Abstract
Experimental streams are bounded and partly enclosed lotic units that facilitate the simulation of certain aspects of natural stream ecosystems under controlled conditions. We summarized the current knowledge regarding experimental streams in order to support researchers in designing and undertaking future studies using experimental streams. We observed an increase in the number of such studies since 1975. The geographically uneven distribution of studies suggests that the generalization of findings to global scale may not be straightforward. Our results indicate that macroinvertebrates, fish, and algae are the most frequently studied organisms and that the size of the experimental streams was related to the focal organism group(s) studied. The size of the units decreased over time, while the number of treatments, interpreted as the combination of the levels of factors, increased. These results suggest that biologically complex studies have gradually been replaced by biologically less complex ones. In contrast, the experimental complexity (the number of treatments) and the statistical power (number of replication) increased. Finally, we identified a number of important, but poorly documented pieces of information regarding experimental stream systems and experimental protocols and made recommendations for future research.
Similar content being viewed by others
Introduction
Controlled experiments are among the most frequently applied approaches in ecology (Orlóci, 1978). Compared to observational studies, where researchers merely describe what is happening, in experiments researchers have the ability to control most of the variables (controlled experimental condition) and manipulate one or more factors of specific interest. As a result, experimental studies are founded on the principles of cause and effect, specifically examining relationships that cannot be demonstrated via observational studies (Rosenbaum, 2017). Experimental streams are typically bounded (i.e., having solid impermeable margins) and partially enclosed experimental units that closely simulate certain/specific elements of natural stream ecosystems. In this respect, they can be regarded as experimental mesocosms (Odum, 1984), which comprise a wide variety of designs ranging from indoor laboratory streams (Cardinale et al., 2002) to outdoor artificial channels (Liess et al., 2009; Piggott et al., 2015a, b, c). Following the definition of mesocosms of Stewart et al. (2013), we considered only experimental streams, where the volume of the stream unit varies between 1 and several thousands of liters. The application of this definition allows us to disregard “microcosm” studies, which are characterized by limited biological complexity.
This study focuses exclusively on ‘experimental streams,’ but not on experiments carried out in streams. Therefore, we disregard, among others, litter breakdown experiments (e.g., Gessner & Chauvet, 2002, Boyero et al. 2011), whole stream manipulations including restoration (Peckarsky et al., 2002; Bond & Lake, 2005), as well as other experiments in real streams (e.g., Bond & Downes, 2000; Thayse & Schmera, 2016). Studies performed in experimental streams have made significant contribution to the fields of fundamental stream ecology and biodiversity research. For instance, they provided evidence that the facilitation among species is a key mechanism by which biodiversity affects the rate of resource use governing the efficiency and productivity of ecosystems (Cardinale et al., 2002). They have been pivotal in demonstrating that lotic communities are not only affected by individual stressors, but also by the interactions among them (Liess et al., 2009; Matthaei et al., 2010; Piggott et al., 2012; Wagenhoff et al., 2012). Therefore, river managers and regulators need to be aware of the potential of interactive multiple-stressor effects, given that ecological outcomes of an increase in a stressor may be amplified more than predicted based on the knowledge of single-stressor effects (Matthaei et al., 2010). In addition, experimental streams have been used for simulating the effect of climate change, drought, flow events (high and low flow), nutrient enrichment, biological invasion, habitat modification, and the specific effect(s) of chemicals (i.e., ecotoxicology studies) on organisms and their functioning (e.g., Humphrey & Stevenson, 1992; Beeson et al., 1998; Woodward et al., 2010; Stewart et al., 2013; Elbrecht et al., 2016).
Review papers focusing on experimental streams provide comprehensive summaries regarding how stream ecosystems respond to the effects of drought (Ledger et al., 2012, 2013), urbanization (Taulbee et al., 2009), and specific toxins (Beeson et al., 1998; Krogh et al., 2003). However, these provide limited information on the design of the experimental streams utilized or the protocols employed to achieve particular objectives, information that may be essential for stream ecologists performing experimental studies in the future using comparable approaches. The latest review on this topic was published more than 20 years ago (Belanger, 1997), and the concept and use of experimental streams might have changed since then. Thematic overviews (e.g., Stewart et al., 2013) focus on specific topics (e.g., climate change) without clear separation of experimental streams from other types of mesocosms (i.e., mesocosms in terrestrial, marine, coastal, estuarine, and lentic systems).
The primary objective of this study is to summarize the current knowledge of experimental stream research, and to support researchers in designing experimental stream systems and carrying out such studies in the future. In particular, we provide the first summary regarding certain aspects of the facilities and protocols used in experimental stream studies. We also identify essential but frequently unreported parameters of experimental facilities and protocols which are required in order to improve our understanding of and ability to replicate studies in the future. Specifically, we were interested in answering the following questions:
-
(1)
How are experimental streams designed?
-
(2)
Which organisms are the most frequently used in experimental streams?
-
(3)
Which are the most frequently applied protocols in experimental stream studies?
-
(4)
Has the use of experimental streams changed over time?
-
(5)
Are experimental facilities and protocols fully documented in published studies?
Literature survey
On March 25, 2019, we carried out a literature search in ISI Science Citation Index Expanded database from 1975 to 2018 using the following combination of keywords: (“stream mesocos*” OR “streamside channel” OR “stream-side channel” OR “experimental stream”). We did not consider the keyword “flume” because it is used in physics and flow studies, and the search resulted in a large amount of irrelevant papers. After examining the abstracts, only papers related to experimental lotic streams were retained, reducing the number of papers to 274. Subsequently, each paper was read carefully to confirm its relevance to the study of experimental streams and lotic ecosystems. Twenty-four papers were considered to be irrelevant and resulted in a total of 250 papers (Electronic Appendix 1). To make our survey more representative, we supplemented the list by 14 additional papers (Electronic Appendix 2), which were known to us but did not appear in the literature search. Consequently, we used 264 papers in the analyses. The number of papers increased over the study period considered (Fig. 1). Although the first paper appeared in the 1970s, there was a gap between 1979 and 1986. The most productive period commenced in 1987 and peaked in the last three studied years (2016–2018). The papers reviewed reported 385 studies (i.e., a piece of scientific work performed for a particular purpose), of which 367 (95.3%) were experimental (i.e., included manipulation of one or more abiotic/biotic factors) and 18 (4.7%) were observational without any experimental manipulation (e.g., describing the behavior of a fish in an experimental stream channel, De Gaudemar & Beall, 1999; Troia et al., 2014).
Methods
During the detailed reading of each paper, we recorded the following information: (i) the size and dimensions of the experimental facility, (ii) the ‘effects’ studied/tested in experimental streams, (iii) the organisms studied, (iv) the type (i.e., behavior, survival, diversity abundance, biomass, ecosystem function, physiology, or other) and response (i.e., individual, population, community, and ecosystem) levels, and (v) details regarding the experimental protocols employed (i.e., duration of conditioning period and number of treatments and replicates, see Electronic Appendix 3 for further details). We recorded the data in a spreadsheet. When information was not available in the original publication (including appendices, supplementary material, or supporting references), we recorded it as ‘missing data’ (coded in R programming environment as “NA”). Calculation of summary statistics and data visualization were performed in R (R Core Team, 2017).
Experimental facility
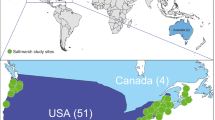
Our study showed a geographically uneven distribution of experimental stream studies indicating a strong bias towards North America. A large number of experimental stream studies were undertaken also in Europe, while Asia, South America, and Australia/New Zealand were underrepresented in comparison (Fig. 2A). There were no published studies available from Africa based on the search terms used. Although this bias is independent from the experimental approach, it does not support inference of broad generalizable conclusions (e.g., response to climate change might be geographically specific, Stewart et al., 2013). However, if research activity, i.e., the number of studies is standardized by the millions of people living on each continent (as a rough proxy of ecologists working with experimental streams, hereafter standardized research activity), then Australia/New Zealand has a leading position in the field followed by North America and Europe (Fig. 2B). Most of the studies were performed in outdoor facilities (54.8%, total N = 279, e.g., Gillespie et al., 1996; Baker et al., 2016) or indoor (37.3%, e.g., Belanger et al., 1995; Cardinale, 2011), with a limited number of studies ran in greenhouses (7.9%, e.g., Larson et al., 2009; Clements & Kotalik, 2016).
Most studies have been carried out using linear experimental facilities (83.2%, total N = 344, e.g., Belanger et al., 1995; Clements & Kotalik, 2016), but circular (11.6%, e.g., Fuller et al., 1998; Wagenhoff et al., 2012) and other configurations (5.2%, e.g., complex systems, Hargrave et al., 2006; Driver & Hoeinghaus, 2016) also exist. Although this observed unevenness indicates the popularity of linear systems, circular systems are receiving increased attention (Nannini & Belk, 2006; Liess et al., 2009; Wagenhoff et al., 2013; Elbrecht et al., 2016). A range of different materials have been used to construct the stream channels with plastic being the most frequently applied (48.9%; Fig. S1). This reflects its high utility as it can be used to construct channels of different sizes, in a variety of settings and it is relatively cheap. The water volume of the individual experimental units ranged between 2 (Pennuto & de Noyelies, 1993) and 302,500 l (Bankey et al., 1995), with the majority varying between 10 and 1,000 l (Fig. 3). In linear experimental streams, the length varied between 34.5 cm (Pennuto & de Noyelies, 1993) and 518 m (Allen, 1991). This range reflects a good trade-off between the simplifications of experimental stream systems and the practicalities of conducting an experiment. Similar values have been reported in earlier reviews (e.g., Belanger, 1997; Mohr et al., 2005), but the size of the experimental streams varied considerably and even smaller experimental unit volumes (< 10 l) have been successfully used in addressing important stream ecology research questions (Cardinale & Palmer, 2002; Cardinale et al., 2004; Matthaei et al., 2010; Cardinale, 2011; Lange et al., 2011; Kulacki et al., 2012; Piggott et al., 2012, 2015a, b, c; Wagenhoff et al., 2012, 2013; Magbanua et al., 2013). Most studies use a through-flow system (58.0%, total N = 307, e.g., Gillespie et al., 1996; Elbrecht et al., 2016) but water may also be re-circulated (38.4%, e.g., Belanger et al., 1995; Driver & Hoeinghaus, 2016) and in some instances a combination of the two has been used (3.6%, e.g., Fairchild et al., 1987; Taylor et al., 1994).
Natural streams are open ecosystems characterized by continuous immigration and emigration of stream biota. Although through-flow experimental streams allow the immigration of stream organisms, this colonization process is strongly size-dependent: the inner diameter of the pipe (Ø = 0.63–16 cm) transferring water and stream biota to the experimental channels can physically restrict the colonization of some organisms. To address this limitation, focal organisms are frequently introduced by the investigators (e.g., Piggott et al., 2015a, b, c note that the colonization of macroinvertebrates was augmented). Such introduction is necessary in the majority of closed re-circulated systems.
Studied effects, organisms, and responses
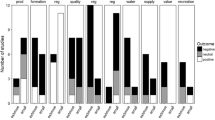
The influence of toxic chemicals, biotic manipulations, nutrient additions, and habitat quality modifications are the most frequently studied effects, while water-level, thermal, and light manipulations and the effects of invasive species were underrepresented in comparison (Fig. 4). Studies addressing the effects of toxic chemical pollutants and nutrients, which are strongly related to human impact on aquatic environments, are frequently tested and reviewed (Beeson et al., 1998; Ferreira et al., 2015). Research on the effects of changes in water temperature, light alteration, and invasive species is crucial for understanding and addressing contemporary challenges such as climate change, and hence should be encouraged (Stewart et al., 2013). Macroinvertebrates, fish and algae were the most frequently studied organisms (Fig. S2). All of these groups have been identified previously as popular model organisms in experimental stream systems (e.g., Belanger, 1997; Ferreira et al., 2015; Dewson et al. 2017). In addition, we found that vascular plants, zooplankton, and amphibians were underrepresented. This finding is not surprising because these organisms are not typical members of stream communities. Some studies, however, submerged zooplankton in small cages in experimental streams for toxicological studies (e.g., Pablo et al., 2008). Finally, we found that the different levels of biological organization are almost equally represented in our survey (individual: 41%, population: 47%, community: 43% and ecosystem: 61%) suggesting that experimental streams are flexible tools and suitable to perform a wide range of experiments.
Experimental protocols
The conditioning period prior to the commencement of the experiment ranged between 0 and 973 days, but typically varied between 10 and 100 days (54% of the studies, Fig. 5A). The duration period of the studies varied between 3 min (behavioral study; Vance, 1996) and 1260 days (e.g., Hall et al., 1991), but typically varied between 10 and 100 days (61% of the studies, Fig. 5B). These duration periods are adequate to examine the responses of stream ecosystems at relevant ecological time-scales, and may allow restricted inference regarding the effects of long-term phenomena such as climate change (but see Piggott et al., 2012, 2015b, c). The number of treatments applied, including control treatments, varied between 1 (no manipulation: observation studies) and 64 (test of two factors, each with 8 levels, e.g., Wagenhoff et al., 2012), whereas the median value was 4 (total N = 319, Fig. S3A). In some of the experiments, the number of replicates was not the same for each treatment. Therefore, we analyzed the smallest and the largest number of replicates used separately (Fig. S3B and S3C) and found that the minimum number of replicates was 1 (no replication) while the median value was 3. The maximum values were 36 for the smallest number of replicates, and 192 for the largest one (the latter being a behavioral study where each individual was considered as replicate, e.g., Higler, 1975, Fig. S3C). These values are similar to those reported previously (e.g., Belanger, 1997), although the range reported here is greater, which is likely a reflection of the higher number of effects considered (e.g., behavioral studies).
Association between research topic, experimental facility, and the studied organisms
We examined the frequency of different groups of organism used to test a range of specific effects (Table 1). Fish were most commonly used in studying the effects associated with invasive species (66%) and biotic effect manipulations (58%). Algae were almost always used when examining the effects of light manipulation (86%). We found that macroinvertebrates were frequently used to test nutrient addition (59%) and the ecotoxicological effects (59%). The responses of other groups of organisms were less frequently investigated. Studies on the effects of climate change (e.g., water temperature manipulations, light manipulations, and invasive species) were underrepresented in microbial investigations (20%, 29%, and 0%, respectively). On the one hand, this knowledge gap is surprising given that microbial communities are likely to respond significantly to climate change. On the other hand, microbial investigations are mostly performed in microcosms that are outside of the scope of the current review. The size of the experimental stream units, expressed as volume, was related to the group of organisms studied: the median value of experimental stream volume was the smallest for algae, followed by macroinvertebrates and fish (Fig. 6). We found that algae, macroinvertebrates, and fish were used in experimental streams of a wide range of sizes (IQR = 10–1,000 l). An obvious explanation for this is that the majority of studies examine several organism groups at the same time, and the size of the experimental stream unit is selected for the needs of the largest organisms. The smallest experimental units had a capacity of 2.5 l for algae, 2 l for macroinvertebrates, and 3.7 l for fish.
Temporal changes
We examined the temporal changes in research protocol and observed a slight increase in the range of the number of treatments through time (Fig. S4). A similar pattern was observed regarding the (smallest) number of replicates (Fig. S5). These findings suggest that experimental stream studies are becoming more complex (increasing number of treatments, Wagenhoff et al., 2012) with increasing statistical power (increasing number of replicates). We also found that the size of the experimental streams (expressed in volume) decreased over time (Fig. 7; values for the log transformed dataset: r = − 0.279, N = 261, P = 0.002).
Lack of data on experimental facilities and protocols
We found a high rate of missing information related to the description of experimental facilities in the published protocols (Table 2). The frequency of missing data was greater than 50% for four experimental stream parameters (sediment depth, maximum and mean water depth, construction material), while data on the experimental protocol parameters were always below 40% indicating that important information regarding both the experimental stream and protocols was not given (Table 2). The high proportion of missing values reduces the repeatability of individual experimental studies. Based on our results, we recommend that the writing of the ‘Methods’ section should be undertaken more carefully in order to improve reproducibility of studies in the future. We feel that this phenomenon might be related to the requests of journals for a reduced ‘Methods’ section. A potential solution would be to move such information to the supplementary material.
Recommendation for future research
-
1.
Experimental stream studies provide a great opportunity to answer a range of scientific questions on stream ecosystems, as they allow for a high degree of realism, control, and replicability.
-
2.
As climate change and other anthropogenic activities have significant impact on lotic ecosystems and their diversity, research effort should be increased in this area. Experimental streams allow for the manipulation of water levels, temperature, and light that can help us study and address such environmental challenges.
-
3.
There is a need for a more even distribution of experimental stream studies on the geographical scale; therefore, the number of studies in Asia and South America should be increased.
-
4.
While linear outdoor stream systems are the most frequently used, other configurations should also be considered. Circular systems, for instance, seem to be highly realistic.
-
5.
There is a tendency towards smaller experimental streams, while the number of treatments and the number of replicates increase with time. We predict that the decrease in the size of stream units will stop in the future because further reduction (< 1 l) is not possible due to the required level of biological complexity. As already demonstrated, the application of several small experimental stream units allows to test the interaction of different factors. The interactions of multiple factors are less known, and therefore we recommend to focus on interactions for a deeper understanding of stream ecology.
-
6.
Alternatively, use larger experimental streams or consider whole stream manipulations for studying large-bodied organisms or more biologically complex systems.
-
7.
Document the details of experimental stream studies more carefully. These efforts would contribute to the replicability of these studies.
References
Allen, K. N., 1991. Seasonal variation of selenium in outdoor experimental stream-wetland systems. Journal of Environmental Quality 20: 865–868.
Baker, L. F., R. S. King, J. M. Unrine, B. T. Castellon, G. V. Lowry & C. W. Matson, 2016. Press or pulse exposures determine the environmental fate of cerium nanoparticles in stream mesocosms. Environmental Toxicology and Chemistry 35: 1213–1223.
Bankey, L. A., P. A. Van Veld, D. L. Borton, L. LaFleur & J. J. Stegeman, 1995. Responses of cytochrome P450IA in freshwater fish exposed to bleached kraft mill effluents in experimental stream channels. Canadian Journal of Fisheries and Aquatic Sciences 52: 439–447.
Beeson, D. R., M. C. Lewis, J. M. Powell & D. R. Nimmo, 1998. Effects of pollutants on freshwater organisms. Water Environment Research 70: 921–931.
Belanger, S. E., 1997. Literature review and analysis of biological complexity in model stream ecosystems – influence of size and experimental design. Ecotoxicology & Environmental Safety 36: 1–16.
Belanger, S. E., E. M. Meiers & R. G. Bausch, 1995. Direct and indirect ecotoxicological effects of alkyl sulfate and alkyl ethoxysulfate on macroinvertebrates in stream mesocosms. Aquatic Toxicology 33: 65–87.
Bond, N. R. & B. J. Downes, 2000. Flow-related disturbance in streams: an experimental test of the role of rock movement in reducing macroinvertebrate population densities. Marine and Freshwater Research 51: 333–337.
Bond, N. R. & P. S. Lake, 2005. Ecological restoration and large-scale ecological disturbance: the effects of drought on the response by fish to habitat restoration experiment. Restoration Ecology 13: 39–48.
Boyero, L., R. G. Pearson, M. O. Gessner, L. A. Barmuta, V. Ferreira, M. A. S. Graça, D. Dudgeon, A. J. Boulton, M. Callisto, E. Chauvet, J. E. Helson, A. Bruder, R. J. Albariño, C. M. Yule, M. Arunachalam, J. N. Davis, R. Figueroa, A. S. Flecker, A. Ramírez, R. G. Death, T. Iwata, J. M. Mathooko, C. Mathuriau, J. F. Gonçalves Jr., M. S. Moretti, T. Jinggut, S. Lamothe, C. M’Erimba, L. Ratnarajah, M. H. Schindler, J. Castela, L. M. Buria, A. Cornejo, V. D. Villanueva & D. C. West, 2011. A global experiments suggest climate warming will not accelerate decomposition in streams but might reduce carbon sequestration. Ecology Letters 14: 289–294.
Cardinale, B. J., 2011. Biodiversity improves water quality through niche partitioning. Nature 472: 86–89.
Cardinale, B. J. & M. A. Palmer, 2002. Disturbance moderates biodiversity-ecosystem function relationships: experimental evidence from caddisflies in stream mesocosms. Ecology 83: 1915–1927.
Cardinale, B. J., M. A. Palmer & S. L. Collins, 2002. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415: 426–429.
Cardinale, B. J., E. R. Gelmann & M. A. Palmer, 2004. Net spinning caddisflies as stream ecosystem engineers: the influence of Hydropsyche on benthic substrate stability. Functional Ecology 18: 381–387.
Clements, W. H. & C. Kotalik, 2016. Effects of major ions on natural benthic communities: an experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshwater Science 35: 126–138.
De Gaudemar, B. & E. Beall, 1999. Reproductive behavioural sequences of single pairs of Atlantic salmon in an experimental stream. Animal Behaviour 57: 1207–1217.
Dewson, Z. S., A. B. W. James & R. G. Death, 2017. A review of the consequences of decreased flow for instream habitat and macroinvertebrates. Journal of the North American Benthological Society 26: 401–415.
Driver, L. J. & D. J. Hoeinghaus, 2016. Fish metacommunity responses to experimental drought are determined by habitat heterogeneity and connectivity. Freshwater Biology 61: 533–548.
Elbrecht, V., A. J. Beermann, G. Goessler, J. Neumann, R. Tollrian, R. Wagner, A. Wlecklik, J. J. Piggott, C. D. Matthaei & F. Leese, 2016. Multiple stressor effects on stream invertebrates: a mesocosm experiment manipulating nutrients, fine sediment and flow velocity. Freshwater Biology 61: 362–375.
Fairchild, J. F., T. Boyle, W. R. English & C. Rabeni, 1987. Effects of sediment and contaminated sediment on structural and functional components of experimental stream ecosystems. Water, Air and Soil Pollution 36: 271–293.
Fairchild, J. F., F. J. Dwyer & T. H. La Point, 1993. Evaluation of a laboratory-generated NOEC for linear alkylbenzene sulfonate in outdoor experimental streams. Environmental Toxicology and Chemistry 12: 1763–1775.
Ferreira, V., B. Castagneyrol, J. Koricheva, V. Gulis, E. Chauvet & M. A. S. Graça, 2015. A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biological Reviews 90: 669–688.
Fuller, R. L., C. Ribble, A. Kelley & E. Gaenzle, 1998. Impact of stream grazers on periphyton communities: a laboratory and field manipulation. Journal of Freshwater Ecology 13: 105–114.
Gessner, M. O. & E. Chauvet, 2002. A case for using litter breakdown to assess functional stream integrity. Ecological Applications 12: 498–510.
Gillespie, W. B., J. H. Rodgers & N. O. Crossland, 1996. Effects of a nonionic surfactant (C14–15AE-7) on aquatic invertebrates in outdoor stream mesocosms. Environmental Toxicology and Chemistry 15: 1418–1422.
Hall T. J., R. K. Haley, & L. E. Lafleur, 1991. Effects of biologically treated bleached kraft mill effluent on cold water stream productivity in experimental stream channels. Environmental Toxicology and Chemistry 10 (8): 1051–1060.
Hargrave, C. W., R. Ramirez, M. Brooks, M. A. Eggleton, K. Sutherland, R. Deaton & H. Galbraith, 2006. Indirect food web interactions increase growth of an algivorous stream fish. Freshwater Biology 51: 1901–1910.
Hayes, J. W., 1989. Social interactions between 0 + brown and rainbow trout in experimental stream troughs. New Zealand Journal of Marine and Freshwater Research 23: 163–170.
Higler, L. W. G., 1975. Reaction of some caddis larvae (Trichoptera) to different types of substrate in an experimental stream. Freshwater Biology 5: 151–158.
Humphrey, K. P. & R. J. Stevenson, 1992. Responses of benthic algae to pulses in current and nutrients during simulations of subscouring spates. Journal of the North American Benthological Society 11: 37–48.
Kovacs, T. G., J. S. Gibbons, L. A. Tremblay, B. I. O’Connor, P. H. Martel & R. Voss, 1995. The effects of a secondary-treated bleached kraft mill effluent on aquatic organisms as assessed by short-term and long-term laboratory tests. Ecotoxicology & Environmental Safety 31: 7–22.
Krogh, K. A., B. Halling-Sorensen, B. B. Mogensen & K. V. Vejrup, 2003. Environmental properties and effects of nonionic surfactant adjuvants in pesticides: a review. Chemosphere 50: 871–901.
Kulacki, K. J., B. J. Cardinale, A. A. Keller, R. Bier & H. Dickson, 2012. How do stream organisms respond to, and influence, the concentration of titanium dioxide nanoparticles? A mesocosm study with algae and herbivores. Environmental Toxicology and Chemisty 31: 2414–2422.
Lange, K., A. Liess, J. J. Piggott, C. R. Townsend & C. D. Matthaei, 2011. Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure. Freshwater Biology 56: 264–278.
Larson, E. R., D. D. Magoulick, C. Turner & K. H. Laycock, 2009. Disturbance and species displacement: different tolerances to stream drying and desiccation in a native and an invasive crayfish. Freshwater Biology 54: 1899–1908.
Ledger, M. E., R. M. L. Harris, P. D. Armitage & A. M. Milner, 2012. Climate change impacts on community resilience: evidence from drought disturbance experiment. Advances in Ecological Research 46: 211–258.
Ledger, M. E., L. E. Brown, F. K. Edwards, L. N. Hudson, A. M. Milner & G. Woodward, 2013. Extreme climatic events alter aquatic food webs: a synthesis of evidence from mesocosm drought experiment. Advances in Ecological Research 48: 343–395.
Liess, A., K. Lange, F. Schulz, J. J. Piggott, C. D. Matthaei & C. R. Townsed, 2009. Light, nutrients and grazing interact to determine diatom species richness via changes to productivity, nutrient state and grazer activity. Journal of Ecology 97: 326–336.
Magbanua, F. S., C. R. Townsend, K. J. Hageman & C. D. Matthaei, 2013. Individual and combined effects of fine sediment and the herbicide glyphosate on benthic macroinvertebrates and stream ecosystem functions. Freshwater Biology 58: 1729–1744.
Matthaei, C. D., J. J. Piggott & C. R. Townsend, 2010. Multiple stressors on agricultural streams: interactions among sediment addition, nutrient enrichment and water abstraction. Journal of Applied Ecology 47: 639–649.
Mohr, S., M. Feibicke, T. Ottenströer, S. Meinecke, R. Berghahn & R. Schmidt, 2005. Enhanced experimental flexibility and control in ecotoxicological mesocosm experiments – A new outdoor and indoor pond and stream system. Environmental Science and Pollution Research 12: 5–7.
Nannini, M. A. & M. C. Belk, 2006. Antipredator responses of two native stream fishes to an introduced predator: does similarity in morphology predict similarity in behavioural response? Ecology of Freshwater Fish 15: 453–463.
Odum, E. P., 1984. The mesocosm. Bioscience 34: 558–562.
Orlóci, L., 1978. Multivariate Analysis in Vegetation Research. Dr. W. Junk B. V. Publishers, The Hague.
Pablo, F., F. R. Krassoi, P. R. F. Jones, A. E. Colville, G. C. Hose & R. P. Lim, 2008. Comparison of the fate and toxicity of chlorpyrifos – laboratory versus a coastal mesocosm system. Ecotoxicology and Environmental Safety 71: 219–229.
Peckarsky, B. L., A. R. McIntosh, B. W. Taylor & J. Dahl, 2002. Predator chemicals induce changes in mayfly life history traits: a whole-stream manipulation. Ecology 83: 612–618.
Pennuto, C. M. & F. de Noyelies Jr., 1993. Behavioural responses of Drunella coloradensis (Ephemeroptera) nymphs to short-term pH reduction. Canadian Journal of Fisheries and Aquatic Sciences 50: 2692–2697.
Piggott, J. J., K. Lange, C. R. Townsend & C. D. Matthaei, 2012. Multiple stressors in agricultural streams: a mesocosm study of interactions among raised water temperature, sediment addition and nutrient enrichment. PLoS ONE 7: e49873.
Piggott, J. J., R. K. Salis, G. Lear, C. R. Townsend & C. D. Matthaei, 2015a. Climate warming and agricultural stressors interact to determine stream periphyton community composition. Global Change Biology 21: 206–222.
Piggott, J. J., C. R. Townsend & C. D. Matthaei, 2015b. Climate warming and artificial stressors interact to determine stream macroinvertebrate community dynamics. Global Change Biology 21: 1887–1906.
Piggott, J. J., D. K. Niyogi, C. R. Townsend & C. D. Matthaei, 2015c. Multiple stressors and stream ecosystem functioning: climate warming and agricultural stressors interact to affect processing of organic matter. Journal of Applied Ecology 52: 1126–1134.
R Core Team, 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Rosenbaum, P., 2017. Observation and Experiment: An Introduction to Causal Inference. Harvard University Press, Cambridge: 400.
Stewart, R. I. A., M. Dossena, D. A. Bohan, E. Jeppesen, R. L. Kordas, M. E. Ledger, M. Meerhoff, B. Moss, C. Mudler, J. B. Shurin, B. Suttle, R. Thompson, M. Trimmer & G. Woodward, 2013. Mesocosm experiments as a tool for ecological climate-change research. Advances in Ecological Research 48: 71–182.
Taulbee, W. K., C. T. Nietch, D. Brown, B. Ramakrishan & M. J. Tompinks, 2009. Ecosystem consequences of contrasting flow regimes in an urban effects stream mesocosm study. Journal of the American Water Resources Association 45: 907–927.
Taylor, E. J., S. J. Maund, D. Bennett & D. Pascoe, 1994. Effects of 3,4-dichloroaniline on the growth of two freshwater macroinvertebrates in a stream mesocosm. Ecotoxicology and Environmental Safety 29: 80–85.
Thayse, N. & D. Schmera, 2016. The effects of top-down and bottom-up controls on macroinvertebrate assemblages in headwater streams. Hydrobiologia 763: 173–181.
Troia, M. J., J. E. Whitney & K. B. Gido, 2014. Alternative spawning strategy and temperature for larval emergence of longfin dace (Agosia chrysogaster) in stream mesocosms. The Southwestern Naturalist 59: 277–280.
Vance, S. A., 1996. The effect of the mermithid parasite Gasteromermis sp (Nematoda: Mermithidae) on the drift behaviour of its mayfly host, Baetis bicaudatus (Ephemeroptera: Baetidae): a trade off between avoiding predators and locating food. Canadian Journal of Zoology-Revue Canadienne De Zoologie 74: 1907–1913.
Wagenhoff, A., C. R. Townsend & C. D. Matthaei, 2012. Macroinvertebrate responses along broad stressor gradients of depositioned fine sediment and dissolved nutrients: a stream mesocosm experiment. Journal of Applied Ecology 49: 892–902.
Wagenhoff, A., K. Lange, C. R. Townsend & C. D. Matthaei, 2013. Patterns of benthic algae and cyanobacteria along twin-stressor gradients of nutrients and fine sediment: a stream mesocosm experiment. Freshwater Biology 98: 1849–1863.
Woodward, G., D. M. Perkins & L. E. Brown, 2010. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365: 2093–2106.
Acknowledgements
Open access funding provided by MTA Centre for Ecological Research (MTA ÖK). We thank Paul J. Wood, Barbara Barta, and the two anonymous reviewers for their invaluable comments on the manuscript. This work was supported by the GINOP (Gazdaságfejlesztési és Innovációs Operatív Program) 2.3.3-15-2016-00019, MTA KEP (Magyar Tudományos Akadémia Társadalmi jólét ökológiai alapjai program), and NKFI (Nemzeti Kutatási, Fejlesztési és Innovációs Hivatal) K 128496 Grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Verónica Ferreira
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Menczelesz, N., Szivák, I. & Schmera, D. How do we construct and operate experimental streams? An overview of facilities, protocols, and studied questions. Hydrobiologia 847, 1–10 (2020). https://doi.org/10.1007/s10750-019-04093-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04093-0