reviews in Aquaculture 8(3): 283–300.
Green, D. S. & T. P. Crowe, 2014. Context- and density-dependent effects of introduced oysters on biodiversity. Biological Invasions 16: 1145–1163.
Green, D. S., B. Boots & T. P. Crowe, 2012. Effects of Non-Indigenous Oysters on Microbial Diversity and Ecosystem Functioning. PLoS ONE 7(10): e48410.
Green, D. S., C. Rocha & T. P. Crowe, 2013. Effects of non-indigenous oysters on ecosystem processes vary with abundance and context. Ecosystems 16(5): 881–893.
Grosholz, E., 2002. Ecological and evolutionary consequences of coastal invasions. Trends in Ecology and Evolution 17(1): 22–27.
Guy, C., A. Blight, D. Smyth & D. Roberts, 2018. The world is their oyster: differences in epibiota on sympatric populations of native Ostrea edulis and non-native Crassostrea gigas (Magallana gigas) oysters. Journal of Sea Research 140: 52–58.
Heard, M. J. & D. F. Sax, 2013. Coexistence between native and exotic species is facilitated by asymmetries in competitive ability and susceptibility to herbivores. Ecology Letters 16(2): 206–213.
Herbert, R. J. H., J. Humphreys, Clare Davies, et al., 2016. Ecological impacts of non-native Pacific oysters (Crassostrea gigas) and management measures for protected areas in Europe. Biodiversity and Conservation 25: 2835–2865.
Hilbish, T. J., 1986. Growth trajectories of shell and soft tissue in bivalves: seasonal variation in Mytilus edulis L. Journal of Experimental Marine Biology and Ecology 96(2): 103–113.
Holm, M. W., J. K. Davids, P. Dolmer, E. Holmes, T. Theis Nielsen, B. Vismann & B. Winding Hansen, 2016. Coexistence of Pacific oyster Crassostrea gigas (Thunberg, 1793) and blue mussels Mytilus edulis Linnaeus, 1758 on a sheltered intertidal bivalve bed? Aquatic Invasions 11(2): 155–165.
Jørgensen, C. B., P. Famme, H. S. Kristensen, P. S. Larsen, F. Møhlenberg & H. U. Riisgård, 1986. The bivalve pump. Marine Ecology Progress Series 34: 69–77.
Joyce, P. W. S., J. T. A. Dick & L. T. Kregting, 2020. Lack of biotic resistance to an invasive bivalve irrespective of season or hydrodynamic disturbance. Journal of Experimental Marine Biology and Ecology 528: 151382.
Joyce, P. W. S., J. W. E. Dickey, R. N. Cuthbert, Jaimie Dick & L. Kregting, 2019a. Using functional responses and prey switching to quantify invasion success of the Pacific oyster, Crassostrea gigas. Marine Environmental Research 145: 66–72.
Joyce, P. W. S., L. Kregting & J. T. A. Dick, 2019b. Relative impacts of the invasive Pacific oyster, Crassostrea gigas, over the native blue mussel, Mytilus edulis, are mediated by flow velocity and food concentration. NeoBiota 45: 19–37.
Knights, A. M. & K. Walters, 2010. Recruit-recruit interactions, density-dependent processes and population persistence in the eastern oyster Crassostrea virginica. Marine Ecology Progress Series 404: 79–90.
Kochmann, J. & T. P. Crowe, 2014. Effects of native macroalgae and predators on survival, condition and growth of non-indigenous Pacific oysters (Crassostrea gigas). Journal of Experimental Marine Biology and Ecology 451: 122–129.
Kochmann, J., C. Buschbaum, N. Volkenborn & K. Reise, 2008. Shift from native mussels to alien oysters: differential effects of ecosystem engineers. Journal of Experimental Marine Biology and Ecology 364(1): 1–10.
Konar, B. & K. Iken, 2005. Competitive dominance among sessile marine organisms in a high Arctic boulder community. Polar Biology 29: 61–64.
Krassoi, F. R., K. R. Brown, M. J. Bishop, B. P. Kelaher & S. Summerhayes, 2008. Condition-specific competition allows coexistence of competitively superior exotic oysters with native oysters. Journal of Animal Ecology 77: 5–15.
Kregting, L. & B. Elsäber, 2014. A hydrodynamic modelling framework for Strangford Lough part 1: tidal model. Journal of Marine Science and Engineering 2(1): 46–65.
Kregting, L. T., C. D. Hepburn & G. Savidge, 2015. Seasonal differences in the effects of oscillatory and uni-directional flow on the growth and nitrate-uptake rates of juvenile Laminaria digitata (Phaeophyceae). Journal of Phycology 51(6): 1116–1126.
Kregting, L., A. J. Blight, B. Elsäber & G. Savidge, 2016. The influence of water motion on the growth rate of the kelp Laminaria digitata. Journal of Experimental Marine Biology and Ecology 478: 86–95.
Kumschick, S., M. Gaertner, M. Vila, F. Essl, J. M. Jeschke, et al., 2014. Ecological impacts of alien species: quantification, scope, caveats, and recommendations. BioScience 65(1): 55–63.
Kuznetsova, A., P. B. Brockhoff & R. H. B. Christensen, 2017. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software 82(13): 1–26.
Laverty, C., J. T. A. Dick, M. E. Alexander & F. E. Lucy, 2015. Differential ecological impacts of invader and native predatory freshwater amphipods under environmental change are revealed by comparative functional responses. Biological Invasions 17(6): 1761–1770.
Millar, R. V., J. D. R. Houghton, B. Elsäber, P. J. Mensink & L. Kregting, 2020. Influence of waves and currents on the growth rate of the kelp Laminaria digitata (Phaeophyceae). Journal of Phycology 56(1): 198–207.
Miller, A. W. & G. M. Ruiz, 2014. Arctic shipping and marine invaders. Nature Climate Change 4(6): 413–416.
Molnar, J. L., R. L. Gamboa, C. Revenga & M. D. Spalding, 2008. Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment 6(9): 485–492.
Nehls, G., I. Hertzler & G. Scheiffarth, 1997. Stable mussel Mytilus edulis beds in the Wadden Sea—They’re just for the birds. Helgoländer Meeresuntersuchungen 51(3): 361–372.
Nehls, Georg, S. Diederich, D. W. Thieltges & M. Strasser, 2006. Wadden Sea mussel beds invaded by oysters and slipper limpets: competition or climate control? Helgoland Marine Research 60(2): 135–143.
Newell, C. R., D. J. Wildish & B. A. MacDonald, 2001. The effects of velocity and seston concentration on the exhalant siphon area, valve gape and filtration rate of the mussel Mytilus edulis. Journal of Experimental Marine Biology and Ecology 262(1): 91–111.
Padilla, D. K., 2010. Context-dependent Impacts of a Non-native Ecosystem Engineer, the Pacific Oyster Crassostrea gigas. Integrative and Comparative Biology 50(2): 213–225.
Paine, R. T., 1974. Intertidal community structure. Oecologia 15(2): 93–120.
R Development Core Team, (2017). R Development Core Team. R: A Language and Environment for Statistical Computing.
Reise, K., C. Buschbaum, H. Büttger & K. M. Wegner, 2017. Invading oysters and native mussels: from hostile takeover to compatible bedfellows. Ecosphere 8(9): e01949.
Ricciardi, A., T. M. Blackburn, J. T. Carlton, J. T. A. Dick, P. E. Hulme, J. C. Iacarella, J. M. Jeschke, A. M. Liebhold, J. L. Lockwood, H. J. MacIsaac, P. Pyšek, D. M. Richardson, G. M. Ruiz, D. Simberloff, W. J. Sutherland, D. A. Wardle & D. C. Aldridge. 2017. Invasion science: a horizon scan of emerging challenges and opportunities. Trends in Ecology and Evolution 32: 464–474.
Salvi, D. & P. Mariottini, 2017. Molecular taxonomy in 2D: a novel ITS2 rRNA sequence-structure approach guides the description of the oysters’ subfamily Saccostreinae and the genus Magallana (Bivalvia: Ostreidae). Zoological Journal of the Linnean Society 179(2): 263–276.
Scherer, A. E. & D. L. Smee, 2017. Eastern oysters Crassostrea virginica produce plastic morphological defenses in response to crab predators despite resource limitation. The Biological Bulletin 233(2): 144–150.
Seebens, H., T. M. Blackburn, E. E. Dyer, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Pagad, P. Pyšek, M. van Kleunen, M. Winter, M. Ansong, M. Arianoutsou, S. Bacher, B. Blasius, E. G. Brockerhoff, G. Brundu, C. Capinha, C. E. Causton, L. Celesti-Grapow & F. Essl, 2018. Global rise in emerging alien species results from increased accessibility of new source pools. Proceedings of the National Academy of Sciences of the United States of America 10: E2264.
Seebens, H., T. M. Blackburn, E. E. Dyer, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Pagad, P. Pyšek, M. Winter, M. Arianoutsou, S. Bacher, B. Blasius, G. Brundu, C. Capinha, L. Celesti-Grapow, W. Dawson, S. Dullinger, N. Fuentes, H. Jäger & F. Essl, 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8: 14435.
Seed, R., & Suchanek, T. H. (1992). Population and community ecology of Mytilus. In E. Gosling (Ed.), The mussel Mytilus: ecology, physiology, genetics and culture (pp. 87–169). Elsevier, Amsterdam.
Simberloff, D., J.-L. Martin, P. Genovesi, V. Maris, D. A. Wardle, J. Aronson, F. Courchamp, B. Galil, E. García-Berthou, M. Pascal, P. Pyšek, R. Sousa, E. Tabacchi & M. Vilà, 2013. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology & Evolution 28(1): 58–66.
Steffani, C. & G. Branch, 2003. Growth rate, condition, and shell shape of Mytilus galloprovincialis: responses to wave exposure. Marine Ecology Progress Series 246: 197–209.
Troost, K., 2010. Causes and effects of a highly successful marine invasion: Case-study of the introduced Pacific oyster Crassostrea gigas in continental NW European estuaries. Journal of Sea Research 64: 145–165.
Troost, K., P. Kamermans & W. J. Wolff, 2008. Larviphagy in native bivalves and an introduced oyster. Journal of Sea Research 60(3): 157–163.
Vismann, B., M. Wejlemann Holm, J. Davids, P. Dolmer, M. Pedersen, E. Blanda, H. Christensen, P. Nielsen & B. Hansen, 2016. Field clearance of an intertidal bivalve bed: relative significance of the co-occurring blue mussel Mytilus edulis and Pacific oyster Crassostrea gigas. Aquatic Biology 25: 107–119.
Walles, B., J. Salvador de Paiva, B. C. van Prooijen, T. Ysebaert & A. C. Smaal, 2015. The Ecosystem Engineer Crassostrea gigas Affects Tidal Flat Morphology Beyond the Boundary of Their Reef Structures. Estuaries and Coasts 38(3): 941–950.
Walne, P. R., 1976. Experiments on the culture in the sea of the butterfish Venerupis decussata L. Aquaculture 8(4): 371–381.
Ward, J. E. & S. E. Shumway, 2004. Separating the grain from the chaff: particle selection in suspension- and deposit-feeding bivalves. Journal of Experimental Marine Biology and Ecology 300(1): 83–130.
Wildish, D. J., D. D. Kristmanson, R. L. Hoar, A. M. DeCoste, S. D. McCormick & A. W. White, 1987. Giant scallop feeding and growth responses to flow. Journal of Experimental Marine Biology and Ecology 113(3): 207–220.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R. Springer, Berlin.
Zwerschke, N., J. Kochmann, Elizabeth Ashton & T. P. Crowe, 2017. Co-occurrence of native Ostrea edulis and non-native Crassostrea gigas revealed by monitoring of intertidal oyster populations. Journal of the Marine Biological Association of the United Kingdom 98(8): 2029–2038.
Zwerschke, N., H. Rein, C. Harrod, C. Reddin, M. C. Emmerson, D. Roberts & N. E. O’Connor, 2018. Competition between co-occurring invasive and native consumers switches between habitats. Functional Ecology 32(12): 2717–2729.
Acknowledgements
The authors thank Jennifer Bowden, Rachel Millar and Nicholas Horne for their enthusiastic assistance with various aspects of the practical work. Necessary licensing was obstained from the Department of Agriculture, Environment and Rural Affairs to perform the experiment. We acknowledge helpful comments from two anonymous reviewers, which have improved the manuscript.
Funding
This work was funded by the Department for the Economy, Northern Ireland and part of SuperGen Marine Energy Research Consortium II, which was funded by the UK Engineering and Physical Science Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Iacopo Bertocci
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joyce, P.W.S., Smyth, D.M., Dick, J.T.A. et al. Coexistence of the native mussel, Mytilus edulis, and the invasive Pacific oyster, Crassostrea (Magallana) gigas, does not affect their growth or mortality, but reduces condition of both species. Hydrobiologia 848, 1859–1871 (2021). https://doi.org/10.1007/s10750-021-04558-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04558-1