Abstract
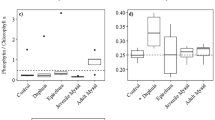
We investigated the seasonal influence of an effective size-selective planktivore, the anadromous alewife (Alosa pseudoharengus Wilson), on summer zooplankton and phytoplankton communities in a series of lakes in Maine, USA (4 with and 4 without alewife) that ranged from oligotrophic to eutrophic to determine the role of lake trophic state in influencing the relative strength of top–down forces on phytoplankton biomass. Predation by young of the year alewife reduced the mean body length of cladoceran and copepod biomass from spring to summer in alewife lakes, while mean body length remained unchanged in nonalewife lakes. Cladoceran biomass decreased substantially from spring to summer in 3 of 4 alewife lakes, increasing only in the most eutrophic lake. Conversely, cladoceran biomass increased from spring to summer in 3 of 4 nonalewife lakes. Predation by alewife on zooplankton did not have consistent cascading effects on phytoplankton biomass. Analysis of planktonic biomass ratios (phytoplankton biomass: zooplankton biomass) suggests that cascading effects were stronger in oligotrophic systems and weakened with increasing trophic status, as ratios in alewife and nonalewife lakes converged at higher total phosphorous levels. Our results suggest that lake trophic status may influence the relative importance of top–down control of both zooplankton and phytoplankton biomass. The mechanisms that explain this pattern remain elusive and likely require additional efforts to estimate alewife densities, rates of zooplankton and phytoplankton production relative to consumption, and the influence of other physical lake features.





Similar content being viewed by others
References
American Public Health Association (APHA), 1995. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC.
Brett, M. T. & C. R. Goldman, 1997. Consumer versus resource control in freshwater pelagic food webs. Science 275: 384–386.
Brocksen, R. W., G. E. Davis & C. E. Warren, 1970. Analysis of trophic processes on the basis of density-dependent functions. In Steele, J. H. (ed.), Marine Food Chains. University of California Press, Los Angeles.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Carpenter, S. R., J. F. Kitchell, K. L. Cottingham, D. E. Schindler, D. L. Christensen, D. M. Post & N. Voichick, 1996. Chlorophyll variability, nutrient input, and grazing: evidence from whole-lake experiments. Ecology 77: 725–735.
Dumont, H. J., I. Van de Velde & S. Dumont, 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepod and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19: 75–97.
Durbin, A. G., S. W. Nixon & C. A. Oviatt, 1979. Effects of the spawning migration of the Alewife, Alosa Pseudoharengus, on freshwater ecosystems. Ecology 60: 8–17.
Garman, G. C. & S. A. Macko, 1998. Contribution of marine-derived organic matter to an Atlantic coast, freshwater, tidal stream by anadromous clupeid fishes. Journal of the North American Benthological Society 17: 277–285.
Hall, C. J., A. Jordaan & M. G. Frisk, 2012. Centuries of anadromous forage fish loss: consequences for ecosystem connectivity and productivity. BioScience 62: 723–731.
Hanson, J. M. & W. C. Leggett, 1982. Empirical prediction of fish biomass and yield. Canadian Journal of Fisheries and Aquatic Science 39: 257–263.
Hanson, J. M. & R. H. Peters, 1984. Empirical prediction of crustacean zooplankton biomass and profundal macrobenthos biomass in lakes. Canadian Journal of Fisheries and Aquatic Science 41: 439–445.
Harman, W. N., M. F. Albright & D. M. Warner, 2002. Trophic changes in Otsego Lake, NY following the introduction of the alewife (Alosa pseudoharengus). Lake and Reservoir Management 18: 215–226.
Havens, K. E., A. C. Elia, M. I. Taticchi & R. S. Fulton III, 2009. Zooplankton-phytoplankton relationships in shallow subtropical versus temperate lakes Apopka (Florida, USA) and Trasimeno (Umbria, Italy). Hydrobiologia 628: 165–175.
Havens, K. E. & J. R. Beaver, 2013. Zooplankton to phytoplankton biomass ratios in shallow Florida lakes: an evaluation of seasonality and hypotheses about factors controlling variability. Hydrobiologia 703: 177–187.
Hessen, D. O., T. Andersen & B. A. Faafeng, 1995. Replacement of herbivore zooplankton species along gradients of ecosystem productivity and fish predation pressure. Canadian Journal of Fisheries and Aquatic Science 52: 733–742.
Hessen, D. O., B. A. Faafeng & P. Brettum, 2003. Autotroph:herbivore biomass ratios; carbon deficits judged from plankton data. Hydrobiologia 491: 167–175.
Hessen, D. O., B. A. Faafeng, P. Brettum & T. Andersen, 2006. Nutrient enrichment and planktonic biomass ration in lakes. Ecosystems 9: 516–527.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top–down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 151–164.
Jeppesen, E., J. P. Jensen, M. Søndergaard & T. Lauridsen, 1999. Trophic dynamics in turbid and Clearwater lakes with special emphasis on the role of zooplankton for water clarity. Hydrobiologia 409: 217–231.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen & F. Landkildehus, 2000. Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshwater Biology 45: 201–218.
Jeppesen, E., J. P. Jensen, C. Jensen, B. Faafeng, D. Hessen, M. Søndergaard, T. Lauridsen, P. Brettum & K. Christoffersen, 2003. The impact of nutrient state and lake depth on top–down control in the pelagic zone of lakes: a study of 466 lakes from the temperate zone to the Arctic. Ecosystems 6: 313–325.
Kamarainen, A. M., E. F. Rowland, R. Biggs & S. R. Carpenter, 2008. Zooplankton and the total phosphorus-chlorophyll a relationship: hierarchical Bayesian analysis of measurement error. Canadian Journal of Fisheries and Aquatic Sciences 65: 2644–2655.
Leibold, M. A., 1989. Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. The American Naturalist 134: 922–949.
Limburg, K. E. & J. R. Waldman, 2009. Dramatic declines in North Atlantic diadromous fishes. BioScience 59: 955–965.
Maine Department of Environmental Protection, 2009. http://www.maine.gov/dep/water/monitoring/lake/lakedata.htm.
Maine Department of Inland Fisheries and Wildlife, 2007. http://www.gulfofmaine.org/kb/2.0/record.html?recordid=9008.
McCauley, E. & J. Kalff, 1981. Empirical relationships between phytoplankton and zooplankton biomass in lakes. Canadian Journal of Fisheries and Aquatic Sciences 38: 458–463.
Mehner, T., J. Padisak, P. Kasprzak, R. Koschel & L. Krienitz, 2008. A test of food web hypotheses by exploring time series of fish, zooplankton and phytoplankton in an oligo-mesotrophic lake. Limnologica 38: 179–188.
Mihuc, T. B., F. Dunlap, C. Binggeli, L. Myers, C. Pershyn, A. Groves & A. Waring, 2012. Long-term patterns in Lake Champlain’s zooplankton: 1992–2010. Journal of Great Lakes Research 38: 49–57.
Naiman, R. J., R. E. Bilby, D. E. Schindler & J. M. Helfield, 2002. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems 5: 399–417.
Nürnberg, G. K., 1996. Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Journal of Lake and Reservoir Management 12: 432–447.
Oksanen, L., S. D. Fretwell, J. Arruda & P. Niemela, 1981. Exploitation ecosystems in gradients of primary productivity. The American Naturalist 118: 240–261.
Pace, M. L., 1986. An empirical analysis of zooplankton community size structure across lake trophic gradients. Limnology and Oceanography 31: 45–55.
Palkovacs, E. P. & D. M. Post, 2009. Experimental evidence that phenotypic divergence in predators drives community divergence n prey. Ecology 90: 300–305.
Persson, L., S. Diehl, L. Johansson, G. Andersson & S. F. Hamrin, 1992. Trophic interactions in temperate lake ecosystems: a test of food chain theory. The American Naturalist 140: 59–84.
Peters, R. H. & J. A. Downing, 1984. Empirical analysis of zooplankton filtering and feeding rates. Limnology and Oceanography 29: 763–784.
Post, D. M., E. P. Palkovacs, E. G. Schielke & S. I. Dodson, 2008. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology 89: 2019–2032.
Quiros, R., 1990. Predictors of relative fish biomass in lakes and reservoirs of Argentina. Canadian Journal of Fisheries and Aquatic Science 47: 928–939.
Quiros, R., 1991. Empirical relationships between nutrients, phyto- and zooplankton and relative biomass in lakes and reservoirs of Argentina. Verhandlungen des Internationalen Verein Limnologie 24: 1198–1206.
R Core Team, 2014. R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. http://www.R-project.org/.
Reed, R. J., 1971. Biology of the fallfish, Semotilus corporalis (Pisces, Cyprinidae). Transactions of the American Fisheries Society 100: 717–725.
Sarnelle, O., 1992. Nutrient enrichment and grazer effects on phytoplankton in lakes. Ecology 73: 551–560.
Schindler, D. E., M. D. Scheuerell, J. W. Moore, S. M. Gende, T. B. Francis & W. J. Palen, 2003. Pacific salmon and the ecology of coastal ecosystems. Frontiers in Ecology and the Environment 1: 31–37.
Schmidt, R. E. & R. Morse, 2011. An historical record of Alewife (Alosa pseudoharengus) in Lake Champlain. Northeastern Naturalist 18: 229–235.
Schoenberg, S. A. & R. E. Carlson, 1984. Direct and indirect effects of zooplankton grazing on phytoplankton in a hypereutrophic lake. Oikos 42: 291–302.
Twining, C. W., D. C. West & D. M. Post, 2013. Historical changes in nutrient inputs from humans and anadromous fishes in New England’s coastal watersheds. Limnology and Oceanography 58: 1286–1300.
Walters, A. W., R. T. Barnes & D. M. Post, 2009. Anadromous alewives (Alosa pseudoharengus) contribute marine-derived nutrients to coastal stream food webs. Canadian Journal of Fisheries and Aquatic Science 66: 439–448.
West, D. C., A. W. Walters, S. Gephard & D. M. Post, 2010. Nutrient loading by anadromous alewife (Alosa pseudoharengus): contemporary patterns and predictions for restoration efforts. Canadian Journal of Fisheries and Aquatic Sciences 67: 1211–1220.
Acknowledgments
This research was supported by funds from the Maine Agricultural and Forest Experiment Station (MAFES), the Department of the Interior US Geological Survey, and the Senator George J. Mitchell Center for Environmental and Watershed Research at the University of Maine, under Grant No. 2007ME152B. This is contribution 3270 to the MAFES. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the US Government. We thank Kristin Ditzler and Lauren Kolb for their hard work and patience in field and laboratory. We also appreciate the constructive comments from 2 anonymous reviewers and Karl E. Havens, which improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Karl E. Havens
Rights and permissions
About this article
Cite this article
Demi, L.M., Simon, K.S., Anderson, D. et al. Trophic status may influence top–down effects of anadromous alewife Alosa pseudoharengus (Actinopterygii, Clupeidae) in lakes. Hydrobiologia 758, 47–59 (2015). https://doi.org/10.1007/s10750-015-2264-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2264-7