Abstract
Climate change projections indicate increased precipitation in northern Europe, leading to increased inflow of allochthonous organic matter to aquatic systems. The food web responses are poorly known, and may differ depending on the trophic structure. We performed an experimental mesocosm study where effects of labile dissolved organic carbon (DOC) on two different pelagic food webs were investigated, one having zooplankton as highest trophic level and the other with planktivorous fish as top consumer. In both food webs, DOC caused higher bacterial production and lower food web efficiency, i.e., energy transfer efficiency from the base to the top of the food web. However, the top-level response to DOC addition differed in the zooplankton and the fish systems. The zooplankton production increased due to efficient channeling of energy via both the bacterial and the phytoplankton pathway, while the fish production decreased due to channeling of energy mainly via the longer and less efficient bacterial pathway. We conclude that the added DOC either acted as a subsidy by increasing the production of the top trophic level (mesozooplankton), or as a sink causing decreased top consumer production (planktivorous fish).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge about pathways and constraints of energy transfer through food webs is fundamental for the understanding of ecosystem function (e.g., Dickman et al., 2008; Wollrab et al., 2012). The complexity of food web dynamics and structure inherently means that both changes in basal production and top–down control can create alternative pathways of energy flow up to the highest trophic level, resulting in patterns of energy transfer and productivity commonly deviating from expectations based on classical food chain theory (Vadeboncoeur et al., 2004, Hulot et al., 2014). For example, the presence or absence of classic trophic cascades in marine food webs has been suggested to be dependent on the size structure of the phytoplankton community (Stibor et al., 2004).
In aquatic systems, phytoplankton (autotrophs) and bacteria (heterotrophs) are basal producers, acting as energy source for higher trophic levels, and thus shape the food webs depending on their production and composition (Azam et al., 1983; Legendre & Rassoulzadegan, 1995, Jansson et al., 2007). The balance between autotrophs and heterotrophic bacteria is governed by both bottom–up factors such as nutrient availability, and top–down effects, e.g., trophic interactions via top predators (Carpenter et al., 1985; Hairston & Hairston, 1993, Vanni & Layne, 1997). These food webs are complex, as many consumers feed on organisms from both the phytoplankton and the bacterial pathway. Both phytoplankton and bacteria are osmotrophic organisms competing for inorganic nutrients, nevertheless phytoplankton utilize inorganic forms of carbon while heterotrophic bacteria are in many systems dependent on autochthonous organic carbon produced by phytoplankton (Cole et al., 1988). However, in aquatic systems influenced by allochthonous dissolved organic carbon (ADOC), bacteria can be decoupled from autochthonous production (Karlsson et al., 2002, Stibor et al., 2004). Accordingly, in systems with high inputs of ADOC, heterotrophic bacteria tend to contribute substantially to the total basal production (Pace et al., 2004; Berglund et al., 2007, Jansson et al., 2007). Phytoplankton are often directly consumed by primary consumers like mesozooplankton, whereas bacteria are too small to be readily eaten by such organisms. Instead, bacteria are consumed by protozoans which in turn are consumed by mesozooplankton (Hessen & Andersen, 1990, Brett et al., 2009). Hence, the heterotrophic-based pathway, i.e., the microbial food web, will have a more complex pattern of energy transfer compared to systems dominated by autotrophic production (Sommer et al., 2002; Berglund et al., 2007).
At the top of the food web, interactions between predator and prey may also yield strong effects on food web function and structure, but the nature and composition of top consumer organisms in the food web will exert different top–down impacts on lower trophic levels (Hairston & Hairston, 1993, Vanni & Layne, 1997, Hulot et al., 2014). For instance, in food webs where mesozooplankton function as top predator, consumption rates on phytoplankton and ciliates have been shown to be high (Johansson et al., 2004; Calbet & Saiz, 2005; Sommer & Sommer, 2006), releasing heterotrophic nanoflagellates (HNF) from predation which in turn cause decreased bacterial abundances (Zöllner et al., 2009). In contrast, if top consumers are planktivorous fish, they suppress the zooplankton abundance which in turn releases phytoplankton from grazing pressure (e.g., Carpenter et al., 1985; Shiomoto et al., 1997; Ordóñez et al., 2010). This may also increase bacterial abundance as zooplankton predation on ciliates decreases, causing increased ciliate density and hence a higher grazing pressure on HNF, the main predator on bacteria (Christoffersen et al., 1993; Nishimura et al., 2011). Effects on food web dynamics and top consumer production can therefore be dependent on both the access to dissolved organic carbon (DOC) for heterotrophic bacteria and the nature and composition of the top consumers in the system.
Food web efficiency (FWE) is a measure of the overall system efficiency and is defined as the proportion of basal production that reaches the top consumers (Rand & Stewart, 1998; Berglund et al., 2007; Lefébure et al., 2013). Changes in the relative importance of autotrophic and heterotrophic basal production, induced either by top–down or bottom–up drivers, will affect the energy transfer to higher trophic levels since bacteria are of poor nutritional quality (i.e., have low C:P ratio, Fagerbakke et al., 1996) and the heterotrophic-based pathway channels the carbon through more trophic levels before it reaches top consumers (Hessen & Andersen, 1990; Sommer et al., 2002; Brett et al., 2009). As it is estimated that each trophic coupling will result in ~70% of the energy/carbon loss due to sloppy feeding and metabolic losses (e.g., Welch, 1968; Straile, 1997), a smaller fraction of the basal production is therefore thought to reach the highest trophic level in food webs dominated by heterotrophic production. Accordingly, it has been shown that FWE in heterotrophic food webs can be up to tenfold lower than in autotrophic webs, due to 1 or 2 extra intermediate trophic levels (Berglund et al., 2007). The FWE can also be affected by other factors, such as the food size preference of the inherent mesozooplankton.
The study of the effects of ADOC on pelagic food webs has gained scientific interest (Pace et al., 2004; Jansson et al., 2007), largely related to climate change predictions of increased run-off of ADOC to aquatic environments (IPCC, 2007; HELCOM, 2007). However, most studies have focused on a limited number of links in the food web to either include only e.g., microbes to zooplankton (Sterner et al., 1998; Berglund et al., 2007; O’Connor et al., 2009) or only phytoplankton or zooplankton to fish (Malzahn et al., 2007; Dickman et al., 2008). With the exception of Faithfull et al. (2011, 2012) and Lefébure et al. (2013), very few studies have experimentally investigated FWE dynamics in systems encompassing food webs with both phytoplankton and bacteria at the base and fish at the top of the food web.
Understanding patterns of energy flow in aquatic systems has proven to be challenging, where even simplistic food chains can yield highly variable results (Dickman et al., 2008; Heath et al., 2014). Furthermore, there is currently a gap in the scientific understanding of how top-down effects can propagate through the food web and impact both biomass and production of lower-level autotrophic and heterotrophic organisms (Hessen & Kaartvedt, 2014). As outlined above, the interplay between top-down predation and bottom-up productivity dynamics can profoundly influence food web structure and efficiency, and the empirical studies that have investigated these dynamics on natural marine food webs are to a large extent lacking. The need to advance food web theory is therefore critical in order to gain a better understanding of how aquatic systems inherently function but also respond to change, be it due to environmental variability or anthropogenic impacts.
We performed an experimental study where effects of variations in primary production (PP) and heterotrophic bacterial production (BP) on FWE and production at higher trophic levels were investigated. A full-factorial experiment was set up in a large-scale mesocosm facility by creating food webs dominated by either bacteria or phytoplankton at the basal level and zooplankton (copepods) or fish as the top predator. Based on the available theory we predicted that: 1) food webs with a higher BP:PP ratio will have lower FWE than food webs with lower BP:PP; 2) consequently, the production of both top consumers will be lower in food webs influenced by DOC, due to an increased dependency on bacteria-produced carbon as energy source; 3) top consumer identity will alter lower trophic level dynamics, potentially resulting in changes in both BP:PB ratios and the pathways of carbon transfer up to higher trophic levels; and 4) DOC enrichment will lead to larger FWE decrease in food webs with fish, due to selective promotion of the bacterial energy transfer route.
Materials and methods
Experimental design
Four experimental treatments, with three replicates each, were used to test differences in food web efficiency and productivity in systems with contrasting structure at the basal trophic level and with food webs of differing length (Fig. 1). The basal production structure was manipulated by adding nitrogen (N) and phosphorous (P) to induce a phytoplankton-based food web and by adding N, P, and carbon (C) to obtain a bacteria-based food web. A labile carbon source, glucose, was added to one of the systems to promote bacterial growth. Inorganic nutrients were added to the other system to promote phytoplankton growth. The trophic position at the top of the food web was manipulated by having either a natural zooplankton assemblage as top predator or by the addition of zooplanktivorous fish, juvenile three-spined stickleback (Gasterosteus aculeatus).
Flow chart of the experimental procedure, showing the incubation temperature and lengths of the acclimation and experimental periods. CNP indicates additions of organic carbon and inorganic nitrogen and phosphorus. F shows presence of fish. F1 and F2 indicate that two different fish assemblages were used, and C1 and C2 that two different concentrations of organic carbon were added
The experiment was carried out in autumn (November), i.e., during the latest part of the productive season in the northern Baltic Sea. Twelve indoor polyethylene mesocosms with a volume of 2000 L and a depth of 5 m were filled with unfiltered coastal water from the northern Baltic Sea (63°34′N, 19°54′E) using a peristaltic pump. The water, collected at the surface, had an in situ temperature of 5°C. The mesocosms were filled to their capacity and air was gently bubbled into the mesocosms (20 ml s−1) at four meters depth to maintain a well-mixed water column. The incubations were kept at a constant temperature of 15°C and a cycle of 12 h light and 12 h dark was established. Five percent of the total volume was replaced twice a week with 0.20 µm filtered seawater. The total length of the study was 63 days, which was divided into three parts. The systems were acclimated for bottom–up (nutrient and DOC enrichment) and top–down (impact of top predator) factors over 30 days before the actual experiment started. Fish growth was then measured twice: once in experimental period 1 (day 30–42; 13 days) and once in period 2 (day 43–63; 22 days).
To obtain phytoplankton-dominated production, nitrogen (N) and phosphorus (P) were added to six of the mesocosms (NP treatment), and to create dominance of bacterial production, N, P, and additional carbon (C) (glucose, originating from a C4 plant) were added to the other six mesocosms (CNP treatment, DOC-enriched systems). Since we were specifically interested in the effect of C enrichments, the NP treatments served as controls. Nutrients were added on a weekly basis and the loading rates were based on a previous study (Berglund et al., 2007): ammonium 2.3 µmol l−1, nitrate 13.8 µmol l−1, phosphate 1.6 µmol l−1, and glucose 75.1 µmol l−1 during the acclimation phase and experimental period 1 and 300.4 µmol l−1 glucose in period 2. The aim of the higher carbon addition in the second experimental period was to create higher BP: PP ratios, in order to study the effect of a larger difference in BP: PP ratio between controls and the DOC-enriched systems. We focused on fish responses to DOC addition during this period since it did not give significant effects during the first experimental period. At the start of the study, the systems were incubated for a week before fish (juvenile sticklebacks) were added to three of the NP- and CNP-enriched mesocosms (CNP = DOC-enriched zooplankton system, NP = control zooplankton system, CNP-F = DOC-enriched fish system, NP-F = control fish system). After an adaptation period of 23 days, all fish were removed from the mesocosms and new fish (6 juvenile sticklebacks per mesocosm, weight 0.069 ± 0.017 g) were gently introduced to the mesocosms, and their growth was measured over 13 days of incubation (period 1). This procedure was repeated with the introduction of new fish on day 43 (fish weight 0.082 ± 0.022 g) for period 2, which lasted for 22 days. We thus obtained two fish experiments.
Sampling and analysis
Water samples were taken once per week starting on day 1 using a 4-m-long PVC hose (D.M. 21-3-37E, inner diameter 2.5 cm, total volume 2.5 l). Two column samples per mesocosm were collected at each sampling occasion. Additionally, 60 l samples were filtered from valves placed on the tank walls at one and three meters depth for zooplankton biomass and composition every second week. Samples for bacterial biomass, and primary and bacterial production were analyzed weekly. Samples for inorganic nutrients, dissolved organic carbon (DOC), and phytoplankton biomass were analyzed at the start, middle, and end of the experiment. Measurements of fish biomass were made at the start and end of the two experimental periods.
DOC was measured using a high-temperature carbon analyzer (Shimadzu TOC-5000) and dissolved inorganic nitrogen (DIN, ammonia, nitrite, and nitrate), dissolved inorganic phosphorus (DIP, phosphate), and total N (Tot N) and P (Tot P) were measured using a Bran and Luebbe TRAACS 800 autoanalyzer.
Samples for heterotrophic bacteria and HNF were preserved with formaldehyde (0.2 µm filtered, 4% final concentration). For enumeration of bacteria, 1 ml of each sample was stained with acridine orange (3.2 mg ml−1 final concentration) and filtered onto a 0.2 µm black polycarbonate filter (Poretics). Bacteria were analyzed using blue excitation light (450–490 nm) on an epifluorescence microscope (Zeiss Axiovert 100) attached to a camera (Hamamatsu ORCA-ER). Cell concentrations and biovolumes were measured using image analysis (Blackburn et al., 1998) and Lab Database (http://www.bioras.com/). Bacterial carbon biomass was estimated as described in Wiklund et al. (2009). For analysis of HNF, 15 ml was filtered onto 0.6 μm black polycarbonate filters and stained with DAPI (Sherr et al., 1992). One diagonal of the filter was counted in UV light at 1000 × magnification using a Nikon TE200 epifluorescence microscope. Flagellate biomass was calculated according to their geometry and using the carbon conversion factor presented by Menden-Deuer and Lessard (2000). Ciliates and phytoplankton samples were fixed with 0.2% alkaline Lugol´s solution. For ciliates, fifty milliliters of each fixed sample were settled in a sedimentation chamber for 48 h and counted according to the Utermöhl technique at 200× magnification. Half of the sedimentation chamber or two diagonals (diameter 26 mm) were scanned. For phytoplankton, 10 ml samples were settled in sedimentation chambers for 12 h and then counted with an inverted microscope (Leica DM IRB) using phase contrast. For cells <10 µm, 1 or 2 diagonals (13.75 or 27.5 mm2) of the sedimentation chamber were counted at 400× magnification and for plankton >10 µm between 10 fields (9.5 mm2) and half of the sedimentation chamber (245 mm2) were scanned at 200× magnification. The cell size was measured using an ocular scale and cell volume biomass and carbon were calculated according to HELCOM guidelines (2007).
Mesozooplankton were collected on a 90 µm mesh and preserved with 0.2% alkaline Lugol’s solution. An inverted microscope was used to identify species and estimate biomass/abundance of each taxon. Length of 10 individuals (all, if fewer were collected) of each taxa was also measured. Copepods were classified into two groups: nauplii and copepodites. Lengths were transformed to body mass using length–mass regressions (Dumont et al., 1975; Hernroth, 1985) and assuming 5% carbon content of wet mass. Mesozooplankton production was estimated in treatments where mesozooplankton was the top consumer. It was calculated from abundance, body mass, and literature data on development time at 15°C (Vijverberg, 1980). Since the mesozooplankton community was dominated by copepods (constituting >89% of the biomass except in one NP replicate day 63), all other groups were ignored in the calculations. The calculation of mesozooplankton production was based on the number and weight of nauplii produced during each time interval and the weight increase of the surviving nauplii that developed to copepodites at each time interval, according to Berglund et al. (2007):
Mesozooplankton net production (MZp) is expressed in µg C l−1 day−1, where n 1 = number of nauplii l−1 at time t 1, w n = nauplii weight in µg C per individual (Hernroth, 1985), n 0 = number of nauplii l−1 at time t 0, and w c = mean copepodite weight in µg C per individual from this study. Mesozooplankton mortality (m) was estimated by the following equation:
where c est = estimated number of nauplii l−1 that have become copepodites, assuming no mortality, and according to development time from literature; c 0 = number of copepodites at t 0; and c 1 = actual number of copepodites present at t 1. During the first experimental period the sampling intervals were set so that nauplii at the start of each time interval had all developed to copepodites at the end of the interval, and all nauplii occurring at the end of each time interval were produced from eggs during that period. During the second experimental period, the sampling frequency was too low to calculate zooplankton production. It was not possible to estimate mesozooplankton production in the fish systems, because the fish efficiently kept the mesozooplankton biomass low all through the experiment.
Primary production was measured according to standard method described in Gargas (1975). For each mesocosm, five light samples (at 0, 1, 2, 3, and 4 m depth) and one dark (control) sample (9 ml each) were incubated at specific depths for 4 h in acid-washed polycarbonate bottles (10 ml leaving 1 ml air) with 3.7 MBq mmol−1 sodium (14C) bicarbonate. After incubation, samples were immediately poured into glass scintillation bottles, acidified with 300 µl of 6 mol l−1 HCl, and bubbled for 30 min to remove the excess sodium (14C) bicarbonate. This process was carried out in the dark. Scintillation cocktail (Optiphase, Hi-Safe 3) was added (10 ml) to each sample and samples were analyzed in a Beckman 6500 scintillation counter. Primary production rates were converted from bottle to mesocosm level by depth-integrating the measured values. Daily values were calculated using a light factor (12/4: 12 h day light, 4 h of incubation).
Bacterial production was measured using the [3H-methyl]-thymidine technique (Fuhrman & Azam, 1982). Triplicate 1 ml samples were incubated for 1 h with 3.1 TBq mmol−1 [3H-methyl]-thymidine. The incubations were stopped by adding 100 µl ice-cold 50% trichloroacetic acid (TCA). Triplicate controls were pre-killed with 100 µl 50% TCA. Non-incorporated thymidine was rinsed out with ice-cold 5% TCA in sequential centrifugation steps. Scintillation cocktail (Optiphase, Hi-Safe 3) was added (1 ml) to each sample and incorporated thymidine was measured with a Beckman 6500 scintillation counter. A conversion factor of 1.4 × 1018 cells per mole of incorporated thymidine was used to calculate cell production (Riemann et al., 1987; Autio, 1998, Wikner & Hagström, 1999, Autio, 2000). Bacterial carbon biomass production was calculated from cell production and cell carbon biomass. Bacterial production rates were converted from tube to mesocosm level by depth-integrating the measured values. Daily values were calculated by multiplying the measured values with a time factor (24/1: 24 h per day, 1 h incubation).
Fish were starved for 24 h before they were introduced to the mesocosms. Their weight was measured at the start and end of the 2 experimental periods. Fish production (FP) was calculated using:
W s is the weight at start and W f the weight at the end of the experiment, t is time.
Fish production (µg carbon day−1) was estimated from weight change of all surviving individuals, and assuming 20% carbon per wet weight (Jobling, 1994). In the first experimental period, a total of three fish died in each treatment and during the second period only one died in the NP-F treatment while four died in the CNP-F treatment. These were assumed to have only minor impact on the food webs, and thus they were excluded from any further analysis.
FWE was defined as the ratio between the production of the top predator and basal production according to the formula:
where production at all levels is given as the average carbon production per day and liter of seawater. Top production indicates zooplankton production in the NP and CNP treatments and fish production in the NP-F and CNP-F treatments. In this calculation we do not consider whether bacterial production was fueled by autochthonous carbon or by added DOC, since at the time of its production it entered the planktivorous food web as the lowest trophic level.
Fish were analyzed for deuterium (δ2H) to get an indication of whether their food source was of autochthonous or allochthonous origin. The fish were collected at the end of the experiment and immediately frozen at −20°C until analysis. Prior to analysis the samples were dried at 60°C for 48 h. Fish were analyzed whole and homogenized using a mortar and pestle and weighed to the nearest µg and placed into silver capsules. Zooplankton samples were directly weighed in the same manner and placed in silver capsules. Because fish were used whole, they had to be acidified with 0.1 M HCL and dried again at 60°C for 24 h to get rid of carbonates (Jacob et al., 2005). Deuterium samples were analyzed at the Colorado Plateau Stable Isotope Laboratory, Northern Arizona University using a Thermo-Electron temperature conversion elemental analyzer (pyrolysis) interfaced to a Delta Plus XL isotope ratio mass spectrometer (via CONFLO II).
Statistical analyses
Two-way ANOVA was used to analyze the effects of nutrient enrichment (NP or CNP) and top consumer (zooplankton or fish) on DIN, DIP, Tot N, Tot P, PP, BP, Tot Prod, bacteria, flagellates, ciliates, phytoplankton, and zooplankton. One-way ANOVA was used to analyze effects of nutrient enrichment (NP or CNP) on zooplankton and fish production. The experimental periods 1 and 2 were analyzed separately. When appropriate, data were log-transformed to obtain homogenous variance. All statistics were performed using IBM SPSS Statistics 22.
The elucidate bottom-up and top-down effects of DOC and fish on the biomass of heterotrophic bacteria, HNF, ciliates, phytoplankton, zooplankton, and fish, we subtracted the average biomass in NP from CNP, the biomass in NP-F from CNP-F, biomass in CNP from CNP-F, and biomass in NP from NP-F. When the average biomass differed and the standard deviation did not overlap we interpreted the factor to affect the plankton biomass.
Results
Two-way ANOVA showed that both nutrient enrichment (NP or CNP) and top-level consumer (zooplankton or fish) affected the DIN and DIP concentrations, and further that there were interactive effects (Table 1). The concentrations of DIN and DIP generally decreased over the time course of the experiment in CNP, CNP-F, and CNP, while the DIN and DIP concentrations were stable or increased in the NP systems (Fig. 2). Tot N and Tot P showed a similar variation as the inorganic nutrients; however, Tot P exhibited lower differences between treatments than Tot N and the inorganic forms (Fig. 2; Table 1). The DOC concentration was similar in all treatments throughout the experiment, ~4 mg l−1 (data not shown).
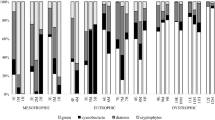
Both nutrient enrichment (NP or CNP) and top-level consumer (zooplankton or fish) affected the total basal production (BP + PP) (Table 2). These factors also affected the bacterial production, while primary production was only influenced by nutrient enrichment (Table 2). In the NP system, the basal production was relatively low (Fig. 3A), and slightly dominated by bacterial production (Fig. 3E). In the CNP system the basal production was higher (Fig. 3B), mainly due to an increase in bacterial production (Fig. 3F). In this case C addition did not cause a markedly decreased primary production. The primary production was highest in NP-F (Fig. 3C), where it constituted a relatively large share (40–80%) of the basal production (Fig. 3G). Highest basal production occurred in the CNP-F system (Fig. 3D), where bacterial production was markedly increased and constituted 95–99% of production (Fig. 3H).
A to D Variation of primary (PP) and bacterial production (BP) during the time course of the mesocosm experiment. Values are means of 3 replicates. Error bars denote 1 SD. E to H Proportion of bacterial and primary production in the different treatments. Arrows show the time span for the two experimental periods
Average bacterial biomass was lowest in NP (Fig. 4). The other treatments had high bacterial biomass, but their order differed between period 1 and 2. A significant effect was only found for top consumer during period 1 (Table 3). Total phytoplankton biomass was lowest in NP and highest in systems where fish was present (NP-F and CNP-F) (Fig. 4). Two-way ANOVA also revealed a significant effect of top consumer during period 1 and 2 (Table 3). The highest HNF biomass occurred in the CNP treatment during the second period (Fig. 4). Two-way ANOVA also showed that nutrient enrichment significantly affected the HNF biomass (Table 3). During both experimental periods, the ciliate biomass was higher in the fish systems than in the zooplankton systems (Fig. 4). In general, the second period yielded similar, although weaker, patterns (Table 3). The mesozooplankton biomass was greatly reduced in the fish systems, while in the zooplankton systems C addition had a positive effect on their biomass (Fig. 4). Thus, both nutrient enrichment and top consumer influenced zooplankton biomass (Table 3).
The analysis of bottom-up effects of DOC and top-down effects of fish showed that in the zooplankton system DOC addition had a positive effect on bacteria, phytoplankton, HNF, and zooplankton, while ciliates were unaffected (Fig. 5). In the fish system, DOC had a negative effect on phytoplankton and fish, while the other organism groups were unaffected. The presence of fish in the DOC-enriched systems caused a clear cascade effect where the zooplankton biomass was reduced, phytoplankton and ciliate biomass increased, and HNF and bacteria biomass was reduced (Fig. 5). The presence of fish in the NP-enriched systems caused a somewhat similar cascade effect, where zooplankton biomass was reduced and phytoplankton and ciliates increased. However, in this case HNF biomass was unaffected while bacterial biomass increased (Fig. 5).
Effect of DOC and fish on the biomass of heterotrophic bacteria (Bact), heterotrophic nanoflagellates (Hnf), ciliates (Cil), phytoplankton (phyto), zooplankton (Zp), and fish. Blue denotes unaffected biomass, green increased biomass, and red decreased biomass. Arrows indicate energy flow in the food web. The causative factor is shown in irregular boxes
DOC enrichment triggered higher zooplankton production in the systems where they constituted the highest trophic level (Fig. 6B; Table 4). Conversely, the data from both experimental periods indicated that DOC caused decreased fish production (Fig. 6B). However, the negative effect on fish production was only significant for the second experimental period (Table 4). The stable isotope data in fish showed lower deuterium values in the NP-F treatment (−168 ± 5.4‰) than in CNP-F (−149 ± 12.5‰) (one-way ANOVA, P < 0.001).
A Food web efficiency in mesocosms where zooplankton (NP and CNP) and fish (NP-F and CNP-F) constituted the highest trophic level, and B average zooplankton (NP and CNP) and fish (NP-F and CNP-F) production in the mesocosm experiment. NP and CNP comprise period 1 and NP-F and CNP-F period 1 and 2, respectively. Values are means of three replicates. Error bars denote 1 SD
DOC additions caused decreased food web efficiency (FWE) in both the zooplankton and the fish systems (Fig. 6A; Table 2). FWE was higher in food webs with zooplankton as top consumer than in the fish systems (Fig. 6A; Table 2). In the zooplankton systems, carbon enrichment caused a decrease in FWE from 17 to ~6%, and in the fish systems carbon enrichment caused a decrease in FWE from 4.4 to 0.4% during the first experimental period and from 5.6 to 0.5% in the second period (Fig. 6A). The FWE decreased ~4 times more from the zooplankton to the fish systems in the carbon-enriched systems than in the NP-enriched systems: The ratio FWENP-F:FWENP was 0.26, while the ratio FWECNP-F:FWECNP: was low as 0.07.
Discussion
The results of this study show that alternative pathways in food webs can be induced both from the bottom and the top of the food chain as they caused complex and unexpected alterations of the productivity and ecological function of the system. We found that depending on which top consumer was present in the system, the response in the production of the top consumer to DOC addition differed substantially. In food webs with zooplankton as top consumer, zooplankton production was higher in the systems receiving DOC, whereas in food webs with fish as top consumer, DOC additions caused reduced growth and production of fish. Hence, the mesozooplankton production did not follow our expectations of higher production in the NP-enriched system. These results highlight that although DOC additions yielded an overall reduction of FWE, this was mainly not a consequence of the changes in the production of the respective top consumer but rather changes in the pathways of energy transfer and interactions at lower trophic levels. Therefore the interaction of top–down and bottom–up drivers can generate similar changes of FWE despite major differences in the absolute production at the top and the bottom of the food web. Our study thus supports the view that ecosystem changes cannot be predicted solely from classical trophic cascades and food web theory (e.g., Wollrab et al., 2012).
In many aquatic ecosystems, the heterotrophic microbial food web is a significant component of the food web and heterotrophic protists, like HNF and ciliates, which constitute an important link in transferring bacteria-produced energy from the microbial food web to zooplankton (Stoecker & Capuzzo, 1990, Jansson et al., 2007). In accordance, we observed that the DOC-induced high bacterial production increased the biomass of HNF in the zooplankton system and increased the biomass of ciliates in the fish system. In the zooplankton system DOC did not cause reduced primary production and phytoplankton biomass, and hence zooplankton gained energy via both the phytoplankton and bacterial pathways. Consequently, the zooplankton production was promoted by channeling energy both via the classical autotrophic and the microbial food web. These results are in agreement with the previous studies reporting that labile transparent DOC promotes zooplankton by enhancing energy transfer via the microbial food web and leaving the phytoplankton pathway unaffected (Faithfull et al., 2012).
The presence of fish led to increased total basal production. In the NP-enriched fish system, both the primary and bacterial production were higher than in the NP-enriched zooplankton system. DOC enrichment caused as strong increase of the bacterial production in the fish system. Thus, fish had different effects on the organisms at the basal level depending on whether DOC was available or not. In agreement with earlier studies we observed a cascade effect in the presence of fish, where zooplankton were reduced, while phytoplankton and ciliates were released from grazing and increased in biomass (e.g., Carpenter et al., 1985; Ordóñez et al., 2010; Karus et al., 2014). Increased ciliate density caused higher grazing on HNF, which decreased in biomass. As a consequence the predation on bacteria was relaxed, which could increase their biomass and production. Our study thus shows that fish can cause cascade effects all the way down to bacteria, as suggested by earlier studies (Christoffersen et al., 1993; Nishimura et al., 2011).
Although the basal production was more than twofold higher in the carbon-enriched than in the NP-enriched fish system, the fish production was lower. When zooplankton constituted highest trophic level, DOC additions acted as a subsidy for top consumer production, while in the presence of fish the trophic cascades were induced and increased the proportion of energy that was allocated to the microbial food web. The increased dependence of higher trophic levels on heterotrophic production translated into lower food web efficiency, due to the longer pathway of energy transfer. This conclusion is supported by a higher fish production in NP-F, having a low ratio of BP: PP and a substantially lower total basal production than the DOC-enriched system. The larger importance of phytoplankton production in the NP-enriched fish system indicates that zooplankton obtained their energy predominantly from the autotrophic pathway, suggesting that carbon transfer comprised a simpler, three-level, food chain from phytoplankton to zooplankton to fish. In contrast, the comparatively much higher basal production in the CNP-F treatment was mainly due to bacterial production, accordingly the energy had to pass through at least 2 additional links before reaching the top of the food web (Berglund et al., 2007). Our estimates of δ2H provide support that fish in the NP-F obtained substantially more energy produced by phytoplankton than in the corresponding DOC amended system. The δ2H value in fish (−149‰) in the DOC-enriched system is in close agreement with data from a number of allochthonous C4 plants (Doucett et al., 2007; Karlsson et al., 2012). The corresponding value, −170‰ in fish, in NP-F suggests a higher reliance of autochthonous carbon, although an autochthonous signal of −290 to −214‰ is commonly reported (Doucett et al., 2007; Karlsson et al., 2012). Thus, the relatively high fish production and FWE in NP-F were probably caused by energy channeling from phytoplankton primary production.
The transfer efficiency from zooplankton to fish was fourfold lower in the carbon-enriched systems than in the NP-enriched systems, indicating that zooplankton were of lower quality in the carbon-enriched systems. In the NP-enriched systems, the transfer efficiency was ~26%, while in the carbon-enriched systems it was only ~7%. The primary production was similar in the two food webs, while the bacterial production was much higher in the carbon-enriched systems. The lower transfer efficiency in the carbon-enriched system can be explained by a higher dependence on heterotrophic bacterial production. Bacteria are known to be of poor food quality compared to phytoplankton (e.g., Ahlgren et al., 1990; Brett & Müller-Navarra, 1997; Dahlgren et al., 2011), resulting in the observed lower transfer from zooplankton to fish in the carbon-enriched system. Our results thus suggest that in DOC-rich systems, cascading effects from planktivorous fish will increase the BP: PP ratios, causing feedbacks in the food web which ultimately negatively influence their own production.
Climate change has been projected to lead to increased precipitation in e.g., northern Europe, which would lead to increased inflow of terrestrial organic matter to lakes and coastal areas (e.g., Andersson et al., 2015 and references therein). This would in turn lead to increased importance of bacteria at the base of aquatic food webs (Karlsson et al., 2009, Kirchman et al., 2009; Wikner & Andersson, 2012), and decreased production at higher trophic levels due to high respiration losses etc. (e.g., Ducklow et al., 1986). In the northern Baltic Sea the riverine freshwater inflow, and thus the DOC inflow, is expected to increase ~30% within the next coming century (Andersson et al., 2015 and references therein). Since the water residence time is long (4–5 years) in this sea area, the DOC concentrations will probably rise concurrently. Our DOC additions compare relatively well to projected climate-induced changes in the northern Baltic Sea. We added 0.13 and 0.51 mg C l−1 day to CNP and CNP-F during period 1 and 2, respectively, which would equal ~60 and 260% of the labile pool of DOC in the coastal system. The DOC concentration was 4 mg C l−1 and assuming ~5% bioavailability (Herlemann et al., 2014; Figueroa et al., 2016), the labile DOC pool would be equivalent to ~0.2 mg C l−1. The additions were thus within the range of expected climate-induced changes. It seems likely that climate change would cause a growth stimulation of bacteria, similar to what we observed in the present experiment. The driving force towards a bacteria-based food web would be even more pronounced considering the browning effect of natural DOC. This will lead to a degraded light climate, which in turn may have additional negative effects on primary production (Diehl et al., 2005; Jansson et al., 2007).
Fishing, habitat loss, and bioaccumulation of toxicants typically affect top consumers whereas eutrophication, land use, and climate change most directly impact the basal producers (e.g., Möllmann et al., 2009). To understand how their effects propagate in food webs and influence whole ecosystem function and productivity is becoming more and more important as anthropogenic forcing on ecosystems accelerates at an unprecedented rate (Harley et al., 2006; Heath et al., 2014; Hessen & Kaartvedt, 2014). Wollrab et al. (2012) argued that responses of consumers in many food webs to changes at the bottom or at the top of the food web can analytically be predicted by reducing the food web to two main energy pathways originating from two distinct types of basal producers. Still, our conceptual experimental study in a relatively simple food web provided unexpected results to simultaneous manipulations at the top and the bottom of the food web. Hence our results provide empirically derived data which are critically needed to understand the complexity in natural systems, but more importantly highlights that even in relatively simple experimental systems the patterns and pathways of energy transfer are complex and rarely follow traditional predictions.
References
Ahlgren, G., L. Lundstedt, M. Brett & C. Forsberg, 1990. Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters. Journal of Plankton Research 12: 809–818.
Andersson, A., H. E. M. Meier, M. Ripszam, O. Rowe, J. Wikner, P. Haglund, K. Eilola, C. Legrand, D. Figueroa, J. Paczkowska, E. Lindehoff, M. Tysklind & R. Elmgren, 2015. Projected future climate change and Baltic Sea ecosystem management. Ambio 44: 345–356.
Autio, R., 1998. Response of seasonally cold-water bacterioplankton to temperature and substrate treatments. Estuarine, Coastal and Shelf Science 46: 465–474.
Autio, R., 2000. Studies on bacterioplankton in the Baltic Sea with special emphasis on the regulation of growth. Ph. D. thesis. University of Helsinki. Walter and Andrée de Nottbeck Foundation Scientific Reports No. 19.
Azam, F., T. Fenchel, J. G. Field, J. S. Gray, L. A. Meyer-Reil & F. Thingstad, 1983. The ecological role of water-column microbes in the sea. Marine Ecology Progress Series 10: 257–263.
Berglund, J., U. Müren, U. Båmstedt & A. Andersson, 2007. Efficiency of a phytoplankton-based and a bacteria-based food web in a pelagic marine system. Limnology and Oceanography 52: 121–131.
Blackburn, N., Å. Hagström, J. Wikner, R. C. Hansson & P. K. Bjørnsen, 1998. Rapid determination of bacterial abundance, biovolume, morphology and growth by neural network-based image analysis. Applied and Environmental Microbiology 64: 3246–3255.
Brett, M. T., M. J. Kainz, S. J. Taipale & H. Seshan, 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences of the United States of America 106: 21197–21201.
Brett, M. T. & D. C. Müller-Navarra, 1997. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biology 38: 483–499.
Calbet, A. & E. Saiz, 2005. The ciliate-copepod link in marine ecosystems. Aquatic Microbial Ecology 38: 157–167.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–639.
Christoffersen, K., B. Riemann, A. Klysner & M. Søndergaard, 1993. Potential role of fish predation and natural populations of zooplankton in structuring a plankton community in eutrophic lake water. Limnology and Oceanography 38: 561–573.
Cole, J. J., S. Findlay & M. L. Pace, 1988. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Marine Ecology Progress Series 43: 1–10.
Dahlgren, K., A.-K. Eriksson Wiklund & A. Andersson, 2011. The influence of autotrophy, heterotrophy and temperature on pelagic food web efficiency in a brackish water system. Aquatic Ecology 45: 307–323.
Dickman, E. M., J. M. Newell, M. J. Gonzáles & M. J. Vanni, 2008. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proceedings of the National Academy of Science of the United States of America 105: 18408–18412.
Diehl, S., S. Berger & R. Wöhrl, 2005. Flexible nutrient stoichiometry mediates environmental influences on phytoplankton and its abiotic resources. Ecology 86: 2931–2945.
Doucett, R. R., J. C. Marks, D. W. Blinn, M. Caron & B. A. Hungate, 2007. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 88: 1587–1592.
Ducklow, H. W., D. A. Purdie, P. J. LeB Williams & J. M. Davies, 1986. Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science 232: 865–867.
Dumont, H. J., I. Van de Velde & S. Dumont, 1975. The dry weight estimate of biomass in a selection of cladocera, copepoda and rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19: 75–97.
Fagerbakke, K. M., M. Heldal & S. Norland, 1996. Content of carbon, nitrogen oxygen, sulfur and phosphours in native aquatic and cultured bacteria. Aquatic Microbial Ecology 10: 15–27.
Faithfull, C. L., M. Huss, T. Vrede & A.-K. Bergström, 2011. Bottom-up carbon subsidies and top-down predation pressure interact to affect aquatic food web structure. Oikos 120: 311–320.
Faithfull, C., M. Huss, T. Vrede, J. Karlsson & A.-K. Bergström, 2012. Transfer of bacterial production based on labile carbon to higher trophic levels in an oligotrophic pelagic system. Canadian Journal of Fisheries and Aquatic Sciences 69: 85–93.
Figueroa, D., O. F. Rowe, J. Paczkowska, C. Legrand & A. Andersson, 2016. Allochthonous Carbon—a Major Driver of Bacterioplankton Production in the Subarctic Northern Baltic Sea. Microbial Ecology 71: 789–801.
Fuhrman, J.-A. & F. Azam, 1982. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Marine Biology 66: 109–120.
Gargas, E., 1975. A manual for phytoplankton production studies in the Baltic. Baltic Marine Biologists, Vol. 2. Water Quality Institute, Hørsholm: 1–88.
Hairston, N. G. & N. G. Hairston, 1993. Cause-Effect relationships in energy flow, trophic structure and interspecific interactions. American Naturalist 142: 379–411.
Harley, C. D. G., A. R. Hughes, K. M. Hultgren, B. G. Miner, C. J. B. Sorte, C. S. Thornber, L. F. Rodriguez, L. Tomanek & S. L. Williams, 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9: 228–241.
Heath, M. R., D. C. Speirs & J. H. Steele, 2014. Understanding patterns and processes in models of trophic cascades. Ecology Letters 17: 101–114.
HELCOM, 2007. Climate change in the Baltic Sea Area- HELCOM thematic assessment in 2007. Baltic Sea Environment Proceedings No. 111: 1–54.
Herlemann, D. P. R., M. Manecki, C. Meskee, F. Pollehne, M. Labrenz, D. Schulz-Bull, T. Dittmar & K. Juergens, 2014. Uncoupling of bacterial and terrigenous dissolved organic matter dynamics in decomposition experiments. Plos One 9(4): e93945.
Hernroth, L., 1985. Recommendations on methods for the marine biological studies in the Baltic Sea. Mesozooplankton biomass assessment. The Baltic Marine Biologists. Publication No. 10. ISSN:0282-8839.
Hessen, D. O. & T. Andersen, 1990. Bacteria as a source of phosphorus for zooplankton. Hydrobiologia 206: 217–223.
Hessen, D. O. & S. Kaartvedt, 2014. Top-down cascades in lakes and oceans: different perspectives but same story? Journal of Plankton Research 36(4): 914–924.
Hulot, F. D., G. Lacroix & M. Loreau, 2014. Differential responses of size-based functional groups to bottom-up and top-down perturbations in pelagic food webs: a meta-analysis. Oikos 123: 1291–1300.
IPCC, 2007. Summary for policymakers. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel in Climate Change (eds S. Solomon, D. Qin, M. Manning et al.), Cambridge University Press, Cambridge: 1–18.
Jacob, U., K. Mintenbeck, T. Brey, R. Knust & K. Beyer, 2005. Stable isotope food web studies: a case for standardized sample treatment. Marine Ecology Progress Series 287: 251–253.
Jansson, M., L. Persson, A. M. De Roos, R. I. Jones & L. J. Tranvik, 2007. Terrestrial carbon and intraspecific size-variation shape lake ecosystems. Trends in Ecology and Evolution 22: 316–322.
Jobling, M., 1994. Fish Bioenergetics. Chapman and Hall, London.
Johansson, M., E. Gorokhova & U. Larsson, 2004. Annual variability in ciliate community structure, potential prey and predators in the open northern Baltic Sea proper. Journal of Plankton Research 26: 67–80.
Karlsson, J., M. Jansson & A. Jonsson, 2002. Similar relationships between pelagic primary and bacterial production in clearwater and humic lakes. Ecology 83: 2902–2910.
Karlsson, J., P. Byström, J. Ask, P. Ask, L. Persson & M. Jansson, 2009. Light limitation of nutrient-poor lake ecosystems. Nature 460: 506–509.
Karlsson, J., M. Berggren, J. Ask, P. Byström, A. Jonsson, H. Laudon & M. Jansson, 2012. Terrestrial organic matter support of lake food webs: evidence from lake metabolism and stable hydrogen isotopes of consumers. Limnology and Oceanography 57: 1042–1048.
Karus, K., T. Paaver, H. Agasild & P. Singel, 2014. The effects of predation by planktivorous juvenile fish on the microbial food web. European Journal of Protistology 50: 109–121.
Kirchman, D. L., X. A. G. Morán & H. Ducklow, 2009. Microbial growth in the polar oceans—role of temperature and potential impact of climate change. Nature Reviews Microbiology 7: 451–459.
Lefébure, R., R. Degerman, A. Andersson, S. Larsson, L.-O. Eriksson, U. Båmstedt & P. Byström, 2013. Impacts of elevated terrestrial nutrient loads and temperature on pelagic food-web efficiency and fish production. Global Change Biology 19: 1358–1372.
Legendre, L. & F. Rassoulzadegan, 1995. Plankton and nutrient dynamics in marine waters. Ophelia 41: 153–172.
Malzahn, A. M., N. Aberle, C. Clemmesen & M. Boersma, 2007. Nutrient limitation of primary producers affects planktivorous fish condition. Limnology and Oceanography 52: 2062–2071.
Menden-Deuer, S. & E. J. Lessard, 2000. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography 45: 569–579.
Möllmann, C., R. Diekmann, B. Müller-Karulis, G. Kornilovs, M. Plikshs & P. Axe, 2009. Reorganization of a large marine ecosystem due to atmospheric and anthropogenic pressure: a discontinuous regime shift in the central Baltic Sea. Global Change Biology 15: 1377–1393.
Nishimura, Y., T. Ohtsuka, K. Yoshiyama, D. Nakai, F. Shibahara & M. Maehata, 2011. Cascading effects of larval Crucian carp introduction on phytoplankton and microbial communities in a paddy field: top-down and bottom-up controls. Ecological Research 26: 615–626.
Ordóñez, J., J. Armengol, E. Moreno-Ostos, L. Caputo, J. C. García & R. Marcé, 2010. On non-Eltonian methods of hunting Cladocera, or impacts of the introduction of planktivorous fish on zooplankton composition and Clearwater phase occurrence in a Mediterranean reservoir. Hydrobiologia 653: 119–129.
O’Connor, M. I., M. F. Piehler, D. M. Leech, A. Anton & J. F. Bruno, 2009. Warming and resource availability shift food web structure and metabolism. PLoS Biology 7(8): e1000178.
Pace, M. L., J. J. Cole, S. R. Carpenter, J. F. Kitchell, J. R. Hodgson, M. C. Van de Bogert, D. L. Bade, E. S. Kritzberg & D. Bastviken, 2004. Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature 427: 240–243.
Rand, P. S. & D. J. Stewart, 1998. Prey fish exploitation, salmonine production, and pelagic food web efficiency in Lake Ontario. Canadian Journal of Fisheries and Aquatic Sciences 55: 318–327.
Riemann, B., P. K. Bjørnsen, S. Newell & R. Fallon, 1987. Calculation of cell production of coastal marine bacteria based on measured incorporation of [3H]thymidine. Limnology and Oceanography 32: 471–476.
Sherr, E. B., D. A. Caron & B. F. Sherr, 1992. Staining of heterotrophic protists for visualization via epifluorescence microscopy. In Kemp, P. F., B. F. Sherr, E. B. Sherr & J. J. Cole (eds), Current Methods in Aquatic Microbial Ecology. Lewis Publishers, Boca Raton, FL: 213–227.
Shiomoto, A., K. Tadokoro, K. Nagasawa & Y. Ishida, 1997. Trophic relations in the subarctic North Pacific eco-system: possible feeding links from pink salmon. Marine Ecology Progress Series 150: 75–85.
Sommer, U. & F. Sommer, 2006. Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton. Oecologia 147: 183–194.
Sommer, U., H. Stibor, A. Katechakis, F. Sommer & T. Hansen, 2002. Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production: primary production. Hydrobiologia 484: 11–20.
Sterner, R. W., J. Clasen, W. Lampert & T. Weisse, 1998. Carbon:phosphorus stoichiometry and food chain production. Ecology Letters 1: 146–150.
Stibor, H., O. Vadstein, S. Diehl, A. Gelzleichter, T. Hansen, F. Hantzsche, A. Katechakis, B. Lippert, K. Loseth, C. Peters, W. Roederer, M. Sandow, L. Sundt-Hansen & Y. Olsen, 2004. Copepods act as a switch between alternative trophic cascades in marine pelagic food webs. Ecology Letters 7: 321–328.
Stoecker, D. K. & J. M. Capuzzo, 1990. Predation on Protozoa: its importance to zooplankton. Journal of Plankton Research 12: 891–908.
Straile, D., 1997. Gross growth efficiencies of protozoan and metazoan zooplankton and their dependence on food concentration, predator-prey weight ratio, and taxonomic group. Limnology and Oceanography 42: 1375–1385.
Vadeboncoeur, Y., K. S. McCann, M. J. V. Zanden & J. B. Rasmussen, 2004. Effects of multi-chain omnivory on the strength of trophic control in lakes. Ecosystems 8: 682–693.
Vanni, M. J. & C. D. Layne, 1997. Nutrient recycling and herbivory as mechanisms in the “top-down” effects of fish on phytoplankton in lakes. Ecology 78: 21–41.
Vijverberg, J., 1980. Effect of temperature in laboratory studies on development and growth of Cladocera and Copepoda from Tjeukemeer, The Netherlands. Freshwater Biology 10: 317–340.
Welch, H. E., 1968. Relationship between assimilation efficiencies and growth efficiencies for aquatic consumers. Ecology 49: 755–759.
Wikner, J. & A. Andersson, 2012. Increased freshwater discharge shifts the trophic balance in the coastal zone of the northern Baltic Sea. Global Change Biology 18: 2509–2519.
Wikner, J. & Å. Hagström, 1999. Bacterioplankton intra-annual variability: importance of hydrography and competition. Aquatic Microbial Ecology 20: 245–260.
Wiklund, A. K. E., K. Dahlgren, B. Sundelin & A. Andersson, 2009. Effects of warming and shifts of pelagic food web structure on benthic productivity in a coastal marine system. Marine Ecology Progress Series 396: 13–25.
Wollrab, S., S. Diehl & A. M. De Roos, 2012. Simple rules describe bottom-up and top-down control in food webs with alternative energy pathways. Ecology Letters 15: 935–946.
Zöllner, E., H.-G. Hoppe, U. Sommer & K. Jürgens, 2009. Effect of zooplankton-mediated trophic cascades on marine microbial food webs. Limnology and Oceanography 54: 262–275.
Acknowledgements
We thank Umeå Marine Sciences Centre for chemical analyses. Dr. Iveta Jurgensone is gratefully acknowledged for phytoplankton analyses. This project was supported by grants from the Swedish Research Council FORMAS to A. Andersson (217-2006-674) and by the marine strategic research environment ECOCHANGE.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Jonne Kotta
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Degerman, R., Lefébure, R., Byström, P. et al. Food web interactions determine energy transfer efficiency and top consumer responses to inputs of dissolved organic carbon. Hydrobiologia 805, 131–146 (2018). https://doi.org/10.1007/s10750-017-3298-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3298-9