Abstract
Cardiac surgical techniques and circulatory supports have strongly evolved in the last years. Right ventricular (RV) function during the post-operatory period is still subject of study, although its relevant prognostic impact has been variably described in different papers. RV post-surgical dysfunction’s underlying mechanisms are still not clear and include a different hypothesis. Echocardiography, with both first and second level parameters, offers the possibility to accurately analyze the right ventricle and optimize these patients’ management. This paper describes the pathophysiology of the right ventricle, the most used echo indexes of RV function, whether they alter after surgery, the different supposed mechanisms of RV dysfunction and its role in the prognosis of patients undergoing cardiac surgery.
Similar content being viewed by others
Background: pathophysiology of the right ventricle
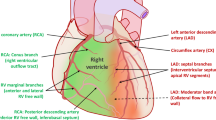
Not much time has passed since right ventricle (RV) was just considered an auxiliary of the left ventricle (LV), mainly consequently to old animal experiments in which ablation or replacement of RV free wall seemed to be quite well tolerated [1, 2]. Recently, RV assessment has gained great relevance in many cardiac diseases and in patients undergoing cardiac surgery, also thanks to the advanced imaging techniques that allow a better evaluation of its anatomy and function. RV has only about one-sixth of LV mass and roughly has the shape of triangular pyramid. In its thin walls, longitudinal myocardial fibers are more represented than circular ones. Surface-to-volume ratio is elevated, and end-diastolic and end-systolic volumes are higher than those of the LV. RV retrosternal position makes its evaluation complex with transthoracic echocardiography (TTE), so it is fundamental to complement different dimensional and functional indexes. Currently, there is no unanimous viewpoint whether RV is always altered during cardiac surgery or regarding which weight should be given to each index during a post-operative routine evaluation. The aim of the present paper is to analyze different characteristics of the main surgical techniques, to comment upon different hypothesis regarding the impact of cardiac surgery on RV function and to describe which parameters can be reduced and their prognostic impact.
Contractility, preload, and afterload
RV output depends on three components: ventricular contractility, preload, and afterload. RV contraction has many peculiarities. Systolic contractility is composed of two sequential phases: in the first, the inlet contracts while the outflow tract broadens, the tricuspid valve plane moves towards the apex, but no RV ejection takes place; in the second, the infundibulum contracts with subsequent RV ejection. In healthy subjects, right-sided pressures are lower than left-sided ones and, given the smaller afterload, RV pressure loop displays an early peaking and a rapid decrease. Differently from LV, RV isometric contraction is much shorter, as it quickly reaches the pressure threshold needed for the ejection. Moreover, RV ejection phase is quite longer than that of LV. RV preload is mostly affected by vascular volume status, heart rate, left-side filling pressure, intra-pericardial pressure, and RV ventricular compliance [3]. RV complies with Frank Starling law: in a healthy heart, an increased RV preload corresponds to improved myocardial contraction and ejection. However, after a compensative phase, persistent volume overload leads to dysfunctional RV filling and contractility, up to RV failure. On the other side, pulmonary vascular resistances are the main determinants of RV afterload. RV can tolerate an increased preload better than an increased afterload. For example, in massive pulmonary embolism, the thin RV walls are not able to adapt to the acute increase of afterload and go through chamber failure due to subendocardial ischemia and subsequent inflammation [4, 5]. On the contrary, a chronic afterload increase, as it occurs in pulmonary hypertension, is followed by RV free wall hypertrophy, the ventricle becomes more rounded-shaped, reducing wall stress in order to maintain an adequate output [6, 7]. However, with time, this compensative process leads to RV dilation and contractile dysfunction.
Ventricular interdependence
About 20–40% of RV stroke volume is dependent on LV contraction [8]. The so-called ventricular interdependence can be explained by the basic concept that size, shape, and compliance of one ventricle affect the hemodynamic properties of the other, both in systole and diastole [9]. This interdependence is due to different factors: the pericardium that embraces both ventricles, the shared blood supply, the continuity between RV myocardial fibers, LV muscular layers, and the interventricular septum (IVS). Moreover, LV stroke volume eventually corresponds to RV preload. Notably, diastolic ventricular interdependence is mainly due to the pericardium [10]. In the case of RV volume or pressure overload, RV dilates and intrapleural pressure increases determining a shift of the IVS towards the left. This phenomenon changes LV geometry and causes upward shifting of LV diastolic pressure curve, raise in LV pressure at end diastole, and reduction of LV stroke volume [9, 11]. Systolic interdependence is mainly mediated by IVS [12].
How to assess RV function by echocardiography: from TAPSE to strain
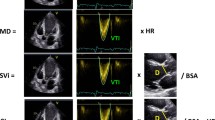
Advanced echocardiographic techniques can integrate standard parameters in order to obtain a complete and satisfying RV evaluation without the need of applying cardiac magnetic resonance (CMR). Table 1 shows normal cutoff values of RV function indexes. RV longitudinal shortening is the main contributor to its ejection and function. Tricuspid annular plane systolic excursion (TAPSE, Fig. 1), measurable by M-mode in apical four-chamber view, is extremely easy to obtain and has low interobserver variability [13, 16]. TAPSE is dependent on the angle of insonation and load conditions. Although being less sensible in presence of important cardiac dysfunction [17], it is the most employed RV parameter and has a key diagnostic and prognostic role in pulmonary hypertension and embolism [18,19,20,21,22,23] and in heart failure [24, 25]. Systolic velocity of the tricuspid annulus (s’, Fig. 2) by tissue Doppler imaging (TDI) in an alternative method for the evaluation of RV longitudinal function. S’ is quick and reproducible, but has the same limitations of TAPSE, it is load dependent and affected by cardiac translational motion as well as by tethering by adjacent diseased myocardial segments. It needs correction if the heart rate is < 70 bpm or > 100 bpm, by multiplying s’ by 75 and dividing it by the heart rate [26]. The main application of s’ is the assessment of RV function in pulmonary hypertension, also in the pediatric population [27]. RV fractional area change (RVFAC) is a quantitative method used to estimate RV systolic function (Fig. 3), which has demonstrated good correlation with RV EF assessed by CMR [28]. In an unselected cohort of more than 400 patients undergoing cardiac surgery [29], RVFAC was more feasible (98% vs 99.3%, respectively), but less reproducible than s’ (coefficients of variation 8.6% for intra-observer and 7.4% for interobserver reproducibility). However, s’ and RVFAC were concordantly impaired just in 39 patients, while they were discordant in 54 patients [29], showing low reliability if used as the only parameters of RV function in an echo report. The so-called RIMP (right ventricular index of myocardial performance) or Tei Index is an index of both systolic and diastolic function. To assess RIMP, isovolumic relaxation time (IVRT), isovolumic contraction time (IVCT), and ejection time intervals are measured by PW Doppler or TDI at lateral tricuspid annulus (the formula is (IVRT + IVCT)/ejection time). The higher the value, the worse the RV function [13]. RIMP is pre-load dependent, being IVRT reduced with elevated right atrial (RA) pressure. Three-dimensional (3D) echocardiography currently allows a better definition of the RV complex anatomy than 2DE, allowing the evaluation, from an apical four-chamber view, of the base, the apex, and the outflow tract. 3D feasibility is quite high during a routine echocardiography, with medium execution time and good quality of images even if it requires a different transducer [30, 31]. Moreover, it has been widely validated against CMR [32]. 3D RV ejection fraction (EF) is not a direct measure of contractile function, but an index of global ventricular performance, being the result of the interaction between RV contractility and load. Irregular heart rhythm, inadequate acoustic window, abnormal septal motion, and load dependency are the main limitations in addition to the poor availability and the complexity of the software. Speckle tracking echocardiography (STE) is another second level echocardiographic technique for an accurate assessment of global and regional cardiac function, independently of the angle of insonation and in-plane translational motion. Born for a selective evaluation of the LV, RV longitudinal strain (RVLS, Fig. 4) has shown good feasibility and reproducibility [15]. The average value of longitudinal deformation of the six segments of the RV represents global RVLS [14, 33]; however, free wall RVLS (Fig. 5) has emerged as an even more accurate parameter in detecting RV dysfunction in some clinical settings, including advanced heart failure [34]. In the absence of second level tools, the combined use of TAPSE, s’, and RVFAC is not just considerable as a basic surrogate but allows a good assessment of RV function in a short time and with high availability.
Global right ventricular longitudinal strain (RVLS). To perform the analysis, an apical four chambers with the complete inclusion of RV wall in the image must be acquired. The operator must accurately trace the endocardial border by a point-and-click approach, obtaining a region of interest composed of six segments (three at interventricular septum, three at free wall). The mean value of the longitudinal deformation of these six segments represents the global RVLS
Insights in cardiac surgery: most common surgical approaches, types of cardioplegia, and extracorporeal circulation
Median sternotomy with pericardiotomy has become the gold standard approach since 1957 [35], with low failure rates and excellent long-term outcomes [36]. Pericardial effusion and onset of supraventricular arrhythmias are the most common complications, with differences in size and location according to the type of surgery [37]. For most of the cardiac interventions, myocardial protection by cardioplegic solutions is needed, while systemic arterial perfusion and oxygenation are transferred to a heart-lung machine in an extracorporeal circulation (ECC). Crystalloid cardioplegic solutions are the most used and are historically divided into two types, intracellular-type and extracellular-type solutions. The types of cardioplegia differ also for delivery method and temperature. Retrograde cardioplegia is an established method of myocardial protection [38,39,40], based on the concept that distribution of an antegrade-delivered cardioplegia might be impaired in case of significant coronary stenosis or ventricular hypertrophy. There are some main concerns, though: the scarce distribution to RV and posterior IVS [41, 42], the extreme variability of cardiac venous system anatomy, and the insufficient capillary flow due to the large amount of cardioplegic solution delivered into the RV through the Thebesian system [43]. Currently, a combined approach is recommended to minimize the potential risk of post-ischemic myocardial damage. Performing surgery at 32–33 °C seems the best compromise to preserve heart and brain metabolism [44, 45]. In fact, tepid cardioplegia seems to overcome the limitations of both cold and warm types, reducing metabolic demands, and allowing an immediate recovery of cardiac function [46]. Cardiopulmonary bypass (CPB) represents the gold standard equipment for ECC in most of the cardiac surgical procedures. It provides optimal conditions (bloodless field and arrested heart) but the blood passage through a foreign, nonendothelial surface and subsequent reintroduction into the body, may lead to an inflammatory response that triggers a powerful thrombotic stimulus and production of vasoactive and cytotoxic substances. A prolonged duration of ECC is correlated to a higher risk of cardiac and non-cardiac damages. To overcome the limitations of standard ECC, a minimally invasive ECC (MECC) has been introduced in clinical practice, with minimized hemodilution and mechanical trauma. In the late 90s, another approach has made inroads in CABG surgery: the beating heart or “off-pump” procedure. As easily perceivable, cardioplegia is not performed, with no need for ECC. Even if patients need a more aggressive anticoagulant therapy in the post-operative period, a lower rate of transfusion or renal failure and improved outcomes after the surgery have been established also in frailty patients [47]. Moreover, off-pump surgery should be the first choice in case of calcified or diseased ascending aorta, where the ECC cannula might put the patient at high risk of dissection or stroke. New thoracic approaches have been developed to reduce the size of the incision. One of the most currently used approaches is the right anterior mini-thoracotomy for mitral valve surgery. Thanks to the limited heart manipulation and trauma, this is a valuable cost-effect strategy associated with reduced morbidity and mortality and has been established as the routine approach in many centers all over the world [48].
Mechanisms underlying right ventricular dysfunction after cardiac surgery: hypothesis and evidences
Different etiological hypotheses have been raised to explain RV dysfunction in the context of cardiac surgery (Fig. 6). Firstly, a suboptimal myocardial protection during surgery is included. Several studies have shown a better protection of the RV using warm rather than cold cardioplegia [49, 50], while differences between anterograde or retrograde administration in terms of post-operative dysfunction did not emerge [51]. Also, duration of ECC has a relevant impact on RV function. A retrospective study conducted by Schuuring et al. on more than 400 patients undergoing cardiac surgery for congenital heart disease revealed that a CPB time over 150 min was a strong determinant of clinical RV failure regardless of the site of surgery [52]. The most accredited theory beyond this relationship would lie in the role of cytokines, mostly endothelin-1, that induce a vasoconstrictive effect on pulmonary arterioles with consequent increase in pulmonary pressures and RV afterload [53]. The adherences following pericardiotomy may impair RV filling and lead to its dysfunction [54]. Coronary embolism or graft acute occlusions can represent other risk factors for RV functional alteration [55]. In particular, if the right coronary artery (RCA) is stenotic, a reduced perfusion during surgery might reduce RV contractility. In a small cohort of patients with different degrees of RCA stenosis referring for CABG, RV volumes increased and RV ejection fraction decreased in the group undergoing only circumflex and left anterior descending arteries revascularization (because the RCA stenosis was considered not significant), but not in those undergoing RCA revascularization [56]. Moreover, the development of ventilatory problems or of acute respiratory distress syndrome (ARDS) during the perioperative respiratory assistance adversely affects RV function. Incidence of ARDS in cardiac surgery patients is estimated around 10% [57]. The type of ventilation is crucial in causing these alterations: the use of high tidal volumes elicits compression on alveolar vessels that raises pulmonary vascular resistance and RV afterload [58] and stretch of alveolar walls, responsible for a ventilator-induced lung injury [59], leading to subsequent RV dysfunction. Finally, the hypothesis of volume overload as a possible mechanism of post-operative RV dysfunction has been proposed. Guinot et al. [60] found that RV dysfunction foreruns renal dysfunction in the early post-operative period, and this was correlated with dilated inferior vena cava and increased central venous pressure, but not with an improved cardiac index, suggesting congestion as the responsible.
Changes in RV function after cardiac surgery: which parameters alter?
Several studies addressing the echocardiographic assessment of RV function after cardiac surgery evaluated the abovementioned parameters. TAPSE and s’ appear to be reduced in subjects undergoing surgery for both congenital and acquired heart diseases [61,62,63]. However, when we consider global RV function, results are mostly variable. In 40 patients evaluated at a 3-month follow-up after mitral valve surgery, Tamborini et al. [64] found that TAPSE and s’ were significantly reduced (25.3 + 4 vs 15.5 + 3 mm and 17.8 + 4 vs 11.9 + 2 cm/s, respectively, all p < 0.0001). On the contrary, 3D echocardiography revealed no differences in RV dimensions and, above all, RVEF did not change accordingly. Interestingly, in this study, at 6- and 12-month follow-up, both TAPSE and s’ returned to normal values. 3D assessment of RV function with TAPSE and s’ in this setting of patients is recommended, when possible, also by Lang et al. [13]. Data regarding RVFAC are still conflicting [65,66,67], while RIMP emerges to improve after surgery [67, 68]. To prove that the main culprit of the loss of RV longitudinal function is the pericardial opening, in 2010, Unsworth and colleagues [69] decided to perform not only transthoracic echocardiography with TAPSE analysis before and after CABG, but also transesophageal echocardiography during surgery, with repetitive measurement of s’ from the onset of general anesthesia to the end of the operation. Compared with preoperative values, s’ was reduced by 44 ± 17% (p < 0.0001) in the first 3 min after pericardium opening, by 54 ± 11% (p < 0.0001) after 5 min and by 61 ± 11% (p < 0.0001) at the time of skin suture, independently of “on-pump” or “off-pump” procedure and cardioplegia. To confirm the hypothesis, successive studies compared parameters of RV longitudinal function in standard sternotomy with anterior pericardiotomy vs mini-thoracotomy with lateral smaller pericardial opening (in which, anyway, CPB and cross-clamp times are quite longer) [66, 70]. TAPSE was lower after surgery but with a systematic greater reduction in the full sternotomy group, again with no changes in 3D RVEF. In 42 patients undergoing cardiac surgery for mitral valve repair, RVLS was evaluated, in addition to the other indexes. With good reproducibility and interobserver agreement, at 6-month follow-up, both free wall RVLS and septal RVLS values were lower than pre-surgical ones (− 26.5% vs − 18% and − 20.8% vs 14.1%, respectively) in accordance with TAPSE and s’ reduction [65]. Analogously, RVLS decreased after correction of congenital heart defects in infants compared with baseline (− 10.7% vs − 19.7%, p < 0.0001) with good correlation between strain and z-score of TAPSE and s’ [71].
Impact of RV dysfunction on prognosis of patients after cardiac surgery
Table 2 lists some of the main papers regarding the impact of RV dysfunction on the short- and long-term prognosis of patients undergoing cardiac surgery. Reduced RV longitudinal or global function is associated with a higher risk of events, mainly of cardiovascular death. In the early post-operative period, incidence of RV dysfunction in patients with hemodynamic compromise was around 50% [77] and a RVFAC below 35% was associated with a high mortality rate [72, 73]. In a population of 50 patients with heart valve disease treated with surgery, both altered RVFAC and RIMP were able to predict a poor long-term outcome, improving risk stratification better than LV function. The composite endpoint included cardiovascular death and circulatory failure and a worse RV function was correlated with the duration of intensive care unit and in-hospital stay [74]. A value of TAPSE < 16 mm predicted 5-year and 8-year mortality rate after ventricular surgical reconstruction in patients with heart failure. Moreover, in the perioperative period, RV dysfunction was associated with higher incidence of low-output syndrome, inotropic support, and intra-aortic balloon pump insertion [75]. The importance of RV longitudinal deformation emerged mostly in the correlation between preoperative RVLS and outcome. In a large cohort of patients referring to different heart diseases, a RVFAC < 35% was associated with the greatest risk of post-operative mortality. In the case of preserved RV global function, mortality rate of patients with a RVLS > − 21% was similar to those with RVFAC < 35% (20% vs 32%; P = 0.12). At multivariate analysis, after adjustment for CPBP duration and EuroSCORE II, only global RVLS was associated with patients’ outcomes [76]. Free wall RVLS has a key role in the selection of patients for the implant of a LV assist device in advanced heart failure as the presence of a pre-implantation reduced longitudinal deformation is indicative of an extremely high risk of developing RV failure in the early or late post-operative period [78, 79].
Echo-guided treatment of RV function after cardiac surgery
In the case of a mild-moderate RV dysfunction without hemodynamic impact, a tailored follow-up of the patient with particular attention on the evolution of biventricular function is indicated. It is not uncommon to find a complete restoration of RV longitudinal deformation with time. In the immediate post-operatory setting, in the case of true RV failure, echocardiography helps the intensivists to validate the diagnosis together with the clinical clues and to guide therapeutic support. Starting from the need for a preload optimization, ultrasounds guide fluids administration in case of low RV filling pressure (i.e., low RA pressure) to optimize cardiac output [80]. Anyways, for the mentioned interventricular interdependence, a rapid fluid overload should be avoided to not reduce LV function. Based on RV dysfunction and pulmonary pressures, inotropic or vasopressor support (e.g., dobutamine, milrinone, norepinephrine) can be required and echocardiography has a key role in their management [81, 82]. Pulmonary embolism should be also excluded and, in addition to vital parameters, arterial blood gas and ECG, evaluation of RV helps in the decision making before a thoracic CT scan.
Conclusions
Right ventricle assumes great importance after cardiac surgery mostly because of its relevant prognostic impact. Beyond all the hypothesized etiologies, a complete assessment of RV size, longitudinal and global function should be performed before and after the intervention to optimize patients’ management. Echocardiography, both with standard indexes and second level techniques including 3D and STE, represents a non-invasive and reliable tool for this purpose. Figure 7 shows a proposed algorithm for a step-by-step evaluation of post-operative RV function. A complete exam systematically including TAPSE, s’, and RVFAC assessment should be performed soon after the surgery and repeated after 3 and 6 months to verify the possible complete restitution of RV longitudinal function. In the case of available second level echocardiographic tools, evaluation of 3DEF and RV longitudinal strains should complete the screening.
Systematic assessment of right ventricular function after cardiac surgery. A complete echocardiogram including TAPSE, s’, and RVFAC should be performed in the early post-operative period and successively at 3 and 6 months. Completing the exam with right ventricular longitudinal strain by speckle tracking echocardiography and the measurement of ejection fraction by 3D echocardiography can be useful, when available
References
Rose JC, Cosimano SJ Jr, Hufnagel CA, Massullo EA (1955) The effects of exclusion of the right ventricle from the circulation in dogs. J Clin Invest 34:1625–1631
Sawatani S, Mandell G, Kusaba E, Schraut W, Cascade P, Wajszczuk WJ, Kantrowitz A (1974) Ventricular performance following ablation and prosthetic replacement of right ventricular myocardium. Trans Am Soc Artif Intern Organs 20(B):629–636
MacNee W (1994) Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part one. Am J Respir Crit Care Med 150:833–852
Watts JA, Zagorski J, Gellar MA, Stevinson BG, Kline JA (2006) Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol 41:296–307
Goldhaber SZ (2004) Pulmonary embolism. Lancet 363:1295–1305
Belik J, Light RB (1989) Effect of increased afterload on right ventricular function in newborn pigs. J Appl Physiol 66:863–869
Giusca S, Jurcut R, Ginghina C, Voigt JU (2010) The right ventricle: anatomy, physiology and functional assessment. Acta Cardiol 65:67–77
Yamaguchi S, Harasawa H, Li KS, Zhu D, Santamore WP (1991) Comparative significance in systolic ventricular interaction. Cardiovasc Res 25:774–783
Santamore WP, Dell'Italia LJ (1998) Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 40:289–308
Feneley MP, Gavaghan TP, Baron DW, Branson JA, Roy PR, Morgan JJ (1985) Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation 71:473–480
Apostolakis S, Konstantinides S (2012) The right ventricle in health and disease: insights into physiology, pathophysiology and diagnostic management. Cardiology 121:263–273
Hoffman D, Sisto D, Frater RW, Nikolic SD (1994) Left-to-right ventricular interaction with a noncontracting right ventricle. J Thorac Cardiovasc Surg 107:1496–1502
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39.e14
Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC (2015) Reference values for right ventricular strain in patients without cardiopulmonary disease: a prospective evaluation and meta-analysis. Echocardiography 32:787–796
Meris A, Faletra F, Conca C, Klersy C, Regoli F, Klimusina J, Penco M, Pasotti E, Pedrazzini GB, Moccetti T, Auricchio A (2010) Timing and magnitude of regional right ventricular function: a speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr 23:823–831
Kaul S, Tei C, Hopkins JM, Shah PM (1984) Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J 107:526–531
Tamborini G, Pepi M, Galli CA, Maltagliati A, Celeste F, Muratori M, Rezvanieh S, Veglia F (2007) Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int J Cardiol 115:86–89
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M (2016) 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37:67–119
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35:3033–3069 69a-69k
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM (2006) Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 174:1034–1041
Schmid E, Hilberath JN, Blumenstock G, Shekar PS, Kling S, Shernan SK, Rosenberger P, Nowak-Machen M (2015) Tricuspid annular plane systolic excursion (TAPSE) predicts poor outcome in patients undergoing acute pulmonary embolectomy. Heart Lung Vessel 7:151–158
Ghio S, Klersy C, Magrini G, D'Armini AM, Scelsi L, Raineri C, Pasotti M, Serio A, Campana C, Viganò M (2010) Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol 140:272–278
Ghio S, Pica S, Klersy C, Guzzafame E, Scelsi L, Raineri C, Turco A, Schirinzi S, Visconti LO (2016) Prognostic value of TAPSE after therapy optimisation in patients with pulmonary arterial hypertension is independent of the haemodynamic effects of therapy. Open Heart 3:e000408
Dini FL, Carluccio E, Simioniuc A, Biagioli P, Reboldi G, Galeotti GG, Raineri C, Gargani L, Scelsi L, Mandoli GE, Cannito A, Rossi A, Temporelli PL, Ghio S, Network Labs Ultrasound (NEBULA) in Heart Failure Study Group (2016) Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail 181:462–471
Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP (2016) Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 18:1472–1487
Howard LS, Grapsa J, Dawson D, Bellamy M, Chambers JB, Masani ND, Nihoyannopoulos P, Simon R, Gibbs J (2012) Echocardiographic assessment of pulmonary hypertension: standard operating procedure. Eur Respir Rev 21:239–248
Koestenberger M, Apitz C, Abdul-Khaliq H, Hansmann G (2016) Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and D6PK. Heart 102(Suppl 2):ii14–ii22
Pavlicek M, Wahl A, Rutz T, de Marchi SF, Hille R, Wustmann K, Steck H, Eigenmann C, Schwerzmann M, Seiler C (2011) Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr 12:871–880
Peyrou J, Parsai C, Chauvel C, Simon M, Dehant P, Abergel E (2014) Echocardiographic assessment of right ventricular systolic function in a population of unselected patients before cardiac surgery: a multiparametric approach is necessary. Arch Cardiovasc Dis 107:529–539
Tamborini G, Brusoni D, Torres Molina JE, Galli CA, Maltagliati A, Muratori M, Susini F, Colombo C, Maffessanti F, Pepi M (2008) Feasibility of a new generation three-dimensional echocardiography for right ventricular volumetric and functional measurements. Am J Cardiol 102:499–505
van der Zwaan HB, Geleijnse ML, McGhie JS, Boersma E, Helbing WA, Meijboom FJ, Roos-Hesselink JW (2011) Right ventricular quantification in clinical practice: two-dimensional vs. three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr 12:656–664
Shimada YJ, Shiota M, Siegel RJ, Shiota T (2010) Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr 23:943–953
Cameli M, Mondillo S, Galderisi M, Mandoli GE, Ballo P, Nistri S, Capo V, D'Ascenzi F, D'Andrea A, Esposito R, Gallina S, Montisci R, Novo G, Rossi A, Mele D, Agricola E (2017) Speckle tracking echocardiography: a practical guide. G Ital Cardiol (Rome) 18:253–269
Lisi M, Cameli M, Righini FM, Malandrino A, Tacchini D, Focardi M, Tsioulpas C, Bernazzali S, Tanganelli P, Maccherini M, Mondillo S, Henein MY (2015) RV longitudinal deformation correlates with myocardial fibrosis in patients with end-stage heart failure. JACC Cardiovasc Imaging 8:514–522
Julian OC, Lopez-Belio M, Dye WS, Javid H, Grove WJ (1957) The median sternal incision in intracardiac surgery with extracorporeal circulation; a general evaluation of its use in heart surgery. Surgery 42:753–761
American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists, Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B (2006) ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 48:e1–e148
Pepi M, Muratori M, Barbier P, Doria E, Arena V, Berti M, Celeste F, Guazzi M, Tamborini G (1994) Pericardial effusion after cardiac surgery: incidence, site, size, and haemodynamic consequences. Br Heart J 72:327–331
Anderson WA, Berrizbeitia LD, Ilkowski DA, Cha R, Gu J, Fernandez J, Laub GW, Adkins MS, Chen C, McGrath LB (1995) Normothermic retrograde cardioplegia is effective in patients with left ventricular hypertrophy. A prospective and randomized study. J Cardiovasc Surg 36:17–24
Dagenais F, Pelletier LC, Carrier M (1999) Antegrade/retrograde cardioplegia for valve replacement: a prospective study. Ann Thorac Surg 68:1681–1685
Menasché P, Tronc F, Nguyen A, Veyssié L, Demirag M, Larivière J, Le Dref O, Piwnica AH, Bloch G (1994) Retrograde warm blood cardioplegia preserves hypertrophied myocardium: a clinical study. Ann Thorac Surg 57:1429–1434
Shiki K, Masuda M, Yonenaga K, Asou T, Tokunaga K (1986) Myocardial distribution of retrograde flow through the coronary sinus of the excised normal canine heart. Ann Thorac Surg 41:265–271
Hochberg MS, Austen WG (1980) Selective retrograde coronary venous perfusion. Ann Thorac Surg 29:578–578
Caldarone CA, Krukenkamp IB, Misare BD, Levitsky S (1994) Perfusion deficits with retrograde warm blood cardioplegia. Ann Thorac Surg 57:403–406
Martin TD, Craver JM, Gott JP, Weintraub WS, Ramsay J, Mora CT, Guyton RA (1994) Prospective, randomized trial of retrograde warm blood cardioplegia: myocardial benefit and neurologic threat. Ann Thorac Surg 57:298–302
Gaillard D, Bical O, Paumier D, Trivin F (2000) A review of myocardial normothermia: its theoretical basis and the potential clinical benefits in cardiac surgery. Cardiovasc Surg 8:198–203
Hayashida N, Ikonomidis JS, Weisel RD, Shirai T, Ivanov J, Carson SM, Mohabeer MK, Tumiati LC, Mickle DA (1994) The optimal cardioplegic temperature. Ann Thorac Surg 58:961–971
Cohn WE (2005) New surgical approaches for coronary disease: what’s coming? Tex Heart Inst J 32:354–357
Mariscalco G, Musumeci F (2014) The minithoracotomy approach: a safe and effective alternative for heart valve surgery. Ann Thorac Surg 97:356–364
Christakis GT, Buth KJ, Weisel RD, Rao V, Joy L, Fremes SE, Goldman BS (1996) Randomized study of right ventricular function with intermittent warm or cold cardioplegia. Ann Thorac Surg 61:128–134
Honkonen EL, Kaukinen L, Pehkonen EJ, Kaukinen S (1997) Right ventricle is protected better by warm continuous than by cold intermittent retrograde blood cardioplegia in patients with obstructed right coronary artery. Thorac Cardiovasc Surg 45:182–189
Menasché P, Fleury JP, Droc L, N'Guyen A, Larivière J, Faris B, Caffarelli F, Piwnica A, Bloch G (1994) Metabolic and functional evidence that retrograde warm blood cardioplegia does not injure the right ventricle in human beings. Circulation 90:II310–II315
Schuuring MJ, van Gulik EC, Koolbergen DR, Hazekamp MG, Lagrand WK, Backx AP, Mulder BJ, Bouma BJ (2013) Determinants of clinical right ventricular failure after congenital heart surgery in adults. J Cardiothorac Vasc Anesth 27:723–727
Bond BR, Dorman BH, Clair MJ, Walker CA, Pinosky ML, Reeves ST, Walton S, Kratz JM, Zellner JL, Crumbley AJ 3rd, Multani MM, Spinale FG (2001) Endothelin-1 during and after cardiopulmonary bypass: association to graft sensitivity and postoperative recovery. J Thorac Cardiovasc Surg 122:358–364
Wranne B, Pinto FJ, Hammarstrom E, St Goar FG, Puryear J, Popp RL (1991) Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J 66:435–442
Haddad F, Couture P, Tousignant C, Denault AY (2009) The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg 108:407–421
Schirmer U, Calzia E, Lindner KH, Hemmer W, Georgieff M (1995) Right ventricular function after coronary artery bypass grafting in patients with and without revascularization of the right coronary artery. J Cardiothorac Vasc Anesth 9:659–664
Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H 3rd, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M, U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS) (2011) Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 183:462–470
Shekerdemian L, Bohn D (1999) Cardiovascular effects of mechanical ventilation. Arch Dis Child 80:475–480
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755
Guinot PG, Abou-Arab O, Longrois D, Dupont H (2015) Right ventricular systolic dysfunction and vena cava dilatation precede alteration of renal function in adult patients undergoing cardiac surgery: an observational study. Eur J Anaesthesiol 32:535–542
Alam M, Hedman A, Nordlander R, Samad B (2003) Right ventricular function before and after an uncomplicated coronary artery bypass graft as assessed by pulsed wave Doppler tissue imaging of the tricuspid annulus. Am Heart J 146:520–526
Hanseus KC, Bjorkhem GE, Brodin LA, Pesonen E (2002) Analysis of atrioventricular plane movements by Doppler tissue imaging and M-mode in children with atrial septal defects before and after surgical and device closure. Pediatr Cardiol 23:152–159
Lindqvist P, Holmgren A, Zhao Y, Henein MY (2012) Effect of pericardial repair after aortic valve replacement on septal and right ventricular function. Int J Cardiol 155:388–393
Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani EG, Alamanni F, Pepi M (2009) Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr 10:630–634
Maffessanti F, Gripari P, Tamborini G, Muratori M, Fusini L, Alamanni F, Zanobini M, Fiorentini C, Caiani EG, Pepi M (2012) Evaluation of right ventricular systolic function after mitral valve repair: a two-dimensional Doppler, speckle-tracking, and three-dimensional echocardiographic study. J Am Soc Echocardiogr 25:701–708
Dalén M, Oliveira Da Silva C, Sartipy U, Winter R, Franco-Cereceda A, Barimani J, Bäck M, Svenarud P (2018) Comparison of right ventricular function after ministernotomy and full sternotomy aortic valve replacement: a randomized study. Interact Cardiovasc Thorac Surg 26:790–797
Hyllen S, Nozohoor S, Ingvarsson A, Meurling C, Wierup P, Sjogren J (2014) Right ventricular performance after valve repair for chronic degenerative mitral regurgitation. Ann Thorac Surg 38:2023–2030
Hashemi N, Brodin LA, Hedman A, Samad AB, Alam M (2018) Improved right ventricular index of myocardial performance in the assessment of right ventricular function after coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 26:798–804
Unsworth B, Casula RP, Kyriacou AA, Yadav H, Chukwuemeka A, Cherian A, Stanbridge Rde L, Athanasiou T, Mayet J, Francis DP (2010) The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J 159:314–322
Zanobini M, Saccocci M, Tamborini G, Veglia F, Di Minno A, Poggio P, Pepi M, Alamanni F, Loardi C (2017) Postoperative echocardiographic reduction of right ventricular function: is pericardial opening modality the main culprit? Biomed Res Int 2017:4808757
Karsenty C, Hadeed K, Dulac Y, Semet F, Alacoque X, Breinig S, Leobon B, Acar P, Hascoet S (2017) Two-dimensional right ventricular strain by speckle tracking for assessment of longitudinal right ventricular function after paediatric congenital heart disease surgery. Arch Cardiovasc Dis 110:157–166
Reichert CL, Visser CA, van den Brink RB, Koolen JJ, van Wezel HB, Moulijn AC, Dunning AJ (1992) Prognostic value of biventricular function in hypotensive patients after cardiac surgery as assessed by transesophageal echocardiography. J Cardiothorac Vasc Anesth 6:429–432
Maslow AD, Regan MM, Panzica P, Heindel S, Mashikian J, Comunale ME (2002) Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth Analg 95:1507–1518
Haddad F, Denault AY, Couture P, Cartier R, Pellerin M, Levesque S, Lambert J, Tardif JC (2007) Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J Am Soc Echocardiogr 20:1065–1072
Garatti A, Castelvecchio S, Di Mauro M, Bandera F, Guazzi M, Menicanti L (2015) Impact of right ventricular dysfunction on the outcome of heart failure patients undergoing surgical ventricular reconstructiondagger. Eur J Cardiothorac Surg 47:333–340
Ternacle J, Berry M, Cognet T, Kloeckner M, Damy T, Monin JL, Couetil JP, Dubois-Rande JL, Gueret P, Lim P (2013) Prognostic value of right ventricular two-dimensional global strain in patients referred for cardiac surgery. J Am Soc Echocardiogr 26:721–726
Costachescu T, Denault A, Guimond JG, Couture P, Carignan S, Sheridan P, Hellou G, Blair L, Normandin L, Babin D, Allard M, Harel F, Buithieu J (2002) The hemodynamically unstable patient in the intensive care unit: hemodynamic vs. transesophageal echocardiographic monitoring. Crit Care Med 30:1214–1223
Cameli M, Sparla S, Focardi M, Righini FM, Solari M, Alvino F, Lisi M, D'Ascenzi F, Bernazzali S, Tsioulpas C, Sassi C, Dokollari A, Sani G, Maccherini M, Mondillo S (2015) Evaluation of right ventricular function in the management of patients referred for left ventricular assist device therapy. Transplant Proc 47:2166–2168
Cameli M, Lisi M, Righini FM, Focardi M, Lunghetti S, Bernazzali S, Marchetti L, Biagioli B, Galderisi M, Maccherini M, Sani G, Mondillo S (2013) Speckle tracking echocardiography as a new technique to evaluate right ventricular function in patients with left ventricular assist device therapy. J Heart Lung Transplant 32:424–430
Kaul TK, Fields BL (2000) Postoperative acute refractory right ventricular failure: incidence, pathogenesis, management and prognosis. Cardiovasc Surg 8:1–9
Haddad F, Couture P, Tousignant C, Denault AY (2009) The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg 108:422–433
Vlahakes GJ (2005) Right ventricular failure following cardiac surgery. Coron Artery Dis 16:27–30
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mandoli, G.E., Cameli, M., Novo, G. et al. Right ventricular function after cardiac surgery: the diagnostic and prognostic role of echocardiography. Heart Fail Rev 24, 625–635 (2019). https://doi.org/10.1007/s10741-019-09785-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09785-2