Abstract
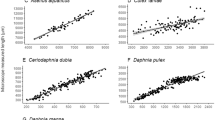
Fully or semi-automated methods are becoming viable, cost-effective alternatives to manual approaches for characterizing zooplankton. The goal of this study was to compare a semi-automated approach (FlowCAM®) and a traditional microscopy method for characterizing zooplankton body size, density, and community structure. We demonstrate that estimating mass from FlowCAM® profile area had similar accuracy to a commonly used length to mass regression model. FlowCAM® and microscopy produced related length measurements for Daphnia, Calanoida, and Cyclopoida. Length measurements of rotifers, nauplii, and Sididae were not significantly related between the two methods, likely because of high morphological variation within taxa. Density comparisons between methods indicated high correlation between the semi-automated approach and microscopy-derived densities with a subtle bias of lower densities with the semi-automated method. After applying a correction factor, independent samples showed similar density estimates between methods, with community composition also not differing between methods. Comparison of processing time between the two methods showed that the semi-automated approach was 11 min (33%) faster per sample. With corrections, semi-automated methods represent a viable and cost-effective alternative to traditional microscopy methods for the processing of zooplankton samples.






Similar content being viewed by others
References
Alcaraz, M., E. Saiz, A. Calbet, I. Trepat & E. Broglio, 2003. Estimating zooplankton biomass through image analysis. Marine Biology 143: 307–315.
Álvarez, E., M. Moyano, A. Lopez-Urrutia, E. Nogueira & R. Scharek, 2014. Routine determination of plankton community composition and size structure: a comparison between FlowCAM and light microscopy. Journal of Plankton Research 36: 170–184.
Benfield, M. C., P. Grosjean, P. F. Culverhouse, X. Irigoien, M. E. Sieracki, A. Lopez-Urrutia, H. G. Dam, Q. Hu, C. S. Davis, A. Hansen, C. H. Pilskaln, E. M. Riseman, H. Schultz, P. E. Utgoff & G. Gorsky, 2007. RAPID: research on automated plankton identification. Oceanography 20: 172–187.
Billones, R. G., M. L. M. Tackx, A. T. Flachier, L. Zhu & M. H. Daro, 1999. Image analysis as a tool for measuring particulate matter concentrations and gut content, body size, and clearance rates of estuarine copepods: validation and application. Journal of Marine Systems 22: 179–194.
Bray, J. R. & J. T. Curtis, 1957. An ordination of upland forest communities of southern Wisconsin. Ecological Monographs 27: 325–349.
Burnham, K. P. & D. R. Anderson, 2002. Model selection and multimodel inference: a practical information theoretic approach, 2nd ed. Springer-Verlag, New York.
Buskey, E. J. & C. J. Hyatt, 2006. Use of the FlowCAM for semi-automated recognition and enumeration of red tide cells (Karenia brevis) in natural plankton samples. Harmful Algae 5: 685–692.
Calbet, A., S. Garrido, E. Saiz, M. Alcaraz & C. M. Duarte, 2001. Annual zooplankton succession in coastal NW Mediterranean waters: the importance of the smaller size fractions. Journal of Plankton Research 23: 319–331.
Collins, S. F., T. M. Detmer, K. A. Kelson, M. A. Nannini, G. G. Sass & D. H. Wahl, 2018. The release and regulation of rotifers: examining the predatory effects of invasive juvenile common and bighead carp. Hydrobiologia 813: 199–211.
Culverhouse, P. F., R. Williams, B. Reguera, V. Henry & S. González-Gil, 2003. Do experts make mistakes? A comparison of human and machine identification of dinoflagellates. Marine Ecological Progress Series 247: 17–25.
Culverhouse, P. F., R. Williams, M. Benfield, P. R. Flood, A. F. Sell, M. G. Mazzochi, I. Buttino & M. Sieracki, 2006. Automatic image analysis of plankton: future perspectives. Marine Ecological Progress Series 312: 297–309.
Davis, C. C., S. M. Gallager & A. R. Solow, 1992. Microaggregations of oceanic plankton observed by towed video microscopy. Science 257: 230–232.
Detmer, T. M., J. H. McCutchan Jr. & W. M. Lewis Jr., 2017a. Trophic interactions across lake-stream boundaries in mountain lakes. Inland Waters 7: 440–448.
Detmer, T. M., J. H. McCutchan Jr. & W. M. Lewis Jr., 2017b. Predator driven changes in prey size distribution stabilize secondary production in lacustrine food webs. Limnology and Oceanography 62: 592–605.
Detmer, T. M., M. J. Diana & D. H. Wahl, 2019. Season and presence of Gizzard Shad influence horizontal spatial distribution of zooplankton in reservoirs of the Midwestern United States. Freshwater Sciences 38: 183–192.
Dumont, H. K., I. Van de Velde & S. Dumont, 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepoda, and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19: 75–97.
Fluid Imaging Technologies Inc., 2011. FlowCAM manual version 3.0 [online]. [available on internet at http://www.ihb.cas.cn/fxcszx/fxcs_xgxz/201203/P020120329576952031804.pdf].
García-Criado, F. & C. Trigal, 2005. Comparison of several techniques for sampling macroinvertebrates in different habitats of a North Iberian pond. Hydrobiologia 545: 103–115.
Garmendia, M., M. Revilla & L. Zarauz, 2013. Testing the usefulness of a simple automatic mathod for particles abundance and size determination to derive cost-effective biological indicators in large monitoring networks. Hydrobiologia 704: 231–252.
Gorsky, G., M. D. Ohman, M. Picheral, S. Gasparini, L. Stemmann, J. Romagnan, A. Cawood, S. Pesant, C. García-Comas & F. Prejger, 2010. Digital zooplankton image analysis using the ZooScan integrated system. Journal of Plankton Research 32: 285–303.
Grosjean, P., M. Picheral, C. Warembourg & G. Gorsky, 2004. Enumeration, measurement, and identification of net zooplankton samples using the ZOOSCAN digital imaging system. ICES Journal of Marine Sciences 61: 518–525.
Hamilton, S. K., S. J. Sippel, W. M. Lewis Jr. & J. F. Saunders III, 1990. Zooplankton abundance and evidence for its reduction by macrophyte mats in two Orinoco floodplain lakes. Journal of Plankton Research 12: 345–363.
Hernández-León, S. & I. Montero, 2006. Zooplankton biomass estimated from digitized images in Antarctic waters: a calibration exercise. Journal of Geophysical Research 111: 3.
Ide, K., K. Takahashi, A. Kuwata, M. Nakamachi & H. Saito, 2008. A rapid analysis of copepod feeding using FlowCAM. Journal of Plankton Research 30: 275–281.
Jakobsen, H. H. & J. Carstensen, 2011. FlowCAM: sizing cells and understanding the impact of size distributions on biovolume of planktonic community structure. Aquatic Microbial Ecology 65: 75–87.
Krueger, D. A. & S. I. Dodson, 1981. Embryological induction and predation ecology in Daphnia pulex. Limnology and Oceanography 26: 219–223.
Kydd, J., H. Rajakaruna, E. Briski & S. Bailey, 2017. Examination of a high resolution laser optical plankton counter and FlowCAM for measuring plankton concentration and size. Journal of Sea Research 133: 2–10.
Le Bourg, B., V. Cornet-Barthaux, M. Pagano & J. Blanchot, 2015. FlowCAM as a tool for studying small (80–1000μm) metazooplankton communities. Journal of Plankton Research 37: 666–670.
Lewis, W. M., 1979. Zooplankton community analysis: studies on a tropical system. Springer-Verlag, New York New York.
Makarewicz, J. C. & G. E. Likens, 1979. Structure and function of the zooplankton community of Mirror Lake, New Hampshire. Ecological Monographs 49: 109–127.
Minchin, P. R., 1987. An evaluation of the relative robustness of techniques for ecological ordination. Theory and Models in Vegetation Science 69: 89–107.
Moberg, E. A. & H. M. Sosik, 2012. Distance maps to estimate cell volume from two-dimensional plankton images. Limnology and Oceanography: Methods 10: 278–288.
Pennak, R. W., 1973. Some evidence for aquatic macrophytes as repellents for a limnetic species of Daphnia. Internationale Revue der gesamten Hydrobiologie 58: 569–576.
Pinel-Alloul, B., C. Guay, N. Angeli, P. Legendre, P. Dutilleul, G. Balvay, D. Gerdeaux & J. Guillard, 1999. Large-scale spatial heterogeneity of macrozooplankton in Lake of Geneva. Canadian Journal of Fisheries and Aquatic Sciences 56: 1437–1451.
Poulton, N. J., 2016. FlowCAM: quantification and classification of phytoplankton by imaging flow cytometry. Imaging Flow Cytometry: Methods and Protocols 1389: 237–247.
R Core Team, 2013. T: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. [available on internet at http://www.R-project.org/.
Saunders III, J. F. & W. M. Lewis Jr., 1988. Composition and seasonality of the zooplankton community of Lake Valencia, Venezuela. Journal of Plankton Research 10: 957–985.
Sieracki, C. K., M. E. Sieracki & C. S. Yentsch, 1998. An imaging-in-flow system for automated analysis of marine microplankton. Marine Ecological Progress Series 168: 285–296.
Siokou-Frangou, I., U. Christaki, M. G. Mazzocchi, M. Montresor, M. Riberad’Alcala, D. Vaque & A. Zingone, 2008. Plankton in the open Mediterranean Sea: a review. Biogeosciences 7: 1543–1586.
Stanislawczyk, K., M. L. Johansson & H. L. MacIsaac, 2018. Microscopy versus automated imaging flow cytometry for detecting and identifying rare zooplankton. Hydrobiologia 807: 53–65.
Thorp, J. H. & A. P. Covich (eds), 1991. Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego.
Turner, A. M. & J. C. Trexler, 1997. Sampling aquatic invertebrates from marshes: evaluating the options. Journal of the North American Benthological Society 16: 694–709.
Welker, M. T., C. L. Pierce & D. H. Wahl, 1994. Growth and survival of larval fishes: roles of competition and zooplankton abundance. Transactions of the American Fisheries Society 123: 703–717.
Wong, E., A. R. Sastri, F. S. Lin & C. H. Hsieh, 2017. Modified FlowCAM procedure for quantifying size distribution of zooplankton with sample recycling capacity. PLoS One 12: e0175235.
Zarauz, L. & X. Irigoien, 2008. Effects of Lugol’s fixation on the size structure of natural nano-microplankton samples, analyzed by means of an automatic counting method. Journal of Plankton Research 30: 1297–1303.
Acknowledgements
We thank M. Diana and B. Diffen for initial setup of the FlowCAM® device and laboratory assistance. We also thank the D. Soucek lab for the use of a microbalance. This study was supported by the Great Lakes Restoration Initiative (CAFWS-93) and the Federal Aid in Sporfish Restoration Act (project F-185-R-6) with funding administered through the Illinois Department of Natural Resources (IDNR). We would like to thank K. Irons and M. McClelland for coordination of this project with the IDNR Division of Fisheries. We would also like to thank three anonymous reviewers for providing constructive comments and helping us to improve our methodology.
Disclaimer
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the University of Illinois, Prairie Research Institute, or the Illinois Natural History Survey.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Piet Spaak
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Detmer, T.M., Broadway, K.J., Potter, C.G. et al. Comparison of microscopy to a semi-automated method (FlowCAM®) for characterization of individual-, population-, and community-level measurements of zooplankton. Hydrobiologia 838, 99–110 (2019). https://doi.org/10.1007/s10750-019-03980-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-03980-w