Abstract
Cardiac magnetic resonance (CMR) has become an essential tool for the evaluation of patients affected or at risk of developing cardiomyopathies (CMPs). In fact, CMR not only provides precise data on cardiac volumes, wall thickness, mass and systolic function but it also a non-invasive characterization of myocardial tissue, thus helping the early diagnosis and the precise phenotyping of the different CMPs, which is essential for early and individualized treatment of patients. Furthermore, several CMR characteristics, such as the presence of extensive LGE or abnormal mapping values, are emerging as prognostic markers, therefore helping to define patients’ risk. Lastly new experimental CMR techniques are under investigation and might contribute to widen our knowledge in the field of CMPs. In this perspective, CMR appears an essential tool to be systematically applied in the diagnostic and prognostic work-up of CMPs in clinical practice. This review provides a deep overview of clinical applicability of standard and emerging CMR techniques in the management of CMPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiomyopathies (CMPs) are a group of myocardial disorders, often affecting young individuals, characterized by the presence of structural and functional abnormalities of the heart muscle, not explained by coronary artery disease, hypertension, valvular disease or congenital heart disease [1]. Advancements in medical treatments and the availability of implantable cardioverter defibrillator to prevent sudden cardiac death (SCD) have allowed a substantial increase in the survival of affected individuals, thus making early diagnosis and prompt treatment mandatory [2].
The non-invasive characterization of cardiomyopathies has received a great boost from the recent advances in cardiovascular magnetic resonance imaging (CMR), which to date represents the gold standard for non-invasive assessment of cardiac morphology, function and myocardial tissue changes. In fact, CMR allows not only the quantification of biventricular volumes, mass, wall thickness, systolic- and diastolic function, intra- and extracardiac flows, but also the detection of myocardial oedema, fibrosis, and the accumulation of other intra/extracellular substances (such as fat, iron, amyloid), providing unique information for the etiological, diagnostic and prognostic definition of the disease. In addition to the conventional sequences, new quantitative techniques are now available and further experimental CMR techniques are under investigation and might contribute to widen our knowledge in the field of CMP. The purpose of this joined document of Working Groups on Myocardial and Pericardial Diseases and on CMR of Italian Society of Cardiology is to provide practical information for the application of both standard and emerging CMR techniques in the clinical management of CMPs, bringing the most recent scientific evidence to daily clinical practice.
Overview of CMR sequences in cardiomyopathies (Table 1 and Figure 1)
CMR is a multiparametric, highly reproducible, non-invasive imaging technique, with a relatively high spatial, temporal and contrast resolution [3,4,5]. This is made possible thanks to a great number of different sequences, each obtained combining specific magnetic gradients and radiofrequency pulses, whose detailed explanation goes beyond the scope of this review (for detailed description see Table 1 and Fig. 1).
The most common conventional sequences in CMR are cine steady state free-precession (SSFP) images for the assessment of cardiac volumes, wall thickness, mass and systolic function [6] and several different static sequences for myocardial tissue characterization. For instance, fatty infiltration can be seen as a dark “India Ink” sign in SSFP images or as a hyperintense area in T1 or PD-weighted fast spin echo (FSE) sequences [7] while myocardial edema appears hyperintense in T2-STIR (short-tau inversion-recovery) sequences. Fibrosis can be seen as a hyperintense area on late gadolinium enhancement (LGE) sequences, which are acquired 10–15 min after gadolinium-based contrast agent administration. The various pattern of LGE have been used to distinguish ischemic cardiomyopathy (characterized by subendocardial or transmural LGE, corresponding to a coronary territory) from primary nonischemic cardiomyopathies (characterized by patchy or mid-wall LGE), myocarditis (sub-epicardial LGE) and cardiac amyloidosis (diffuse subendocardial-to-transmural LGE).
As compared to the wide range of information derived from CMR, there are only few contraindications, mostly related to MR-unsafe metal implants, severe renal failure (which limits the use of several gadolinium-based contrast agents), patient discomfort (claustrophobia) and tachyarrhythmias or poor breath-holding (with consequent impairment of image quality) [8, 9].
Compared to conventional imaging, the novel mapping sequences allow the absolute quantification of T1, T2, and T2* relaxation times (ms) for each tissue generating pixel-wise quantitative myocardial maps [10, 11], reflecting changes due to several myocardial diseases [12].
Native (pre-contrast) T1 mapping encompasses both intracellular and extracellular changes: myocardial infarction, inflammation, edema, fibrosis or amyloid all demonstrate prolonged native T1 values compared with normal myocardium, while iron (in cardiac hemochromatosis) or lipids (as in Fabry disease) shorten pre-contrast T1 [12, 13].
-
The myocardial extracellular volume (ECV) is calculated from pre- and post-contrast T1 mapping and hematocrit and correlates with the extent of interstitial space (where gadolinium-based contrast agents accumulate). Myocardial necrosis, interstitial oedema, fibrosis and amyloidosis are the most common causes of an increased ECV [14, 15]. Differently from LGE, ECV mapping does not require the presence of local differences in the myocardium, thus allowing the detection of diffuse myocardial changes (i.e. diffuse interstitial fibrosis), which can hardly be detected with the sole LGE technique.
-
T2 mapping detects myocardial oedema, with a higher sensitivity and reproducibility than T2-STIR sequences [16], in both ischemic and non-ischemic cardiac diseases.
-
T2* differs from T2 mapping because it accounts for magnetic field inhomogeneities, and it has emerged as a valuable tool in the detection and quantification of myocardial iron deposits, such as in myocardial hemorrhage and hemochromatosis [17, 18].
Further experimental CMR techniques (resumed in Supplemental Table 1) are under investigation and may become available for clinical practice in the near future.
Non-ischemic dilated cardiomyopathies
Non-ischemic dilated cardiomyopathy (DCM) is characterized by the presence of a poorly contractile and frequently dilated left and/or right ventricle, resulting from a complex interplay between individual genetic background and environmental factor [19].
In this context, CMR is now acknowledged as the gold standard technique for the quantification of chamber volumes, mass, and ejection fraction (EF) [20, 21]. Furthermore, CMR has the ability to characterize myocardial tissue and to detect myocardial fibrosis, which has been recognized to have a prognostic relevance in patients with DCM, thus improving risk stratification and patients’ outcome. Therefore, it is widely accepted that all DCMs should undergo an early CMR as a part of the diagnostic and prognostic workup.
Histological studies have pointed out that in DCM fibrosis can occur in two forms [22]. One is irreversible replacement fibrosis, corresponding to the presence of LGE, which depicts areas of myocardial scarring developed as a consequence of cell death [22, 23]. LGE can be found in about 30–40% of DCM patients, the most typical pattern being in the midwall of the interventricular septum, even if also a subepicardial pattern can be found, especially in post inflammatory DCM [24]. Since the first prospective longitudinal study conducted in 2006 by Assomull et al. [25], midwall fibrosis detected by LGE has emerged as a predictor of adverse prognosis in patients with DCM, including all-cause mortality, hospitalization and SCD/VT. Subsequent studies have confirmed these data, pointing out that the presence of myocardial scar allows to identify a subgroup of patients at a higher risk of adverse outcome independently from LVEF [24, 26]. A recent meta-analysis [24], confirmed that the presence of LGE is significantly associated with arrhythmic endpoint, such as SCD, sustained VT and appropriate ICD therapy (pooled OR 4.3, 95% CI 3.3 to 5.8, p = 0.001). Moreover, in this meta-analysis LVEF was not able to predict arrhythmic events in DCM, while a significant association between LGE and VA or SCD was observed also in patients with LVEF above 35%. On these bases, the recently published ESC guidelines [20], which have reduced ICD recommendation class for patients with non-ischemic CMP and severely reduced EF (i.e. class IIA, level of evidence A), encompass the use of LGE as a tool with additional value to LVEF for the identification of the best candidates to ICD implantation in primary prevention [26, 27]. However, no specific cut off have been validated and patients should be counseled on individual basis. Furthermore, whether LGE localization, pattern of distribution or LGE extension could have a prognostic impact is still not clear and further investigations are needed. CMR could also be useful in patients receiving cardiac resynchronization therapy (CRT) thanks to its capability to guide LV lead placement away from scarred tissue [28, 29].
The second form of fibrosis is interstitial and it is due to the accumulation of collagen even in the absence of cell death [30]. This form of fibrosis may be detected and quantified by native myocardial T1 relaxation times and ECV, and it has recently emerged as an independent marker of poor outcome [31,32,33].
CMR can be a valuable tool also in the analysis of right ventricle, often poorly visualized by echocardiography, which has emerged as an important tool in DCM risk stratification [34].
Finally, another promising CMR derived parameter is represented by global longitudinal strain (GLS) measured by feature-tracking analysis which was found to correlate better than LVEF and BNP with the composite of cardiac death, heart transplantation and appropriate ICD shock due to VT or VF, in a DCM population [35, 36].
Arrhythmogenic cardiomyopathy
Arrhythmogenic cardiomyopathy (ACM) is a genetically-determined heart muscle disease characterized by fibro-fatty myocardial replacement, clinically associated with malignant ventricular arrhythmias and SCD [37]. Although originally described as a disease with predominant right ventricular (RV) involvement, subsequent increasing recognition of biventricular and left dominant phenotypic variants has led to broad the concept of arrhythmogenic cardiomyopathy as a disease potentially involving both right and left ventricles [38].
CMR has always been considered as a non-invasive tool for the demonstration of morpho-functional abnormalities. In the recently published “Padua Criteria” [39] CMR has gained further importance. In fact, while according to the previous diagnostic criteria the presence of structural myocardial abnormalities could only be detected by endomyocardial biopsy, it is now contemplated to detect these abnormalities also with CMR (LGE). Accordingly, it is now mandatory to perform CMR in patients with known or suspected ACM.
The T1 weighted images, once considered useful to identify fatty infiltration, have limited sensitivity and specificity because of poor resolution and partial volume artifacts [40,41,42] and might be replaced by the detection of “India Ink” artifacts in conventional cine-SSFP images [7]. The routine use of T2-weighted images for the depiction of myocardial edema is also not recommended, unless in case of “hot-phase” presentation (chest pain and troponin release), which are common for instance in pediatric patients and carriers of desmoplakin gene mutations [43]. It is instead mandatory to acquire LGE images which allows the detection of areas of fibro-fatty myocardial replacement, that are the hallmark lesions of ACM and which adds valuable information for arrhythmic risk stratification, particularly in left-dominant forms [44]. In RV diseases, LGE assessment can be challenging and limited by a high intra-interobserver variability; however, when considered together with wall motion abnormalities, it increases CMR accuracy for the diagnosis of ACM [45]. In LV arrhythmogenic diseases, LGE is commonly found in the subepicardial layers of the LV free wall, especially in the inferolateral region, with or without septal involvement [42]. The presence of circumferential LV subepicardial LGE in short axis view (“ring pattern”) has been consistently reported in left-dominant variants with specific genotype [46, 47]. As in DCM, it is clearly the emerging impact of CMR (and specifically LGE) on top of standard risk scores to identify high arrhythmic risk patients, candidates to primary prevention ICD implantation when a LV dominant form is present, regardless the amount of systolic dysfunction [44].
The new CMR techniques, such as T1 and T2 mapping, still have limited applications in patients with ACM. Conversely, feature-tracking CMR has recently raised interest given its potential capability to detect subtle segmental impairment of wall contraction, useful to early identify ACM patients in concealed phases of disease, as well as family members and asymptomatic gene carriers [48]. Supplementary materials, case 1.
Acute myocarditis
Acute myocarditis (AM) is an inflammatory disease of the myocardium with different aetiology and with a heterogeneous presentation and clinical course that make patients’ management and risk stratification challenging. [49]. The diagnosis of AM can be confirmed only when histological Dallas Criteria are met, being therefore endomyocardial biopsy (EMB) necessary. Despite being an invasive examination with potentially life-threatening complications, EMB is indicated in selected myocarditis patients with hemodynamic instability not responsive to conventional medical treatment as well as when specific myocarditis aetiologies are suspected, also in hemodynamically stable patients [20, 50]. The limited availability of EMB has been compensated for by the increased use of CMR, which is able to characterize myocardial tissue and to identify areas of myocardial oedema and fibrosis/necrosis, thus allowing a non-invasive diagnosis of AM.
According to the original Lake Louise Criteria (LLC) the diagnosis of myocarditis could be made in the presence of “any 2 out of 3” CMR markers, consisting of T2-weighted, Early Gadolinium Enhancement and Late Gadolinium Enhancement (LGE) sequences, assessing myocardial edema, hyperemia and fibrosis/necrosis, respectively [51]. LLC have been shown to be very sensitive in the diagnosis of AM in patients presenting with chest pain, while sensitivity was reduced in those presenting with arrhythmias or heart failure [52].
The advent of parametric mapping has allowed overcoming some of the limitations of standard T2-weighted and T1-weighted sequences. In fact, each tissue has a characteristic range of T1 and T2 values which are altered in case of increase in the free water content (such as in myocardial inflammation) [10, 53].
Consequently, the LLC criteria have been recently updated so that, in order to achieve the diagnosis of AM, it is now necessary the presence of both a “T1 criterion” (presence of LGE, increased native T1-mapping or extracellular volume values) and a “T2 criterion” (hyperintensity on T2 weighted sequences or increased T2 mapping values) [54].
While T1 mapping and ECV seem to be altered both in acute as well as chronic myocarditis, T2 mapping has proved to be better correlated with the disease activity (inflammation), thus allowing the detection of AM and its differentiation from chronic inflammation with better accuracy [55].
Although limitations for the applicability of parametric mapping still exist (i.e. the lack of universal reference values), the evaluation of native T1 and T2 mapping, has been shown to led to an increase in CMR diagnostic accuracy, therefore advanced tissue characterization comprehensive of T1 and T2 mapping is now highly recommended by international consensus in all patients with suspected myocarditis, whenever feasible. [3, 56]
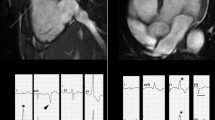
Apart from the role of CMR in the diagnosis of AM, several studies have investigated the potential contribution of tissue characterization by CMR in patients’ risk stratification. While a normal CMR correlates with a favorable outcome, several studies have confirmed the negative prognostic value of LGE as well as the correlation between abnormal T2-weighted imaging and worse outcome [57, 58]. Feature tracking analysis, thanks to a better assessment of systolic function and LV kinetic, has already demonstrated both to be helpful in detecting AM with preserved ejection fraction, and to be promising tool in patients’ risk stratification, even if more studies are needed to confirm these preliminary data [59,60,61] Fig. 2.
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a genetic disease characterized by inappropriate hypertrophy, myocardial fibrosis and diffuse disarray with diverse phenotypic expressions, clinical course and prognosis [62].
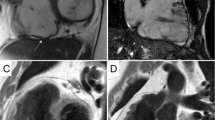
Cardiovascular magnetic resonance (CMR) is capable to provide assessment of ventricular mass, chamber volume, cardiac function, pattern and distribution of hypertrophy and tissue characterization without ionizing radiation [63, 64] thus representing an essential tool for the diagnosis and morphological assessment of HCM [64,65,66,67]. CMR allows the detection of unusual pattern of LV hypertrophy, such as lateral and apical distribution, which are not always easily visualized by echocardiography. Furthermore, CMR is a useful tool to evaluate the extent and severity of the hypertrophy in terms of mass quantification [5, 68] and to recognize right ventricular as well as papillary muscles hypertrophy, and mitral valve anomalies [69]. Moreover, CMR has also emerged as a valuable instrument to detect markers of the disease in patients with positive genotype but without LV hypertrophy (negative phenotype), such as myocardial crypts, elongated anterior mitral leaflet, abnormal apical trabeculae and smaller LV ventricular volumes [70] (Fig. 2).
CMR is helpful in the differential diagnosis between sarcomeric HCM and phenocopies or secondary hypertrophy, showing important differences in pattern and location of LV hypertrophy as well as in pattern and distribution of LGE and different values of native T1 [56, 64, 68, 71, 72]. CMR has also become an essential tool in the preoperative planning in patients undergoing septal reduction surgery [64, 73].
Areas of myocardial LGE representing replacement fibrosis [67, 68] are a common finding in this disease, expressed in up to 80% of HCM population [69], so that only quantitative analysis is a robust marker of unfavourable prognosis, in terms of progressive systolic dysfunction and malignant arrhythmias. A LGE threshold of 10–15% of LV mass have proved to be a possible cut off to identify patients at high risk of SCD, even in the absence of other major risk factors, who may benefit of primary prevention therapy [64 74,75,76,77,78,79]. Not surprisingly, the presence of LGE has been listed among the criteria to be considered in ICD patients selection in the recently updated HCM guidelines by AHA/ACC [64].
Also high signal intensity on T2-Weighted images has been demonstrated to predict arrhythmic events in the setting of HCM [80].
Although area of low ECV have been described in areas remote from hypertrophy, ECV is usually elevated in the hypertrophied areas both in patients with HCM as well as in phenotype-negative carriers of the disease [64, 81].
Diffusion Tensor (DT) CMR, visualizing microstructure of myocardial fibers, is an innovative sequence with the potential to represent myocardial disarray [82]. The latter technique, despite its complexity and limited availability, has the potentiality to provide further histopathological insights in the study of HCM and to offer additional markers of arrhythmic risk in HCM.
Finally, advanced analyses of standard technique might have clinical impact in the next future: a CMR Virtual Native Enhancement (VNE) can be generated from “cine” and native T1 mapping images using artificial intelligence, resembling conventional LGE without contrast administration [83]. Heterogeneity of scar, expressed as “dispersion map of LGE” may be a better marker of poor prognosis than its extent [84]. Another innovative post-processing analysis of LGE images enables to differentiate between the scar core and the border zone and to isolate corridors connecting the areas of normal myocardium to the scar core areas [85]. Lastly, we have to mention the role of bSSFP analysis in differentiating the different etiologies of HCM [86].
Cardiac amyloidosis
Cardiac amyloidosis (CA) is a restrictive cardiomyopathy characterized by a pseudo-hypertrophy resulting by extracellular deposition of abnormal proteins in the myocardium [87]. Recently developed disease-modifying therapies increase the need of an early diagnosis [88]. Until recently, a positive biopsy was the only way to diagnose CA [88]. However, the combination of several imaging modalities has made possible a non-invasive diagnosis of CA, thus restricting the indication for EMB to those patients with equivocal or discordant clinical and imaging findings [88].
Although echocardiography remains the first line imaging modality in patients with suspected CA, CMR has shown to provide incremental information thanks to accurate morpho-functional evaluation, and tissue characterization [88]. Among recently published consensus documents, only one have proposed a “CMR” based pathway for the diagnosis of CA [89]. According to an ESC position paper, CMR can be used to implement the diagnostic algorithm of CA both in the “scintigraphy-based” and in the “laboratory-based” pathways, being particularly useful in patients with positive hematologic test and a negative scintigraphy (grade zero) [90].
To date, the key CMR technique to image CA is LGE, being the presence of diffuse subendocardial LGE highly specific for CA (94%). LGE imaging in patients with CA can be challenging in advanced stages due to the diffuse nature of LGE and to the equalization of myocardial and blood pool nulling point [22, 88]. However, the characteristic alterations in inversion times responsible of the aforementioned challenges in myocardial nulling, partially overcome by the development of phase sensitive inversion recovery (PSIR) sequences, are also strongly suggestive of the presence of amyloid deposits, supporting the diagnosis of CA [88, 93, 94].
Native T1 demonstrated high diagnostic accuracy in suspected CA with high positive and negative predictive values [95]. However, being T1 a composite signal from both the extra and intracellular space, it has turned out to be less specific than ECV, which to date represents the best parameter for quantifying amyloid and which has showed the best diagnostic accuracy when compared to other CMR parameters [96].
Beyond its role in the diagnostic workup of CA, CMR is important for prognostic information. The presence of LGE, especially when transmural, is a significant and independent predictor of mortality [88, 91, 97]. Furthermore, the aforementioned alterations in myocardial inversion times have also been found to be a negative prognostic marker thanks to their correlation with amyloid burden [22, 88, 92, 93].
ECV was found to be the parameter with the highest hazard ratio (as compared to LGE and native T1) in predicting patients’ prognosis, and its changes over the time could allow the assessment patients’ response to treatments [96, 98, 99]. The role of T2 mapping, adenosine stress perfusion and CMR-FT strain imaging have also showed to provide additional information in patients with CA, but further studies are needed to validate these findings in order to allow the application of these new techniques in daily clinical practice [100,101,102,103,104,105]. Supplementary material, case 2.
Anderson fabry disease and other rare CMPs
Apart from sarcomeric HCM and amyloidosis, there are several other CMPs characterized by LV hypertrophy and therefore defined HCM mimics of phenocopies. Despite this overlapping phenotype, it is of extreme importance to correctly differentiate these entities, especially since specific treatments have become available to treat these conditions.
Anderson fabry disease
Anderson-Fabry disease (AFD) is a rare X-linked inherited disorder caused by deficiency or absence of the enzyme α-galactosidase A (GLA), with subsequent accumulation of glycosphingolipids in several districts included the heart muscle cells and coronary circulation. The AFD clinical phenotype encompasses several scenarios due to the presence of different pathogenetic mutations in the GLA genes as well as to the X-linked inheritance of the disease, with homozygous males presenting with early signs and symptoms and heterozygous females experiencing milder phenotypes with later onset [106].
Although echocardiography remains the first line imaging examination in suspected AFD, CMR can help both in the differential diagnosis between AFD and sarcomeric HCM, as well as in the detection of subclinical stages of the disease. The main CMR findings in AFD are concentric LV hypertrophy [107] and non-ischemic mid-wall or subepicardial LGE pattern mainly involving the basal inferolateral LV segment [108]. In males, it seems that LGE does not precede the development of LV hypertrophy, while its presence has been reported in a significant proportion of female patients without hypertrophy [109]. The recently developed mapping techniques also provide useful data for the diagnosis of AFD. Indeed, intracellular accumulation of sphingolipids causes a typical shortening of native T1 relaxation times, even before the development of hypertrophy, and allows also to distinguish AFD from other hypertrophic diseases, typically characterized by elevated T1 values [109, 110]. However, it is also important to remember that during the disease course, the development of myocardial fibrosis, secondary to myocardial inflammation mediated by sphingolipid, balances the effect of sphingolipid on T1 relaxation times leading to a pseudo-normalization of native T1, at least in myocardial regions involved by fibrosis. Among parameters derived from mapping analysis, ECV is typically normal in AFD because of the intracellular accumulation of sphingolipids, as compared to other CMPs characterized by interstitial infiltration (e.g., amyloidosis). In fact, ECV values reflect the increase of the extracellular space, typically not affected in AFD [56]. Finally, T2 mapping has been used to demonstrate the presence of myocardial inflammation, which is thought to contribute to disease progression [111,112,113].
Recently, both enzyme replacement therapy (ERT) and chaperone therapy have demonstrated to be safe and effective in stabilizing the disease course and improving symptoms in patients affected by AFD. The initiation of ERT treatment is yet recommend for patients exhibiting symptoms and LV hypertrophy. CMR techniques hold strong potential in AFD not only for guiding the appropriate timing for ERT introduction and prognostic classification, but also for monitoring response to therapy. For instance, several studies reported more effective results of ERT in terms of LV mass regression when little or no LGE was present at baseline evaluation [114] thus suggesting that specific treatment should be initiated earlier, as soon as the first structural or functional cardiac abnormalities become detectable and before development of myocardial fibrosis. Supplementary, material case 3.
Cardiac siderosis
Iron overload cardiomyopathy can occur in patients affected by genetic haemochromatosis or, more commonly, it can be secondary to excessive iron administration in subjects requiring repeated blood transfusion as it happens in the setting of hereditary anemias. When left untreated, it can lead to heart failure and even death. After the introduction of mapping techniques, CMR has become an essential tool in the diagnosis and risk stratification of this condition. In fact, the myocardial iron deposits affect T2* relaxation time, thus allowing the diagnosis of cardiac siderosis. Furthermore, a linear relationship between the reduction in T2* and the amount of iron in myocardium and an increased risk of ventricular arrhythmias has been demonstrated. Therefore, to date different cut offs of T2* are used to diagnose iron overload CMP and to guide the initiation of iron chelation therapy, as well as to monitor patients’ response to medical treatment, with a dramatic improvement in the prognosis of these patients [115]. Native T1 is also decreased in 10 and can be used for diagnosis [116].
Glycogen storage disease
Glycogen storage diseases (e.g., Pompe, PRKAG2, Danon) may determine severe increase in LV mass with rapid progression toward heart failure. CMR may be helpful also in the assessment of these rare CMP, for instance Danon disease is characterized by extensive LV subendocardial LGE, particularly at apical level, with sparing of basal septum [117]. However, because of the scarce amount of data, the role of CMR in determining prognosis in these rare conditions still needs to be defined.
LV noncompaction—anatomical phenotype or a distinct entity?
LV noncompaction (LVNC) is a heterogeneous entity characterized by the presence of extensive myocardial trabeculations and currently listed among “not classified CMPs.” Traditionally, the presence of this characteristic ventricular pattern has been attributed to the arrest of normal embryogenesis of the endocardium and myocardium or to an abnormal myocardial development, which recognize a genetic background in one third of cases, with mutation in genes encoding for sarcomeric and cytoskeletal proteins being the most represented [1, 118, 119]. Furthermore, several genetic mutations have been associated with the presence of LV systolic dysfunction and a more severe prognosis [120]. Despite those proved genetic determinants, there are growing data demonstrating the presence of reversible forms of LVNC related to overload conditions (i.e., strenuous training, pregnancy), thus suggesting that LVNC should be considered as an anatomical phenotype rather than a real CMP [119]. The definition of this entity in clinical practice has always been challenging especially due to an overlap with other cardiomyopathies and with normal LV trabeculation [22]. CMR has become a valuable tool for the non-invasive assessment of patients with a suspected LVNC. Several diagnostic criteria have been proposed, among these the two most widely used are those proposed by Petersen and Jacquier which require the presence of a NC to C ratio of 2.3/1 and the detection of a trabeculated LV mass > 20% of the LV global mass, respectively [22, 121, 122]. All these proposed CMR diagnostic criteria have showed to be highly sensitive but also non-specific, with several normal individuals meeting at least one criterion for LVNC according to a recent study [123]. Furthermore, in asymptomatic subjects the presence of LVNC as diagnosed by the aforementioned CMR criteria have showed no progression at 10 years follow up [124]. Similarly, 1,4% of athletes meet the diagnostic criteria for LVNC at CMR but only a small percentage of them (0,1%) have also LV dysfunction or a positive family history. Therefore, since it has been demonstrated that in absence of symptoms, positive family history, left ventricular systolic dysfunction or LGE, the event-rate during follow up is very low [125], CMR criteria should be integrated with clinical data in order to improve the specificity of LVNC diagnosis [86]. Recently, an individualized model for prognostic risk stratification has been proposed. This model, which considers also the presence of LGE on CMR, is based on a multicenter retrospective study enrolling 585 patients and showing that LVNC was associated with a higher risk of adverse outcome during follow-up in the presence of LV systolic dysfunction or in patients with preserved LVEF but with LGE at CMR [126].
At the same time, additional CMR markers could be validated in the future to discriminate individuals with an increased risk of events at follow up, among these the presence of LV systolic dysfunction and LGE has already demonstrated to correlate with a worse prognosis especially when associated with LV dysfunction [125, 127, 128].
Table 2 resumes the main diagnostic and prognostic CMR findings for both dilated and hypertrophic phenotype.
Conclusion
Today, more than ever before, a patient-tailored approach is mandatory in every medical field, and particularly in CMPs. In fact, the growing body of knowledge on patho-physiological pathways, diagnostic and prognostic work-up of CMPs as well as the availability of an increasing number of targeted disease-modifying therapies make it mandatory to achieve a timely diagnosis and a precise characterization of the different phenotypes of CMP.
Recent advances in CMR and its increased accessibility allow a precise assessment of ventricular dimension and function as well as a non-invasive tissue characterization of the myocardium. However, the growing knowledge deriving from CMR studies should always be interpreted in light of clinical elements and integrated with information derived by other imaging techniques (Table 3), such as echocardiography (which remains the first line imaging tool to guide the diagnosis in patients with suspected CMP) and genotype or histological information. CMR, thanks to its ability to add information about tissue characterization, appears to be particularly relevant in subclinical and recently onset CMPs, as well as in genotype positive phenotype negative subjects [129]. New imaging techniques both for echo and for CMR (i.e. diffusion tension imaging, speckle and feature tracking and myocardial work, T1/T2 mapping) are increasingly used in experienced labs to help clinicians in the differential diagnosis and management of specific CMP subtypes (i.e. Amyloid or Anderson Fabry disease) [129]. Although the increased enthusiasm for the use of CMR in the diagnosis and characterization of CMP, it has to be recognized that a multimodality imaging approach remains the gold-standard, mostly for challenging settings such as infiltrative cardiomyopathies [129, 130].
In conclusion, an integrated clinical and imaging approach seems to be essential to guide diagnosis, define the different CMP phenotypes (HCM, DCM, arrhythmogenic cardiomyopathy, restricted cardiomyopathy, LVNC) and unravel specific underlying aetiologies as well as to ensure a tailored therapeutic management and predict disease prognosis.
Change history
04 September 2022
The original version of this paper was updated to add funding note.
References
Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P et al (2007) Classification of the cardiomyopathies: a position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J 29(2):270–276
Merlo M, Cannatà A, Pio Loco C, Stolfo D, Barbati G, Artico J et al (2020) Contemporary survival trends and aetiological characterization in non-ischaemic dilated cardiomyopathy. Eur J Heart Fail 22(7):1111–1121
Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E (2020) Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 22(1):17
Francone M, Aquaro GD, Barison A, Castelletti S, de Cobelli F, de Lazzari M et al (2021) Appropriate use criteria for cardiovascular MRI: SIC – SIRM position paper Part 2 (myocarditis, pericardial disease, cardiomyopathies and valvular heart disease). J Cardiovasc Med 22(7):515–529
Pontone G, Di Cesare E, Castelletti S, De Cobelli F, De Lazzari M, Esposito A et al (2021) Appropriate use criteria for cardiovascular magnetic resonance imaging (CMR): SIC—SIRM position paper part 1 (ischemic and congenital heart diseases, cardio-oncology, cardiac masses and heart transplant). Radiol med 126(3):365–379
Aquaro GD, Camastra G, Monti L, Lombardi M, Pepe A, Castelletti S et al (2017) Reference values of cardiac volumes, dimensions, and new functional parameters by MR: A multicenter, multivendor study: Reference Range of Normality for CMR. J Magn Reson Imaging 45(4):1055–1067
Aquaro GD, Nucifora G, Pederzoli L, Strata E, De Marchi D, Todiere G et al (2012) Fat in left ventricular myocardium assessed by steady-state free precession pulse sequences. Int J Cardiovasc Imaging 28(4):813–821
Ferreira PF, Gatehouse PD, Mohiaddin RH, Firmin DN (2013) Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson 15(1):41
Barison A, Baritussio A, Cipriani A, De Lazzari M, Aquaro GD, Guaricci AI et al (2021) Cardiovascular magnetic resonance: What clinicians should know about safety and contraindications. Int J Cardiol 331:322–328
Greulich S, Ferreira VM, Dall’Armellina E, Mahrholdt H (2015) Myocardial Inflammation—Are We There Yet? Curr Cardiovasc Imaging Rep 8(3):6
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M (2016) T1 Mapping. J Am Cardiol Img 9(1):67–81
Bulluck H, Maestrini V, Rosmini S, Abdel-Gadir A, Treibel TA, Castelletti S et al (2015) Myocardial T1 Mapping: – Hope or Hype? –. Circ J 79(3):487–494
Radenkovic D, Weingärtner S, Ricketts L, Moon JC, Captur G (2017) T1 mapping in cardiac MRI. Heart Fail Rev 22(4):415–430
Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G et al (2012) Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 98(19):1436–1441
Ugander M, Oki AJ, Hsu L-Y, Kellman P, Greiser A, Aletras AH et al (2012) Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J 33(10):1268–1278
Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP et al (2011) Direct T2 Quantification of Myocardial Edema in Acute Ischemic Injury. J Am Cardiol Img 4(3):269–78
Lota AS, Gatehouse PD, Mohiaddin RH (2017) T2 mapping and T2* imaging in heart failure. Heart Fail Rev 22(4):431–440
Positano V, Meloni A, Santarelli MF, Gerardi C, Bitti PP, Cirotto C et al (2015) Fast generation of T2⁎ maps in the entire range of clinical interest: Application to thalassemia major patients. Comput Biol Med 56:200–210
McNally EM, Mestroni L (2017) Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ Res 121(7):731–748
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–3726
Petersen SE, Khanji MY, Plein S, Lancellotti P, Bucciarelli-Ducci C (2019) European Association of Cardiovascular Imaging expert consensus paper: a comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Heart J Cardiovasc Imaging 20(12):1321–1331
Patel AR, Kramer CM (2017) Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. J Am Cardiol Img 10(10):1180–93
Iles LM, Ellims AH, Llewellyn H, Hare JL, Kaye DM, McLean CA et al (2015) Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur Heart J Cardiovasc Imaging 16(1):14–22
Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA et al (2017) Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy. J Heart Failure. 5(1):28–38
Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M et al (2006) Cardiovascular Magnetic Resonance, Fibrosis, and Prognosis in Dilated Cardiomyopathy. J Am Coll Cardiol 48(10):1977–1985
Pontone G, Guaricci AI, Andreini D, Solbiati A, Guglielmo M, Mushtaq S et al (2016) Prognostic Benefit of Cardiac Magnetic Resonance Over Transthoracic Echocardiography for the Assessment of Ischemic and Nonischemic Dilated Cardiomyopathy Patients Referred for the Evaluation of Primary Prevention Implantable Cardioverter–Defibrillator Therapy. Circ: Cardiovasc Imaging [Internet]. 2016 Oct [cited 2021 Nov 4];9(10). Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.115.004956
Guaricci AI, Masci PG, Lorenzoni V, Schwitter J, Pontone G (2019) DERIVATE Study Group, 4327 Results of the DERIVATE study in non-ischemic dilated cardiomyopathy (NICM), European Heart J 40(Issue Supplement_1, October 2019):ehz745.0164
Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F et al (2011) Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 13(1):29
Barison A, Aimo A, Ortalda A, Todiere G, Grigoratos C, Passino C et al (2018) Late gadolinium enhancement as a predictor of functional recovery, need for defibrillator implantation and prognosis in non-ischemic dilated cardiomyopathy. Int J Cardiol 250:195–200
Halliday BP, Gulati A, Ali A, Guha K, Newsome S, Arzanauskaite M et al (2017) Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 135(22):2106–2115
Puntmann VO, Carr-White G, Jabbour A, Yu C-Y, Gebker R, Kelle S et al (2016) T1-Mapping and Outcome in Nonischemic Cardiomyopathy. J Am Cardiol Img 9(1):40–50
Barison A, Del Torto A, Chiappino S, Aquaro GD, Todiere G, Vergaro G et al (2015) Prognostic significance of myocardial extracellular volume fraction in nonischaemic dilated cardiomyopathy. J Cardiovasc Med 16(10):681
Vita T, Gräni C, Abbasi SA, Neilan TG, Rowin E, Kaneko K et al (2019) Comparing CMR Mapping Methods and Myocardial Patterns Toward Heart Failure Outcomes in Nonischemic Dilated Cardiomyopathy. J Am Cardiol Img (8):1659–69
Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA et al (2013) The Prevalence and Prognostic Significance of Right Ventricular Systolic Dysfunction in Nonischemic Dilated Cardiomyopathy. Circulation 128(15):1623–1633
Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D et al (2015) Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 16(3):307–315
Farzaneh-Far A, Romano S (2020) Imaging and Impact of Myocardial Strain in Myocarditis. J Am Cardiol Img (9):1902–1905
Corrado D, Basso C, Judge DP (2017) Arrhythmogenic Cardiomyopathy. Circ Res 121(7):784–802
Corrado D, Zorzi A, Cipriani A, Bauce B, Bariani R, Beffagna G et al (2021) Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy. JAHA [Internet]. 2021 Sep 21 [cited 2021 Nov 5];10(18). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.121.021987
Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari MD et al (2020) Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int J Cardiol 319:106–114
Kellman P, Hernando D, Shah S, Zuehlsdorff S, Jerecic R, Mancini C et al (2009) Multiecho dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium: Fibrofatty Infiltration in Myocardium. Magn Reson Med 61(1):215–221
Tandri H, Castillo E, Ferrari VA, Nasir K, Dalal D, Bomma C et al (2006) Magnetic Resonance Imaging of Arrhythmogenic Right Ventricular Dysplasia. J Am Coll Cardiol 48(11):2277–2284
Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin S et al (2020) Arrhythmogenic Right Ventricular Cardiomyopathy: Characterization of Left Ventricular Phenotype and Differential Diagnosis With Dilated Cardiomyopathy. JAHA [Internet]. 2020 Mar 3 [cited 2021 Nov 5];9(5). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.119.014628
Bariani R, Cipriani A, Rizzo S, Celeghin R, Bueno Marinas M, Giorgi B et al (2021) ‘Hot phase’ clinical presentation in arrhythmogenic cardiomyopathy. EP Europace 23(6):907–917
Aquaro GD, De Luca A, Cappelletto C, Raimondi F, Bianco F, Botto N et al (2020) Prognostic Value of Magnetic Resonance Phenotype in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. J Am Coll Cardiol 75(22):2753–2765
Pfluger HB, Phrommintikul A, Mariani JA, Cherayath JG, Taylor AJ (2008) Utility of myocardial fibrosis and fatty infiltration detected by cardiac magnetic resonance imaging in the diagnosis of arrhythmogenic right ventricular dysplasia–a single centre experience. Heart Lung Circ 17(6):478–483
Augusto JB, Eiros R, Nakou E, Moura-Ferreira S, Treibel TA, Captur G et al (2019) Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: a comprehensive genotype-imaging phenotype study. European Heart J - Cardiol Img jez188
Castelletti S, Vischer AS, Syrris P, Crotti L, Spazzolini C, Ghidoni A et al (2017) Desmoplakin missense and non-missense mutations in arrhythmogenic right ventricular cardiomyopathy: Genotype-phenotype correlation. Int J Cardiol 249:268–273
Muscogiuri G, Fusini L, Ricci F, Sicuso R, Guglielmo M, Baggiano A et al (2021) Additional diagnostic value of cardiac magnetic resonance feature tracking in patients with biopsy-proven arrhythmogenic cardiomyopathy. Int J Cardiol 339:203–210
Sinagra G, Anzini M, Pereira NL, Bussani R, Finocchiaro G, Bartunek J et al (2016) Myocarditis in Clinical Practice. Mayo Clin Proc 91(9):1256–1266
Seferović PM, Tsutsui H, McNamara DM, Ristić AD, Basso C, Bozkurt B et al (2021) Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur J Heart Fail 23(6):854–871
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al (2009) Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J Am Coll Cardiol 53(17):1475–1487
Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R et al (2014) CMR Sensitivity Varies With Clinical Presentation and Extent of Cell Necrosis in Biopsy-Proven Acute Myocarditis. J Am Cardiol Img 7(3):254–63
Gannon MP, Schaub E, Grines CL, Saba SG (2019) State of the art: Evaluation and prognostication of myocarditis using cardiac MRI. J Magn Reson Imaging 49(7):e122–e131
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U et al (2018) Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation. J Am Coll Cardiol 72(24):3158–3176
Kotanidis CP, Bazmpani M-A, Haidich A-B, Karvounis C, Antoniades C, Karamitsos TD (2018) Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis. J Am Cardiol Img 11(11):1583–90
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19(1):75
Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G (2017) Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J Am Coll Cardiol 70(16):1977–1987
Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K et al (2017) Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol 70(16):1964–1976
Baeßler B, Treutlein M, Schaarschmidt F, Stehning C, Schnackenburg B, Michels G et al (2017) A novel multiparametric imaging approach to acute myocarditis using T2-mapping and CMR feature tracking. J Cardiovasc Magn Reson 19(1):71
Porcari A, Merlo M, Crosera L, Stolfo D, Barbati G, Biondi F et al (2020) Strain analysis reveals subtle systolic dysfunction in confirmed and suspected myocarditis with normal LVEF. A cardiac magnetic resonance study. Clin Res Cardiol 109(7):869–80
Fischer K, Obrist SJ, Erne SA, Stark AW, Marggraf M, Kaneko K et al (2020) Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. J Am Cardiol Img 13(9):1891–901
Clinical Course and Management of Hypertrophic Cardiomyopathy (2018) N Engl J Med 379(20):1976–1977
Pontone G, Di Bella G, Castelletti S, Maestrini V, Festa P, Ait-Ali L et al (2017) Clinical recommendations of cardiac magnetic resonance, Part II: inflammatory and congenital heart disease, cardiomyopathies and cardiac tumors. J Cardiovasc Med 18(4):209–222
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P et al (2020) 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy. J Am Coll Cardiol 76(25):e159-240
Quarta G, Aquaro GD, Pedrotti P, Pontone G, Dellegrottaglie S, Iacovoni A et al (2018) Cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy: the importance of clinical context. Eur Heart J Cardiovasc Imaging 19(6):601–610
Kozor R, Nordin S, Treibel TA, Rosmini S, Castelletti S, Fontana M, Captur G, Baig S, Steeds RP, Hughes D, Manisty C, Grieve SM, Figtree GA, Moon JC (2017) Insight into hypertrophied hearts: a cardiovascular magnetic resonance study of papillary muscle mass and T1 mapping. Eur Heart J Cardiovasc Imaging 18(9):1034–1040
Reant P, Captur G, Mirabel M, Nasis A, M. Sado D, Maestrini V et al (2015) Abnormal septal convexity into the left ventricle occurs in subclinical hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 17(1):64
Todiere G, Aquaro GD, Piaggi P, Formisano F, Barison A, Masci PG et al (2012) Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 60(10):922–929
Harrigan CJ, Appelbaum E, Maron BJ, Buros JL, Gibson CM, Lesser JR et al (2008) Significance of Papillary Muscle Abnormalities Identified by Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. Am J Cardiol 101(5):668–673
Captur G, Lopes LR, Mohun TJ, Patel V, Li C, Bassett P et al (2014) Prediction of sarcomere mutations in subclinical hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 7(6):863–871
Kato S, Nakamori S, Bellm S, Jang J, Basha T, Maron M et al (2016) Myocardial Native T1 Time in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol 118(7):1057–1062
Treibel TA, Kozor R, Menacho K, Castelletti S, Bulluck H, Rosmini S et al (2017) Left Ventricular Hypertrophy Revisited: Cell and Matrix Expansion Have Disease-Specific Relationships. Circulation 136(25):2519–2521
Maron MS, Rowin EJ, Maron BJ (2017) How to Image Hypertrophic Cardiomyopathy. Circ: Cardiovascular Imaging [Internet]. 2017 Jul [cited 2021 Nov 6];10(7). Available from: https://www.ahajournals.org/doi/ 10.1161/CIRCIMAGING.116.005372
Moon JCC, Reed E, Sheppard MN, Elkington AG, Ho S, Burke M et al (2004) The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 43(12):2260–2264
Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M et al (2009) Cardiac Magnetic Resonance Detection of Myocardial Scarring in Hypertrophic Cardiomyopathy. J Am Coll Cardiol 54(3):242–249
Aquaro GD, Masci P, Formisano F, Barison A, Strata E, Pingitore A et al (2010) Usefulness of Delayed Enhancement by Magnetic Resonance Imaging in Hypertrophic Cardiomyopathy as a Marker of Disease and Its Severity. Am J Cardiol 105(3):392–397
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T et al (2014) Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 130(6):484–495
Todiere G, Nugara C, Gentile G, Negri F, Bianco F, Falletta C et al (2019) Prognostic Role of Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Low-to-Intermediate Sudden Cardiac Death Risk Score. Am J Cardiol 124(8):1286–1292
Freitas P, Ferreira AM, Arteaga-Fernández E, de Oliveira AM, Mesquita J, Abecasis J et al (2019) The amount of late gadolinium enhancement outperforms current guideline-recommended criteria in the identification of patients with hypertrophic cardiomyopathy at risk of sudden cardiac death. J Cardiovasc Magn Reson 21(1):50
Hen Y, Takara A, Iguchi N, Utanohara Y, Teraoka K, Takada K et al (2018) High Signal Intensity on T2-Weighted Cardiovascular Magnetic Resonance Imaging Predicts Life-Threatening Arrhythmic Events in Hypertrophic Cardiomyopathy Patients. Circ J 82(4):1062–1069
Castelletti S, Menacho K, Davies RH, Maestrini V, Treibel TA, Rosmini S et al (2021) Hypertrophic cardiomyopathy: insights from extracellular volume mapping. European J Prevent Cardiol zwaa083
Ariga R, Tunnicliffe EM, Manohar SG, Mahmod M, Raman B, Piechnik SK et al (2019) Identification of Myocardial Disarray in Patients With Hypertrophic Cardiomyopathy and Ventricular Arrhythmias. J Am Coll Cardiol 73(20):2493–2502
Zhang Q, Burrage MK, Lukaschuk E, Shanmuganathan M, Popescu IA, Nikolaidou C et al (2021) Toward Replacing Late Gadolinium Enhancement With Artificial Intelligence Virtual Native Enhancement for Gadolinium-Free Cardiovascular Magnetic Resonance Tissue Characterization in Hypertrophic Cardiomyopathy. Circulation 144(8):589–599
Aquaro GD, Grigoratos C, Bracco A, Proclemer A, Todiere G, Martini N et al (2020) Late Gadolinium Enhancement–Dispersion Mapping: A New Magnetic Resonance Imaging Technique to Assess Prognosis in Patients With Hypertrophic Cardiomyopathy and Low-Intermediate 5-Year Risk of Sudden Death. Circ: Cardiovascular Imaging [Internet]. 2020 Jun [cited 2021 Nov 6];13(6). Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.120.010489
Sánchez-Somonte P, Quinto L, Garre P, Zaraket F, Alarcón F, Borràs R et al (2021) Scar channels in cardiac magnetic resonance to predict appropriate therapies in primary prevention. Heart Rhythm 18(8):1336–1343
Schofield R, Ganeshan B, Fontana M, Nasis A, Castelletti S, Rosmini S et al (2019) Texture analysis of cardiovascular magnetic resonance cine images differentiates aetiologies of left ventricular hypertrophy. Clin Radiol 74(2):140–149
Kittleson MM, Maurer MS, Ambardekar AV, Bullock-Palmer RP, Chang PP, Eisen HJ et al (2020) Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation [Internet]. 2020 Jul 7 [cited 2021 Nov 6];142(1). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000792
Dorbala S, Cuddy S, Falk RH (2020) How to Image Cardiac Amyloidosis. J Am Cardiol Img 13(6):1368–83
Yilmaz A, Bauersachs J, Bengel F, Büchel R, Kindermann I, Klingel K et al (2021) Diagnosis and treatment of cardiac amyloidosis: position statement of the German Cardiac Society (DGK). Clin Res Cardiol 110(4):479–506
Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A et al (2021) Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 42(16):1554–1568
Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM et al (2015) Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 132(16):1570–1579
Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS et al (2017) Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol 70(4):466–477
Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD et al (2010) Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 3(2):155–164
Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK et al (2020) Native T1 Mapping in Transthyretin Amyloidosis. J Am Cardiol Img (2):157–65
Baggiano A, Boldrini M, Martinez-Naharro A, Kotecha T, Petrie A, Rezk T et al (2020) Noncontrast Magnetic Resonance for the Diagnosis of Cardiac Amyloidosis. J Am Cardiol Img 13(1 Pt 1):69–80
Pan JA, Kerwin MJ, Salerno M (2020) Native T1 Mapping, Extracellular Volume Mapping, and Late Gadolinium Enhancement in Cardiac Amyloidosis. J Am Cardiol Img 13(6):1299–310
Vogelsberg H, Mahrholdt H, Deluigi CC, Yilmaz A, Kispert EM, Greulich S et al (2008) Cardiovascular Magnetic Resonance in Clinically Suspected Cardiac Amyloidosis. J Am Coll Cardiol 51(10):1022–1030
Martinez-Naharro A, Abdel-Gadir A, Treibel TA, Zumbo G, Knight DS, Rosmini S et al (2018) CMR-Verified Regression of Cardiac AL Amyloid After Chemotherapy. J Am Cardiol Img 11(1):152–4
Fontana M, Martinez-Naharro A, Chacko L, Rowczenio D, Gilbertson JA, Whelan CJ et al (2021) Reduction in CMR Derived Extracellular Volume With Patisiran Indicates Cardiac Amyloid Regression. J Am Cardiol Img 14(1):189–99
Kotecha T, Martinez-Naharro A, Treibel TA, Francis R, Nordin S, Abdel-Gadir A et al (2018) Myocardial Edema and Prognosis in Amyloidosis. J Am Coll Cardiol 71(25):2919–2931
Chacko L, Kotecha T, Martinez-Naharro A, Brown J, Knight D, Steriotis A et al (2019) 1171Myocardial perfusion mapping in cardiac amyloidosis - exploring the spectrum from infiltration to ischaemia. European Heart J 40(Supplement_1):ehz748.0013
Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC et al (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98(19):1442–1448
Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M et al (2014) Left Ventricular Structure and Function in Transthyretin-Related Versus Light-Chain Cardiac Amyloidosis. Circulation 129(18):1840–1849
Mohty D, Boulogne C, Magne J, Varroud-Vial N, Martin S, Ettaif H et al (2016) Prognostic value of left atrial function in systemic light-chain amyloidosis: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging 17(9):961–969
Nochioka K, Quarta CC, Claggett B, Roca GQ, Rapezzi C, Falk RH et al (2017) Left atrial structure and function in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 18(10):1128–1137
Di Toro A, Favalli V, Arbustini E (2018) Anderson-Fabry disease. J Cardiovasc Med 19:e1-5
Kozor R, Grieve SM, Tchan MC, Callaghan F, Hamilton-Craig C, Denaro C et al (2016) Cardiac involvement in genotype-positive Fabry disease patients assessed by cardiovascular MR. Heart 102(4):298–302
Moon J (2003) Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J 24(23):2151–2155
Niemann M, Herrmann S, Hu K, Breunig F, Strotmann J, Beer M et al (2011) Differences in Fabry Cardiomyopathy Between Female and Male Patients. J Am Cardiol Img 4(6):592–601
Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G et al (2013) Identification and Assessment of Anderson-Fabry Disease by Cardiovascular Magnetic Resonance Noncontrast Myocardial T1 Mapping. Circ Cardiovasc Imaging 6(3):392–398
Perry R, Shah R, Saiedi M, Patil S, Ganesan A, Linhart A et al (2019) The Role of Cardiac Imaging in the Diagnosis and Management of Anderson-Fabry Disease. JACC Cardiovasc Imaging 12(7 Pt 1):1230–1242
Nordin S, Kozor R, Bulluck H, Castelletti S, Rosmini S, Abdel-Gadir A et al (2016) Cardiac Fabry Disease With Late Gadolinium Enhancement Is a Chronic Inflammatory Cardiomyopathy. J Am Coll Cardiol 68(15):1707–1708
Augusto JB, Nordin S, Vijapurapu R, Baig S, Bulluck H, Castelletti S et al (2020) Myocardial Edema, Myocyte Injury, and Disease Severity in Fabry Disease. Circ: Cardiovasc Imaging [Internet]. 2020 Mar [cited 2021 Nov 29];13(3). Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.119.010171
Azevedo O, Cordeiro F, Gago MF, Miltenberger-Miltenyi G, Ferreira C, Sousa N et al (2021) Fabry Disease and the Heart: A Comprehensive Review. IJMS 22(9):4434
Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH et al (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22(23):2171–2179
Sado DM, Maestrini V, Piechnik SK, Banypersad SM, White SK, Flett AS et al (2015) Noncontrast myocardial T 1 mapping using cardiovascular magnetic resonance for iron overload: Myocardial T 1 in Iron Overload. J Magn Reson Imaging 41(6):1505–1511
Wei X, Zhao L, Xie J, Liu Y, Du Z, Zhong X et al (2021) Cardiac Phenotype Characterization at MRI in Patients with Danon Disease: A Retrospective Multicenter Case Series. Radiology 299(2):303–310
Towbin JA, Lorts A, Jefferies JL (2015) Left ventricular non-compaction cardiomyopathy. The Lancet 386(9995):813–825
Arbustini E, Favalli V, Narula N, Serio A, Grasso M (2016) Left Ventricular Noncompaction: A Distinct Genetic Cardiomyopathy? J Am Coll Cardiol 68(9):949–966
van Waning JI, Caliskan K, Hoedemaekers YM, van Spaendonck-Zwarts KY, Baas AF, Boekholdt SM et al (2018) Genetics, Clinical Features, and Long-Term Outcome of Noncompaction Cardiomyopathy. J Am Coll Cardiol 71(7):711–722
Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH et al (2005) Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 46(1):101–105
Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert J-Y et al (2010) Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J 31(9):1098–1104
Weir-McCall JR, Yeap PM, Papagiorcopulo C, Fitzgerald K, Gandy SJ, Lambert M et al (2016) Left Ventricular Noncompaction. J Am Coll Cardiol 68(20):2157–2165
Zemrak F, Ahlman MA, Captur G, Mohiddin SA, Kawel-Boehm N, Prince MR et al (2014) The relationship of left ventricular trabeculation to ventricular function and structure over a 9.5-year follow-up: the MESA study. J Am Coll Cardiol 64(19):1971–80
Grigoratos C, Barison A, Ivanov A, Andreini D, Amzulescu M-S, Mazurkiewicz L et al (2019) Meta-Analysis of the Prognostic Role of Late Gadolinium Enhancement and Global Systolic Impairment in Left Ventricular Noncompaction. J Am Cardiol Img 12(11):2141–51
Casas G, Limeres J, Oristrell G, Gutierrez-Garcia L, Andreini D, Borregan M et al (2021) Clinical Risk Prediction in Patients With Left Ventricular Myocardial Noncompaction. J Am Coll Cardiol 78(7):643–662
Guaricci AI, Masci PG, Muscogiuri G, Guglielmo M, Baggiano A, Fusini L et al (2021) CarDiac magnEtic Resonance for prophylactic Implantable-cardioVerter defibrillAtor ThErapy in Non-Ischaemic dilated CardioMyopathy: an international Registry. EP Europace 23(7):1072–1083
Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, Schwitter J et al (2016) Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction. J Am Coll Cardiol 68(20):2166–2181
Vidula MK, Bravo PE (2021) Multimodality imaging for the diagnosis of infiltrative cardiomyopathies. Heart heartjnl-2020–318001
Genovesi D, Vergaro G, Giorgetti A, Marzullo P, Scipioni M, Santarelli MF et al (2021) [18F]-Florbetaben PET/CT for Differential Diagnosis Among Cardiac Immunoglobulin Light Chain, Transthyretin Amyloidosis, and Mimicking Conditions. J Am Cardiol Img 14(1):246–55
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors: draft of the manuscript; Sinagra, Basso, Indolfi and Perrone Filardi: critical revision and final approval; Merlo, Barison and Autore: draft of the manuscript and final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Camillo Autore and Andrea Barison share the senior author position. Marco Merlo, Giulia Gagno, Anna Baritussio, Barbara Bauce, Elena Biagini, Alberto Cipriani, Giuseppe Limongelli, Maria Beatrice Musumeci, Vanda Parisi, Silvia Pica, Cristina Basso, and Gianfranco Sinagra are members of the European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Merlo, M., Gagno, G., Baritussio, A. et al. Clinical application of CMR in cardiomyopathies: evolving concepts and techniques. Heart Fail Rev 28, 77–95 (2023). https://doi.org/10.1007/s10741-022-10235-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10235-9