Abstract
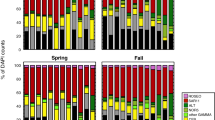
Although temperature is a key parameter in the control of the activity and growth of all microorganisms, little is known about how temperature might simultaneously affect viral lysis and nanoflagellate grazing on prokaryotes. This study investigated the effect of rising temperatures on nanoflagellate grazing, viral lysis, and prokaryotic growth in coastal waters of the western subtropical Pacific Ocean during winter (<25°C) and summer (>25°C) in 2013. To do this, water samples were incubated for 24 h at ambient and experimentally increased temperatures (averages 2–3°C higher than in situ values) and analyzed for prokaryotic gross growth, grazing, and viral lysis using a modified dilution method. The experimental warming conditions were found to cause an increase in prokaryotic gross growth rates in the winter and summer season, respectively. Although the experimental warming conditions also caused an increase in the average rate at which prokaryotes were being grazed, they also caused a general decrease in viral lysis during summer. These findings suggest that short-term warming influenced the function of the microbial food web by increasing the rates of prokaryotic production and flux in prokaryotic carbon to predators.








Similar content being viewed by others
References
Almeida, M. A., M. A. Cunha & F. Alcântara, 2001. Factors influencing bacterial production in a shallow estuarine system. Microbial Ecology 42: 416–426.
Adams, H. E., B. C. Crump & G. W. Kling, 2010. Temperature controls on aquatic bacterial production and community dynamics in arctic lakes and streams. Environmental Microbiology 12: 1319–1333.
Ameryk, A., B. Podgórska & Z. Witek, 2005. The dependence between bacterial production and environmental conditions in the Gulf of Gdaúsk. Oceanologia 47: 27–45.
Apple, J. K., P. A. del Giorgio & M. W. Kemp, 2006. Temperature regulation of bacterial production, respiration, and growth efficiency in a temperate salt-marsh estuary. Aquatic Microbial Ecology 43: 243–254.
Bettarel, Y., T. Sime-Ngando, M. Bouvy, R. Arfi & C. Amblard, 2005. Low consumption of virus-sized particles by heterotrophic nanoflagellates in two lakes the French Massif Central. Aquatic Microbial Ecology 39: 205–209.
Boras, J. A., M. M. Sala, E. Vázquez-Domínguez, M. G. Weinbauer & D. Vaqué, 2009. Annual changes of bacterial mortality due to viruses and protists in an oligotrophic coastal environment (NW Mediterranean). Environmental Microbiology 11: 1181–1193.
Castillo, M. M., J. D. Allan, R. L. Sinsabaugh & G. W. Kling, 2004. Seasonal and interannual variation of bacterial production in lowland rivers of the Orinoco basin. Freshwater Biology 49: 1400–1414.
Choi, J. W. & F. Peters, 1992. Effects on temperature in two psychrophilis ecotypes of a heterotrophic nanoflagellate, Paraphysomonas imperforate. Applied and Environmental Microbiology 58: 593–599.
Cole, J. J., S. Findley & M. L. Pace, 1988. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Marine Ecology Progress Series 43: 1–10.
Ducklow, H. W. & C. A. Carlson, 1992. Oceanic bacterial production. Advances in Microbial Ecology 12: 113–181.
Evans, C., S. D. Archer, S. Jacquet & W. H. Wilson, 2003. Direct estimates of the contribution of viral lysis and microzooplankton grazing to the decline of a Micromonas spp. population. Aquatic Microbial Ecology 30: 207–219.
Fischer, U. R. & B. Velimirov, 2002. High control of bacterial production by viruses in a eutrophic oxbow lake. Aquatic Microbial Ecology 27: 1–12.
Garza, D. R. & C. A. Suttle, 1998. The effect of cyanophages on the mortality of Synechococcus spp. and selection for UV resistant viral communities. Microbial Ecology 36: 281–292.
Gasol, J. M., P. A. Del Giorgio & C. M. Duarte, 1997. Biomass distribution in marine planktonic communities. Limnology and Oceanorgaphy 42: 1353–1363.
Gasol, J. M., J. Pinhassi, L. Alonso-Sáez, H. Ducklow, G. J. Herndl, M. Koblízek, M. Labrenz, Y. Luo, X. A. G. Morán, T. Reinthaler & M. Simon, 2008. Towards a better understanding of microbial carbon flux in the sea. Aquatic Microbial Ecology 53: 21–38.
Gobler, C. J., D. A. Hutchins, N. S. Fisher, E. M. Cosper & S. A. Sañudo-Wilhelmy, 1997. Release and bioavailability of C, N, P, Se and Fe following viral lysis of marine chrysophyte. Limnology and Oceanography 42: 1492–1504.
Goosen, N. K., J. Kromkamp, J. Peene, P. van Rijswijk & P. van Breugel, 1999. Bacterial and phytoplankton production in the maximum turbidity zone of three European estuaries: the Elbe, Westerschelde and Gironde. Journal of Marine System 22: 151–171.
Hoch, K. P. & D. L. Kirchman, 1993. Seasonal and inter-annual variability in bacterial production and biomass in a temperate estuary. Marine Ecology Progress Series 98: 283–295.
Holding, J. M., C. M. Duarte, J. M. Arrieta, R. Vaquer-Suyner, A. Coello-Camba, P. Wassmann & A. Agustí, 2013. Experimentally determined temperature thresholds for Arctic plankton community metabolism. Biogeosciences 10: 357–370.
Hoppe, H. G., K. Gocke, R. Koppe & C. Begler, 2002. Bacterial growth and primary production along a north-south transect of the Atlantic Ocean. Nature 416: 168–171.
Hwang, C. Y. & B. C. Cho, 2002. Virus-infected bacteria in oligotrophic open waters of the East Sea, Korea. Aquatic Microbial Ecology 30: 1–9.
Jacquet, S., I. Domaizon, S. Personnic, A. Sriram, P. Ram, M. Hedal, S. Duhamel & T. Sime-Ngando, 2005. Estimates of protozoan- and viral-mediated mortality of bacterioplankton in Lake Bourget (France). Freshwater Biology 50: 627–645.
Jiang, S. C. & J. H. Paul, 1994. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Marine Ecology Progress Series 104: 163–172.
Keil, R. G. & D. L. Kirchman, 1991. Contribution of dissolved free amino acids and ammonium to the nitrogen requirements of heterotrophic bacterioplankton. Marine Ecology Progress Series 73: 1–10.
Kirchman, D. L., J. H. Rich & R. T. Barber, 1995. Biomass and biomass production of heterotrophic bacteria along 140°W in the equatorial Pacific: effect of temperature on the microbial loop. Deep-Sea Research II 42: 603–619.
Kirchman, D. L. & J. H. Rich, 1997. Regulation of bacterial growth rates by dissolved organic carbon and temperature in the equatorial Pacific Ocean. Microbial Ecology 33: 11–20.
Kimmance, S. A., W. H. Wilson & S. D. Archer, 2007. Modified dilution technique to estimate viral versus grazing mortality of phytoplankton: limitations associated with method sensitivity in natural waters. Aquatic Microbial Ecology 49: 207–222.
Kisand, V. & P. Zingel, 2000. Dominance of ciliate grazing on bacteria during spring in a shallow eutrophic lake. Aquatic Microbial Ecollogy 22: 135–142.
Landry, M. R. & R. P. Hassett, 1982. Estimating the grazing impact of marine microzooplankton. Marine Biology 67: 283–288.
Lara, E., J. M. Arrieta, I. Garcia-Zarandona, J. A. Boras, C. M. Duarte, S. Agustí, P. F. Wassmann & D. Vaqué, 2013. Experimental evaluation of the warming effect on viral, bacterial and protistan communities in two contrasting Arctic systems. Aquatic Microbial Ecology 70: 17–32.
Li, W. K. W., 1998. Annual average abundance of heterotrophic bacteria and Synechococcus in surface ocean waters. Limnology and Oceanography 43: 1746–1753.
Mathias, C. B., K. T. Kirschner & B. Velimirov, 1995. Seasonal variations of virus abundance and virus control of bacterial population in backwater system of Danube River. Applied and Environmental Microbiology 61: 3734–3740.
Miki, T. & S. Jacquet, 2008. Complex interactions in the microbial world: underexplored key links between viruses, bacteria and protozoan grazers in aquatic environments. Aquatic Microbial Ecology 51: 195–208.
McManus, G. B., P. M. Griffin & J. R. Pennock, 2004. Bacterioplankton abundance and growth in a river-dominated estuary: relationships with temperature and resources. Aquatic Microbial Ecology 37: 23–32.
Murrell, M. C., 2003. Bacterioplankton dynamics in a subtropical-estuary: evidence for substrate limitation. Aquatic Microbial Ecology 32: 239–250.
Nedwell, D. B., 1999. Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiology Ecology 30: 101–111.
Nobel, R. T. & J. A. Fuhrman, 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquatic Microbial Ecology 14: 113–118.
Pace, M. L., 1988. Bacterial mortality and the fate of bacterial production. Hydrobiologia 159: 41–49.
Porter, K. G. & Y. S. Feig, 1980. The use of DAPI for identifying and counting aquatic microflora. Limnology and Oceanography 25: 943–948.
Pradeep Ram, A. S. & T. Sime-Ngando, 2008. Functional responses of prokaryotes and viruses to grazer effects and nutrient additions in freshwater microcosms. The ISME Journal 2: 498–509.
Pradeep Ram, A. S., D. Boucher, T. Sime-Ngando, D. Debroas & J. C. Romagoux, 2005. Phage bacteriolysis, protistan bacterivory potential, and bacterial production in a freshwater reservoir: coupling with temperature. Microbial Ecology 50: 64–72.
Rivkin, R. B., M. R. Anderson & C. Lajzerowicz, 1996. Microbial processes in cold oceans. I. Relationship between temperature and bacterial growth rate. Aquatic Microbial Ecology 10: 243–254.
Rose, J. M. & D. A. Caron, 2007. Dose low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnology and Oceanography 52: 886–895.
Rose, J. M., N. M. Vora, P. D. Countway, R. J. Gast & D. A. Caron, 2009. Effects of temperature on growth rate and gross growth efficiency of an Antarctic bacterivorous protest. The ISME Journal 3: 252–260.
Sherr, E. B. & B. F. Sherr, 2002. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81: 293–308.
Shiah, F. K. & H. W. Ducklow, 1994. Temperature and substrate regulation of bacterial abundance, production, and specific growth rate in temperate estuarine ecosystem. Marine Ecology Progress Series 103: 297–308.
Sommer, U. & H. Stibor, 2002. Copepoda-Cladocera-Tunicata: the role of three major mesozooplankton groups in pelagic food webs. Ecology Research 17: 161–174.
Steward, G. F., D. C. Smith & F. Azam, 1996. Abundance and production of bacteria and viruses in the Bering and Chukchi Sea. Marine Ecology Progress Series 131: 287–300.
Suttle, C. A. & F. Chen, 1992. Mechanisms and rates of decay of marine viruses in seawater. Applied and Environmental Microbiology 58: 3721–3729.
Suttle, C. A. & A. M. Chan, 1993. Marine cyanophages infecting oceanic and costal strains of Synechococcus: abundance, morphology, cross-infectivity and growth characteristics. Marine Ecology Progress Series 92: 99–109.
Thouvenot, A., D. Debroas, M. Richardot & J. Devaux, 1999. Impact of natural metazooplankton assemblage on planktonic microbial communities in a newly flooded reservoir. Journal of Plankton Research 21: 179–199.
Tsai, A. Y., K. P. Chiang, J. Chang & G. C. Gong, 2005. Seasonal diel variations of picoplankton and nanoplankton in a subtropical western Pacific coastal ecosystem. Limnology and Oceanography 50: 1221–1231.
Tsai, A. Y., K. P. Chiang, J. Chang & G. C. Gong, 2008. Seasonal variations in trophic dynamics of nanoflagellates and picoplankton in coastal waters of the western subtropical Pacific Ocean. Aquatic Microbial Ecology 51: 263–274.
Tsai, A. Y., G. C. Gong, R. W. Sanders, W. H. Chen, C. F. Chao & K. P. Chiang, 2011. Importance of bacterivory by pigmented and heterotrophic nanoflagellates during the warm season in a subtropical western Pacific coastal ecosystem. Aquatic Microbial Ecology 63: 9–18.
Tsai, A. Y., G. C. Gong & J. Hung, 2013. Seasonal variations of virus- and nanoflagellate-mediated mortality of heterotrophic bacteria in the coastal ecosystem of subtropical western Pacific. Biogeosciences 10: 3055–3065.
Tunin-Ley, A., F. Ibañez, J. P. Labat, A. Zingone & R. Lemée, 2009. Phytoplankton biodiversity and NW Mediterranean Sea warming: changes in the dinoflagellate genus Ceratium in the 20th century. Marine Ecology Progress Series 375: 85–99.
Unrein, F., R. Massana, L. Alonso-Sáez & J. M. Gasol, 2007. Significant year-round effect of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnology and Oceanography 52: 456–469.
Vaqué, D., Ó. Guadayol, F. Peters, J. Felipe, A. Malits & C. Pedrós-Alió, 2009. Differential response of grazing and bacterial heterotrophic production to experimental warming in Antarctic waters. Aquatic Microbial Ecology 54: 101–112.
Vázquez-Domínguez, E., D. Vaqué & J. M. Gasol, 2012. Temperature effects on the heterotrophic bacteria, heterotrophic nanoflagellates and microbial top predators of the NW Mediterranean. Aquatic Microbial Ecology 67: 107–121.
Weinbauer, M. G. & M. G. Höfle, 1998. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Applied and Environmental Microbiology 64: 431–438.
Weinbauer, M. G., K. Hornak, J. Jezbera, J. Nedoma, J. R. Dolan & K. Simek, 2007. Synergistic and antagonistic effects of viral lysis and protistan grazing on bacterial biomass, production and diversity. Environmental Microbiology 9: 777–788.
Wells, L. E. & J. W. Deming, 2006. Significance of bacterivory and viral lysis in bottom waters of Franklin Bay, Canadian Arctic, during winter. Aquatic Microbial Ecology 43: 209–221.
White, P. A., J. Kalff, J. B. Rasmussen & J. M. Gasol, 1991. The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microbiology Ecology 21: 99–118.
Wilken, S., J. Huisman, S. Naus-Wiezer & V. Donk, 2012. Mixotrophic organisms became more heterotrophic with rising temperature. Ecology Letter. doi:10.111/ele.12033.
Work, K., K. Havens, B. Sharstein & E. Therese, 2005. How important is bacterial carbon to planktonic grazers in a turbid, subtropical lake? Journal of Plankton Research 27: 357–372.
Zubkov, M. V. & G. A. Tarran, 2008. High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature 455: 224–226.
Acknowledgments
This study was supported by a grant (NSC 101-2611-M-019-009) from the National Science Council, ROC. We are most grateful to Mr. James Steed for his suggestions regarding the presentation of material and language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Stefano Amalfitano
Rights and permissions
About this article
Cite this article
Tsai, A.Y., Gong, GC. & Shiau, W. Viral lysis and nanoflagellate grazing on prokaryotes: effects of short-term warming in a coastal subtropical marine system. Hydrobiologia 751, 43–54 (2015). https://doi.org/10.1007/s10750-014-2170-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2170-4