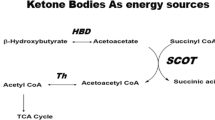
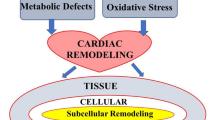
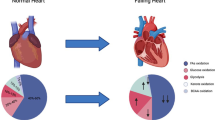
Abstract
Heart failure (HF) often coexists with insulin resistance (IR), and the incidence of HF in type 2 diabetes mellitus (T2DM) patients is significantly higher. The reciprocal relationship between HF and IR has long been recognized, and the integration complicates the therapy of both. A number of mechanisms ascribe to the progression of cardiac IR, in which the main factors are the shift of myocardial substrate metabolism. Studies have found that SGLT2 inhibitors, an anti-diabetic drug, can improve the cardiac prognosis of patients with T2DM, which may be at least partially due to the relief of cardiac IR. Basic and clinical studies have revealed the important role of cardiac IR in the pathogenesis and progression of HF, and studies suggest that energy metabolism plays an important role in the pathogenesis of cardiac IR and HF. SGLT2 inhibitors mediated cardiovascular benefits through various mechanisms such as improving substrate utilization and improving myocardial energy. The regulation of SGLT2 inhibitors on cardiac energy status including carbohydrates, fatty acids (FA), amino acids and ketones, ATP transfer to the cytoplasm, and mitochondrial functional status have received extensive attention in HF, but its specific mechanism of action is still unclear. Therefore, this article reviews the relationship between IR and HF from the perspective of energy metabolism; subsequently, targeting energy metabolism discusses the pivotal role of SGLT2 inhibitors in improving cardiac IR and HF based on basic and clinical research evidences, and sought to clarify the molecular mechanism involved. (Fig. 1).




Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl CoA carboxylase
- ADA:
-
The American Diabetes Association
- AMPK:
-
AMP-activated protein kinase
- BCATm:
-
Branched chain aminotransferase in mitochondrial
- BCKDH:
-
Branched chain ketoacid dehydrogenase
- BMPR2:
-
Bone morphogenetic protein receptor type 2
- BDH1:
-
β-Hydroxybutyrate dehydrogenase 1
- βOHB:
-
β-Hydroxybutyrate
- CPT1/2:
-
Carnitine palmitoyltransferase 1/2
- DPP-4 inhibitors:
-
Dipeptidyl peptidase-4 inhibitors
- EASD:
-
The European Association for the Study of Diabetes
- ESC:
-
The European Society of Cardiology
- FA:
-
Fatty acid
- G-6-P:
-
Glucose-6-phosphate
- GLP-1:
-
Glucagon-like peptide-1
- MACE:
-
Major adverse cardiovascular events
- MCD:
-
Malonyl-CoA decarboxylase
- mTOR:
-
Mechanistic target of rapamycin
- MCT:
-
Monocarborxylat transporter
- MPC:
-
Mitochondrial pyruvate carrier
- NT-proBNP:
-
Amino-terminal pro-B-type natriuretic peptide
- PDH:
-
Pyruvate dehydrogenase
- SBP:
-
Systolic blood pressure
- SCOT:
-
Succinyl-CoA transferase
- SNS:
-
Sympathetic nervous system
References
Horwich TB, Fonarow GC (2010) Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol 55(4):283–293. https://doi.org/10.1016/j.jacc.2009.07.029
Nikolaidis LA, Levine TB (2004) Peroxisome proliferator activator receptors (PPAR), insulin resistance, and cardiomyopathy. Cardiol Rev 12(3):158–170. https://doi.org/10.1097/01.crd.0000102419.52594.90
Boonman-de Winter L J et al (2012) High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 55(8):2154–62. https://doi.org/10.1111/1753-0407.12645
Riehle C, Abel ED (2016) Insulin signaling and heart failure. Circ Res 118(7):1151–1169. https://doi.org/10.1161/circresaha.116.306206
Bertoni AG et al (2004) Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 27(3):699–703. https://doi.org/10.2337/diacare.27.3.699
Cosmi F et al (2018) Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail 20(5):888–895. https://doi.org/10.1002/ejhf.1146
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34(1):29–34. https://doi.org/10.1016/0002-9149(74)90089-7
Fox CS, Coady S, Sorlie PD, D’Agostino RB Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ (2007) Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 115(12):1544–1550. https://doi.org/10.1161/CIRCULATIONAHA.106.658948
Chilton R et al (2015) Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 17(12):1180–1193. https://doi.org/10.1111/dom.12572
Kaplan A, Abidi E, El-Yazbi A, Eid A, Booz GW, Zouein FA (2018) Direct cardiovascular impact of SGLT2 inhibitors: mechanisms and effects. Heart Fail Rev 23(3):419–437. https://doi.org/10.1007/s10741-017-9665-9
Zinman B et al (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373(22):2117–2128. https://doi.org/10.1056/NEJMoa1504720
Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP (2016) The failing heart relies on ketone bodies as a fuel. Circulation 133(8):698–705. https://doi.org/10.1161/CIRCULATIONAHA.115.017355
Ferrannini E, Mark M, Mayoux E (2016) CV protection in the EMPA-REG OUTCOME Trial: a “thrifty substrate” hypothesis. Diabetes Care 39(7):1108–1114. https://doi.org/10.2337/db15-1356
Mizuno Y et al (2017) The diabetic heart utilizes ketone bodies as an energy source. Metabolism 77:65–72. https://doi.org/10.1016/j.metabol.2017.08.005
Benjamin EJ et al (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 136(10):e196. https://doi.org/10.1161/cir.0000000000000530
Roth GA, Nguyen G, Forouzanfar MH, Mokdad AH, Naghavi M, Murray CJ (2015) Estimates of global and regional premature cardiovascular mortality in 2025. Circulation 132(13):1270–1282. https://doi.org/10.1161/circulationaha.115.016021
Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63(12):1123–1133. https://doi.org/10.1016/j.jacc.2013.11.053
Tanaka H (2019) Utility of strain imaging in conjunction with heart failure stage classification for heart failure patient management. J Echocardiogr 17(1):17–24. https://doi.org/10.1007/s12574-018-0408-2
Farre N, Vela E, Cleries M, Bustins M, Cainzos-Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdu-Rotellar JM, Comin-Colet J (2017) Real world heart failure epidemiology and outcome: a population-based analysis of 88,195 patients. PLoS ONE 12(2):e0172745. https://doi.org/10.1371/journal.pone.0172745
Chen YT, Wong, LL, Liew OW, Richards AM (2019) Heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF): the diagnostic value of circulating MicroRNAs. Cells 8(12). https://doi.org/10.3390/cells8121651
Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R, Komuro I, Kobayashi Y, Minamino T (2012) p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15(1):51–64. https://doi.org/10.1016/j.cmet.2011.12.006
Karwi QG, Uddin GM, Ho KL, Lopaschuk GD (2018) Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 5:68. https://doi.org/10.3389/fcvm.2018.00068
Goldberg IJ, Trent CM, Schulze PC (2012) Lipid metabolism and toxicity in the heart. Cell Metab 15(6):805–812. https://doi.org/10.1016/j.cmet.2012.04.006
Birkenfeld AL, Jordan J, Dworak M, Merkel T, Burnstock G (2019) Myocardial metabolism in heart failure: purinergic signalling and other metabolic concepts. Pharmacol Ther 194:132–144. https://doi.org/10.1016/j.pharmthera.2018.08.015
Quinaglia T, Oliveira DC, Matos-Souza JR, Sposito A (1992) C (2019) Diabetic cardiomyopathy: factual or factoid? Rev Assoc Med Bras 65(1):61–69. https://doi.org/10.1590/1806-9282.65.1.69
Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD (2013) Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail 6(5):1039–1048. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000228
Hemnes AR et al (2020) BMPR2 dysfunction impairs insulin signaling and glucose homeostasis in cardiomyocytes. Am J Physiol Lung Cell Mol Physiol 318(2):L429–L441. https://doi.org/10.1152/ajplung.00555.2018
Alrob OA et al (2014) Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res 103(4):485–497. https://doi.org/10.1093/cvr/cvu156
Sankaralingam S et al (2015) Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: implications for the obesity paradox. Diabetes 64(5):1643–1657. https://doi.org/10.2337/db14-1050
Hattori Y (2020) Insulin resistance and heart failure during treatment with sodium glucose cotransporter 2 inhibitors: proposed role of ketone utilization. Heart Fail Rev 1(20). https://doi.org/10.1007/s10741-020-09921-3
Kolwicz SC Jr, Airhart S, Tian R (2016) Ketones step to the plate: a game changer for metabolic remodeling in heart failure? Circulation 133(8):689–691. https://doi.org/10.1161/CIRCULATIONAHA.116.021230
Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, Monzani V, Saripalli C, Mascagni P, Reece TB, Ambardekar AV, Granzier HL, Dinarello CA, McKinsey TA (2018) Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med 10(427). https://doi.org/10.1126/scitranslmed.aao0144
Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JR, Alberta H (2015) Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 10(5):e0124844. https://doi.org/10.1371/journal.pone.0124844
Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi Jr, KC, Margulies KB, Coon JJ, Kelly P (2016) Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2 JCI Insight 2(1). https://doi.org/10.1172/jci.insight.84897
Karlstaedt A et al (2016) Oncometabolite d-2-hydroxyglutarate impairs alpha-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A 113(37):10436–10441. https://doi.org/10.1073/pnas.1601650113
Taegtmeyer H (2016) Failing heart and starving brain: ketone bodies to the rescue. Circulation 134(4):265–266. https://doi.org/10.1161/CIRCULATIONAHA.116.022141
Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, Deberardinis R, Cox JE, Kfoury AG, Selzman CH, Stehlik J, Fang JC, Li DY, Drakos SG (2016) Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: implications for cardiac reloading and conditioning. JACC Basic Transl Sci 1(6):432–444. https://doi.org/10.1016/j.jacbts.2016.06.009
Noordali H, Loudon BL, Frenneaux MP, Madhani M (2018) Cardiac metabolism—a promising therapeutic target for heart failure. Pharmacol Ther 182:95–114. https://doi.org/10.1016/j.pharmthera.2017.08.001
Le Page LM et al (2015) Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 64(8):2735–2743. https://doi.org/10.2337/db14-1560
Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY (2013) ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol 304(8):H1103–H1113. https://doi.org/10.1152/ajpheart.00636.2012
Bloomgarden Z (2018) Diabetes and branched-chain amino acids: what is the link? J Diabetes 10(5):350–352. https://doi.org/10.1111/1753-0407.12645
Matthews VB et al (2017) Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens 35(10):2059–2068. https://doi.org/10.1097/hjh.0000000000001434
Takata M, Amiya E, Watanabe M, Hosoya Y, Nakayama A, Fujiwara T, Taya M, Oguri G, Hyodo K, Takayama N, Takano N, Mashiko T, Uemura Y, Komuro I (2017) An exploratory study on the efficacy and safety of a BCAA preparation used in combination with cardiac rehabilitation for patients with chronic heart failure. BMC Cardiovasc Disord 17(1):205. https://doi.org/10.1186/s12872-017-0639-6
Wende AR, Brahma MK, McGinnis GR, Young ME (2017) Metabolic origins of heart failure. JACC Basic Transl Sci 2(3):297–310. https://doi.org/10.1016/j.jacbts.2016.11.009
Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui WJ, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y (2016) Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133(21):2038–2049. https://doi.org/10.1161/CIRCULATIONAHA.115.020226
Verma S (2019) Potential mechanisms of sodium-glucose co-transporter 2 inhibitor-related cardiovascular benefits. Am J Cardiol 124(Suppl 1):S36-s44. https://doi.org/10.1016/j.amjcard.2019.10.028
Santos-Gallego CG et al (2019) Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol 73(15):1931–1944. https://doi.org/10.1016/j.jacc.2019.01.056
Verma S et al (2018) Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci 3(5):575–587. https://doi.org/10.1016/j.jacbts.2018.07.006
Durak A, Olgar Y, Degirmenci S, Akkus E, Tuncay E, Turan B (2018) A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular-repolarization through augmentation of mitochondrial function in insulin-resistant metabolic syndrome rats. Cardiovasc Diabetol 17(1):144. https://doi.org/10.1186/s12933-018-0790-0
Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T (2019) Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol 18(1):165. https://doi.org/10.1186/s12933-019-0964-4
Adingupu DD, Göpel SO, Grönros J, Behrendt M, Sotak M, Miliotis T, Dahlqvist U, Gan LM, Jönsson-Rylander AC (2019) SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob(-/-) mice. Cardiovasc Diabetol 18(1):16. https://doi.org/10.1186/s12933-019-0820-6
Maejima Y (2019) SGLT2 inhibitors play a salutary role in heart failure via modulation of the mitochondrial function. Front Cardiovasc Med 6(2019):186. https://doi.org/10.3389/fcvm.2019.00186
Bertero E, Prates Roma L, Ameri P, Maack C (2018) Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovasc Res 114(1):12–18. https://doi.org/10.1093/cvr/cvx149
Jun S, Aon MA, Paolocci N (2019) Empagliflozin and HFrEF: known and possible benefits of NHE1 inhibition. JACC Basic Transl Sci 4(7):841–844. https://doi.org/10.1016/j.jacbts.2019.10.005
Oshima H, Miki T, Kuno A, Mizuno M, Sato T, Tanno M, Yano T, Nakata K, Kimura Y, Abe K, Ohwada W, Miura T (2019) Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther 368, no. 3 (Mar 2019): 524–34. https://doi.org/10.1124/jpet.118.253666
Shintani H, Shintani T, Ashida, H, Sato M (2018) Calorie restriction mimetics: upstream-type compounds for modulating glucose metabolism. Nutrients 10, no. 12 (Nov 22 2018). https://doi.org/10.3390/nu10121821
Koike Y, Shirabe SI, Maeda H, Yoshimoto A, Arai K, Kumakura A, Hirao K, Terauchi Y (2019) Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 149:140–146. https://doi.org/10.1016/j.diabres.2019.01.029
Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E (2015) Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 38(9):1730–1735. https://doi.org/10.2337/dc15-0355
Lundkvist P, Pereira MJ, Kamble PG, Katsogiannos P, Langkilde AM, Esterline R, Johnsson E, Eriksson JW (2019) Glucagon levels during short-term SGLT2 inhibition are largely regulated by glucose changes in patients with type 2 diabetes. J Clin Endocrinol Metab 104(1):193–201. https://doi.org/10.1210/jc.2018-00969
Iuchi H, Sakamoto M, Matsutani D, Suzuki H, Kayama Y, Takeda N, Minamisawa S, Utsunomiya K. Time-dependent effects of ipragliflozin on behaviour and energy homeostasis in normal and type 2 diabetic rats: continuous glucose telemetry analysis. Sci Rep 7(1):11906. https://doi.org/10.1038/s41598-017-12106-y
Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 8(4):416–27. https://doi.org/10.1111/jdi.12644
Joubert M, Jagu B, Montaigne D, Marechal X, Tesse A, Ayer A, Dollet L, Le May C, Toumaniantz G, Manrique A, Charpentier F, Staels B, Magré J, Cariou B, Prieur X (2017) The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes 66(4):1030–1040. https://doi.org/10.2337/db16-0733
Yoshii A, Nagoshi T, Kashiwagi Y, Kimura H, Tanaka Y, Oi Y, Ito K, Yoshino T, Tanaka TD, Yoshimura M (2019) Cardiac ischemia-reperfusion injury under insulin-resistant conditions: SGLT1 but not SGLT2 plays a compensatory protective role in diet-induced obesity. Cardiovasc Diabetol 18(1):85. https://doi.org/10.1186/s12933-019-0889-y
Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA (2014) Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124(2):509–514. https://doi.org/10.1172/JCI70704
Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B, Pattou F (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21(5):512–517. https://doi.org/10.1038/nm.3828
Xu L et al (2017) SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine 20:137–149. https://doi.org/10.1016/j.ebiom.2017.05.028
Komiya C et al (2016) Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS ONE 11(3):e0151511. https://doi.org/10.1371/journal.pone.0151511
Nakatsu Y, Kokubo H, .Bumdelger B, Yoshizumi M, Yamamotoya T, Matsunaga Y, Ueda K, Inoue Y, Inoue MK, Fujishiro M, Kushiyama A, Ono H, Sakoda H, Asano T (2017) The SGLT2 inhibitor luseogliflozin rapidly normalizes aortic mRNA levels of inflammation-related but not lipid-metabolism-related genes and suppresses atherosclerosis in diabetic ApoE KO mice. Int J Mol Sci 18(8). https://doi.org/10.3390/ijms18081704
Lopaschuk GD, Verma S (2020) Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 5(6):632–644. https://doi.org/10.1016/j.jacbts.2020.02.004
O’Brien TP, Jenkins EC, Estes SK, Castaneda AV, Ueta K, Farmer TD, Puglisi AE, Swift LL, Printz RL, Shiota M (2017) Correcting postprandial hyperglycemia in Zucker diabetic fatty rats with an SGLT2 inhibitor restores glucose effectiveness in the liver and reduces insulin resistance in skeletal muscle. Diabetes 66(5):1172–1184. https://doi.org/10.2337/db16-1410
Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ (2014) Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124(2):499–508. https://doi.org/10.1172/jci72227
Kalra S, Gupta Y (2016) The insulin: glucagon ratio and the choice of glucose-lowering. Diabetes Ther 7(1):1–9. https://doi.org/10.1007/s13300-016-0160-4
Yabe D, Iwasaki M, Kuwata H, Haraguchi T, Hamamoto Y, Kurose T, Sumita K, Yamazato H, Kanada S, Seino Y (2017) Sodium-glucose co-transporter-2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: a randomized, open-label, 3-arm parallel comparative, exploratory study. Diabetes Obes Metab 19(5):739–743. https://doi.org/10.1111/dom.12848
Vallon V, Thomson SC (2017) Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60(2):215–225. https://doi.org/10.1007/s00125-016-4157-3
Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. (2017) Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol 16, no. 1 (Mar 3 2017):32. https://doi.org/10.1186/s12933-017-0516-8
Takasu T, Hayashizaki Y, Hirosumi J, Minoura H, Amino N, Kurosaki E, Takakura S (2017) The sodium glucose cotransporter 2 inhibitor ipragliflozin promotes preferential loss of fat mass in non-obese diabetic Goto-Kakizaki rats. Biol Pharm Bull 40(5):675–680. https://doi.org/10.1248/bpb.b16-00964
Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, Haring HU, Stefan N, Fritsche AF (2019) Artunc Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol 18, no. 1 (Apr 5 2019):46. https://doi.org/10.1186/s12933-019-0852-y
Riggs K, Ali H, Taegtmeyer H, Gutierrez AD (2015) The use of SGLT-2 inhibitors in type 2 diabetes and heart failure. Metab Syndr Relat Disord 13(7):292–297. https://doi.org/10.1089/met.2015.0038
Garcia-Ropero A, Vargas-Delgado AP, Santos-Gallego CG, Badimon JJ (2019) Inhibition of sodium glucose cotransporters improves cardiac performance. Int J Mol Sci 20(13). https://doi.org/10.3390/ijms20133289
Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E (2016) Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 65(5):1190–1195. https://doi.org/10.2337/db15-1356
Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, Rim JH, Hwang I, Lee CJ, Lee M, Oh CM, Jeon JY, Gee HY, Kim JH, Lee BW, Kang ES, Cha BS, Lee MS, Yu JW, Cho JW, Kim JS, Lee YH (2020) SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 11(1):2127. https://doi.org/10.1038/s41467-020-15983-6
Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q (2004) Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 10(11):1245–1250. https://doi.org/10.1038/nm1116
Bjorkegren J, Veniant M, Kim SK, Withycombe SK, Wood PA, Hellerstein MK, Neese RA, Young SG (2001) Lipoprotein secretion and triglyceride stores in the heart. J Biol Chem 276(42):38511–38517. https://doi.org/10.1074/jbc.M106839200
Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, . Dreyfuss JM, Pan H, Tangcharoenpaisan Y, Morningstar J, Gerszten R, Patti ME (2019) SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 4(5). https://doi.org/10.1172/jci.insight.123130
Day EA, Ford RJ, Lu JH, Lu R, Lundenberg L, Desjardins EM, Green AE, Lally JSV, Schertzer JD, Steinberg GR (2020) The SGLT2 inhibitor canagliflozin suppresses lipid synthesis and interleukin-1 beta in ApoE deficient mice. Biochem J 477(12):2347–2361. https://doi.org/10.1042/bcj20200278
Sasaki T, Sugawara M, Fukuda M (2019) Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig 10(1):108–117. https://doi.org/10.1111/jdi.12851
Baker HE, Kiel AM, Luebbe ST, Simon BR, Earl CC, Regmi A, Roell WC, Mather KJ, Tune JD, Goodwill AG (2019) Inhibition of sodium-glucose cotransporter-2 preserves cardiac function during regional myocardial ischemia independent of alterations in myocardial substrate utilization. Basic Res Cardiol 114(3):25. https://doi.org/10.1007/s00395-019-0733-2
Abdurrachim D, Manders E, Nicolay K, Mayoux E, Prompers JJ (2018) Single dose of empagliflozin increases in vivo cardiac energy status in diabetic db/db mice. Cardiovasc Res 114(14):1843–1844. https://doi.org/10.1093/cvr/cvy246
Martinez-Moreno J, Fontecha-Barriuso MM, Martin-Sanchez D, Guerrero-Mauvecin J, Goma-Garces E, Fernandez-Fernandez B, Carriazo S, Sanchez-Niño MD, Ramos AM, Ruiz-Ortega M, Ortiz A, Sanz AB (2020) Epigenetic modifiers as potential therapeutic targets in diabetic kidney disease. Int J Mol Sci 21(11). https://doi.org/10.3390/ijms21114113
White PJ, Newgard CB (2019) Branched-chain amino acids in disease. Science 363(6427):582–583. https://doi.org/10.1126/science.aav0558
Sivanand S, Vander Heiden MG (2020) Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell 37(2):147–156. https://doi.org/10.1016/j.ccell.2019.12.011
Kappel BA, Lehrke M, Schütt K, Artati A, Adamski J, Lebherz C, Marx N (2017) Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation 136(10):969–972. https://doi.org/10.1161/circulationaha.117.029166
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166):31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Marton A, Kaneko T, Kovalik JP, Yasui A, Nishiyama A, Kitada K et al (2021) Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol 17(1):65–77. https://doi.org/10.1038/s41581-020-00350-x
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373(22):2117–2128. https://doi.org/10.1056/NEJMoa1504720
Neal B, Perkovic V, Matthews DR (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377(21):2099. https://doi.org/10.1056/NEJMc1712572
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM et al (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380(24):2295–2306. https://doi.org/10.1056/NEJMoa1811744
Marx N, Davies MJ, Grant PJ, Mathieu C, Petrie JR, Cosentino F et al (2021) Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol 9(1):46–52. https://doi.org/10.1016/s2213-8587(20)30343-0
Serenelli M, Böhm M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA et al (2020) Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J 41(36):3402–3418. https://doi.org/10.1093/eurheartj/ehaa496
Docherty KF, Jhund PS, Anand I, Bengtsson O, Böhm M, de Boer RA et al (2020) Effect of Dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA-HF. Circulation 142(17):1623–1632. https://doi.org/10.1161/circulationaha.120.047480
Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL Jr, Kalyani RR et al (2020) 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 76(9):1117–1145. https://doi.org/10.1016/j.jacc.2020.05.037
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A et al (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380(4):347–357. https://doi.org/10.1056/NEJMoa1812389
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U et al (2020) Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 383(15):1425–1435. https://doi.org/10.1056/NEJMoa2004967
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK et al (2021) Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 384(2):117–128. https://doi.org/10.1056/NEJMoa2030183
Filion KB, Lix LM, Yu OH, Dell’Aniello S, Douros A, Shah BR et al (2020) Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: multi-database retrospective cohort study. BMJ 370:m3342. https://doi.org/10.1136/bmj.m3342
Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q et al (2021) Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 372:m4573. https://doi.org/10.1136/bmj.m4573
Kohsaka S, Lam CSP, Kim DJ, Cavender MA, Norhammar A, Jørgensen ME et al (2020) Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol 8(7):606–615. https://doi.org/10.1016/s2213-8587(20)30130-3
Hiruma S, Shigiyama F, Hisatake S, Mizumura S, Shiraga N, Hori M et al (2021) A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: the ASSET study. Cardiovasc Diabetol 20(1):32. https://doi.org/10.1186/s12933-021-01228-3
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303
Cowie MR, Fisher M (2020) SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 17(12):761–772. https://doi.org/10.1038/s41569-020-0406-8
Vaduganathan M, Greene SJ, Zhang S, Grau-Sepulveda M, DeVore AD, Butler J et al (2020) Applicability of US Food and Drug Administration labeling for Dapagliflozin to patients with heart failure with reduced ejection fraction in US clinical practice: The Get With the Guidelines-Heart Failure (GWTG-HF) Registry. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.5864
Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ et al (2021) Effect of Empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-reduced trial. Circulation 143(4):326–336. https://doi.org/10.1161/circulationaha.120.051783
Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT et al (2021) Effect of Empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation 143(6):516–525. https://doi.org/10.1161/circulationaha.120.052186
Januzzi JL Jr, Ibrahim NE (2020) Understanding the mechanistic benefit of heart failure drugs matters. J Am Coll Cardiol 76(23):2752–2754. https://doi.org/10.1016/j.jacc.2020.10.026
Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, de Boer RA et al (2020) Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation 142(11):1040–1054. https://doi.org/10.1161/circulationaha.120.047077
Kang A, Jardine MJ (2021) SGLT2 inhibitors may offer benefit beyond diabetes. Nat Rev Nephrol 17(2):83–84. https://doi.org/10.1038/s41581-020-00391-2
Ferrannini E, Murthy AC, Lee YH, Muscelli E, Weiss S, Ostroff RM et al (2020) Mechanisms of sodium-glucose cotransporter 2 inhibition: insights from large-scale proteomics. Diabetes Care 43(9):2183–2189. https://doi.org/10.2337/dc20-0456
Douros A, Lix LM, Fralick M, Dell’Aniello S, Shah BR, Ronksley PE et al (2020) Sodium-glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis : a multicenter cohort study. Ann Intern Med 173(6):417–425. https://doi.org/10.7326/m20-0289
Acknowledgements
We kindly thank Mr. Guanwei Fan. for editing the manuscript.
Funding
This review was supported by grants from the National Key Subject of Drug Innovation (2019ZX09201005-007), the National Key R&D Program of China (2018YFC1704500), the National Natural Science Foundation of China (81,774,050), and Tianjin Science Foundation for Distinguished Young Scholars (17JCJQJC46200).
Author information
Authors and Affiliations
Contributions
Xiaodan Wang, Guanwei Fan, and Jingyu Ni—concept and original draft; Xiaodan Wang, Jingyu Ni, Rui Guo, and Lan Li—literature search and data extraction; Xiaodan Wang, Guanwei Fan, Jing Su, and Feng He—assessed quality of evidence; Xiaodan Wang, Jing Su, Feng He, and Guanwei Fan—spell check and grammar check; Xiaodan Wang, Guanwei Fan, Jingyu Ni, Rui Guo, Lan Li, Jing Su, and Feng He—writing and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Ni, J., Guo, R. et al. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail Rev 27, 961–980 (2022). https://doi.org/10.1007/s10741-021-10096-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10096-8