Abstract
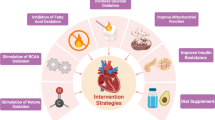
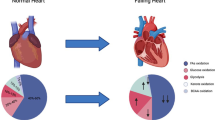
The heart failure accounts for the highest mortality rate all over the world. The development of preventive therapeutic approaches is still in their infancy. Owing to the extremely high energy demand of the heart, the bioenergetics pathways need to respond efficiently based on substrate availability. The metabolic regulation of such heart bioenergetics is mediated by various rate limiting enzymes involved in energy metabolism. Although all the pertinent mechanisms are not clearly understood, the progressive decline in the activity of metabolic enzymes leading to diminished ATP production is known to cause progression of the heart failure. Therefore, metabolic therapy that can maintain the appropriate activities of metabolic enzymes can be a promising approach for the prevention and treatment of the heart failure. The flavonoids that constitute various human dietary ingredients also effectively offer a variety of health benefits. The flavonoids target a variety of metabolic enzymes and facilitate effective management of the equilibrium between production and utilization of energy in the heart. This review discusses the broad impact of metabolic enzymes in the heart functions and explains how the dysregulated enzyme activity causes the heart failure. In addition, the prospects of targeting dysregulated metabolic enzymes by developing flavonoid-based metabolic approaches are discussed.

Similar content being viewed by others
References
Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL (2001) Mitochondrial dysfunction in cardiac disease: ischemia—reperfusion, aging, and heart failure. J Mol Cell Cardiol 33:1065–1089
Kannel WB (2000) Incidence and epidemiology of heart failure. Heart Fail Rev 5:167–173
Sliwa K, Damasceno A, Mayosi BM (2005) Epidemiology and etiology of cardiomyopathy in Africa. Circulation 112:3577–3583
Ingwall JS, Weiss RG (2004) Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 95:135–145
Neubauer S (2007) Cardiac physiology investigated by new methods of imaging. Clin Med (Lond) 7:189–191
Huss JM, Kelly DP (2005) Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115(3):547–555
Blache D, Devaux S, Joubert O, Loreau N, Schneider M, Durand P, Prost M, Gaume V, Adrian M, Laurant P, Berthelot A (2006) Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic Biol Med 41:277–284
Cantu D, Schaack J, Patel M (2009) Oxidative inactivation of mitochondrial aconitase results in iron and H2O2-mediated neurotoxicity in rat primary mesencephalic cultures. PLoS One 4:e7095
Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Korner R, Greff Z, Keri G, Stemmann O, Mann M (2008) Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell 31:438–448
Vahid F, Zand H, Nosrat-Mirshekarlou E, Najafi R, Hekmatdoost A (2015) The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: a review. Gene 562:8–15
Maron DJ (2004) Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep 6:73–78
Huxley RR, Neil HA (2003) The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 57:904–908
Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC (2002) Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr 75:880–886
Knekt P, Jarvinen R, Reunanen A, Maatela J (1996) Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ 312:478–481
Zern TL, Fernandez ML (2005) Cardioprotective effects of dietary polyphenols. J Nutr 135:2291–2294
Jeong YJ, Choi YJ, Kwon HM, Kang SW, Park HS, Lee M, Kang YH (2005) Differential inhibition of oxidized LDL-induced apoptosis in human endothelial cells treated with different flavonoids. Br J Nutr 93:581–591
Fuhrman B, Volkova N, Coleman R, Aviram M (2005) Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient (E0) mice and reduce macrophage atherogenicity. J Nutr 135:722–728
Hubbard GP, Wolffram S, de Vos R, Bovy A, Gibbins JM, Lovegrove JA (2006) Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: a pilot study. Br J Nutr 96:482–488
Ludwig A, Lorenz M, Grimbo N, Steinle F, Meiners S, Bartsch C, Stangl K, Baumann G, Stangl V (2004) The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun 316:659–665
Hallund J, Bugel S, Tholstrup T, Ferrari M, Talbot D, Hall WL, Reimann M, Williams CM, Wiinberg N (2006) Soya isoflavone-enriched cereal bars affect markers of endothelial function in postmenopausal women. Br J Nutr 95:1120–1126
Hodgson JM (2006) Effects of tea and tea flavonoids on endothelial function and blood pressure: a brief review. Clin Exp Pharmacol Physiol 33:838–841
Mao TK, van de Water J, Keen CL, Schmitz HH, Gershwin ME (2002) Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev Immunol 9:135–141
Rein D, Paglieroni TG, Pearson DA, Wun T, Schmitz HH, Gosselin R, Keen CL (2000) Cocoa and wine polyphenols modulate platelet activation and function. J Nutr 130:2120S–2126S
Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD (1999) Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 100:1050–1055
Schulze PC (2009) Myocardial lipid accumulation and lipotoxicity in heart failure. J Lipid Res 50:2137–2138
Sanbe A, Tanonaka K, Hanaoka Y, Katoh T, Takeo S (1993) Regional energy metabolism of failing hearts following myocardial infarction. J Mol Cell Cardiol 25:995–1013
Kluge MA, Fetterman JL, Vita JA (2013) Mitochondria and endothelial function. Circ Res 112:1171–1188
Erecinska M, Wilson DF (1982) Regulation of cellular energy metabolism. J Membr Biol 70:1–14
Leverve XM (2007) Mitochondrial function and substrate availability. Crit Care Med 35:S454–S460
Finck BN (2007) The PPAR regulatory system in cardiac physiology and disease. Cardiovasc Res 73:269–277
Rosca MG, Hoppel CL (2013) Mitochondrial dysfunction in heart failure. Heart Fail Rev 18:607–622
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258
Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S (2002) Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40:1267–1274
Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK (1991) Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338:973–976
Nascimben L, Friedrich J, Liao R, Pauletto P, Pessina AC, Ingwall JS (1995) Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation 91:1824–1833
Tian R, Nascimben L, Kaddurah-Daouk R, Ingwall JS (1996) Depletion of energy reserve via the creatine kinase reaction during the evolution of heart failure in cardiomyopathic hamsters. J Mol Cell Cardiol 28:755–765
Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T (2010) Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3:420–430
Lei B, Lionetti V, Young ME, Chandler MP, d’Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA (2004) Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36:567–576
Duncan JG, Finck BN (2008) The PPARalpha-PGC-1alpha axis controls cardiac energy metabolism in healthy and diseased myocardium. PPAR Res 2008:253817
Wang S, Fu C, Wang H, Shi Y, Xu X, Chen J, Song X, Sun K, Wang J, Fan X, Wang H, Yang X, Huan T, Hui R (2007) Polymorphisms of the peroxisome proliferator-activated receptor-gamma coactivator-1alpha gene are associated with hypertrophic cardiomyopathy and not with hypertension hypertrophy. Clin Chem Lab Med 45:962–967
Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS (2011) Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 108:837–846
Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP (2000) Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest 105:1723–1730
Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD (2011) Targeting fatty acid and carbohydrate oxidation—a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 1813:1333–1350
Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, Melki J, Hindelang C, Matyas R, Rustin P, Koenig M (2001) Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet 27:181–186
Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R (2003) Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 551:491–501
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO (1994) Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213:263–276
Stanley WC, Lopaschuk GD, McCormack JG (1997) Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res 34:25–33
Lopaschuk GD (2002) Metabolic abnormalities in the diabetic heart. Heart Fail Rev 7:149–159
Desvergne B, Michalik L, Wahli W (2006) Transcriptional regulation of metabolism. Physiol Rev 86:465–514
Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W (2006) From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45:120–159
Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821–827
Lee TI, Kao YH, Chen YC, Pan NH, Chen YJ (2010) Oxidative stress and inflammation modulate peroxisome proliferator-activated receptors with regional discrepancy in diabetic heart. Eur J Clin Investig 40:692–699
Yu BC, Chang CK, Ou HY, Cheng KC, Cheng JT (2008) Decrease of peroxisome proliferator-activated receptor delta expression in cardiomyopathy of streptozotocin-induced diabetic rats. Cardiovasc Res 80:78–87
Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ (2007) Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest 117(10):2791–2801
Koonen DP, Glatz JF, Bonen A, Luiken JJ (2005) Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta 1736:163–180
Nickerson JG, Momken I, Benton CR, Lally J, Holloway GP, Han XX, Glatz JF, Chabowski A, Luiken JJ, Bonen A (2007) Protein-mediated fatty acid uptake: regulation by contraction, AMP-activated protein kinase, and endocrine signals. Appl Physiol Nutr Metab 32:865–873
Harmon CM, Abumrad NA (1993) Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol 133:43–49
Luiken JJ, Coort SL, Koonen DP, van der Horst DJ, Bonen A, Zorzano A, Glatz JF (2004) Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch 448:1–15
Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JR (2004) Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation 109:1550–1557
Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A (2005) The subcellular compartmentation of fatty acid transporters is regulated differently by insulin and by AICAR. FEBS Lett 579:2428–2432
Fukuchi K, Nozaki S, Yoshizumi T, Hasegawa S, Uehara T, Nakagawa T, Kobayashi T, Tomiyama Y, Yamashita S, Matsuzawa Y, Nishimura T (1999) Enhanced myocardial glucose use in patients with a deficiency in long-chain fatty acid transport (CD36 deficiency). J Nucl Med 40:239–243
Nozaki S, Tanaka T, Yamashita S, Sohmiya K, Yoshizumi T, Okamoto F, Kitaura Y, Kotake C, Nishida H, Nakata A, Nakagawa T, Matsumoto K, Kameda-Takemura K, Tadokoro S, Kurata Y, Tomiyama Y, Kawamura K, Matsuzawa Y (1999) CD36 mediates long-chain fatty acid transport in human myocardium: complete myocardial accumulation defect of radiolabeled long-chain fatty acid analog in subjects with CD36 deficiency. Mol Cell Biochem 192:129–135
Watanabe K, Ohta Y, Toba K, Ogawa Y, Hanawa H, Hirokawa Y, Kodama M, Tanabe N, Hirono S, Ohkura Y, Nakamura Y, Kato K, Aizawa Y, Fuse I, Miyajima S, Kusano Y, Nagamoto T, Hasegawa G, Naito M (1998) Myocardial CD36 expression and fatty acid accumulation in patients with type I and II CD36 deficiency. Ann Nucl Med 12:261–266
Neely JR, Morgan HE (1974) Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 36:413–459
Wisneski JA, Gertz EW, Neese RA, Mayr M (1987) Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest 79:359–366
Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA (2010) Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab 12:53–64
Li LO, Ellis JM, Paich HA, Wang S, Gong N, Altshuller G, Thresher RJ, Koves TR, Watkins SM, Muoio DM, Cline GW, Shulman GI, Coleman RA (2009) Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J Biol Chem 284:27816–27826
Martin MA, Gomez MA, Guillen F, Bornstein B, Campos Y, Rubio JC, de la Calzada CS, Arenas J (2000) Myocardial carnitine and carnitine palmitoyltransferase deficiencies in patients with severe heart failure. Biochim Biophys Acta 1502:330–336
He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q (2012) Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation 126:1705–1716
Paulson DJ (1998) Carnitine deficiency-induced cardiomyopathy. Mol Cell Biochem 180:33–41
Atar D, Spiess M, Mandinova A, Cierpka H, Noll G, Luscher TF (1997) Carnitine—from cellular mechanisms to potential clinical applications in heart disease. Eur J Clin Investig 27:973–976
Arsenian MA (1997) Carnitine and its derivatives in cardiovascular disease. Prog Cardiovasc Dis 40:265–286
Regitz V, Shug AL, Fleck E (1990) Defective myocardial carnitine metabolism in congestive heart failure secondary to dilated cardiomyopathy and to coronary, hypertensive and valvular heart diseases. Am J Cardiol 65:755–760
Awan MM, Saggerson ED (1993) Malonyl-CoA metabolism in cardiac myocytes and its relevance to the control of fatty acid oxidation. Biochem J 295(Pt 1):61–66
Lopaschuk GD, Witters LA, Itoi T, Barr R, Barr A (1994) Acetyl-CoA carboxylase involvement in the rapid maturation of fatty acid oxidation in the newborn rabbit heart. J Biol Chem 269:25871–25878
Lopaschuk GD, Gamble J (1994) The 1993 Merck Frosst Award. Acetyl-CoA carboxylase: an important regulator of fatty acid oxidation in the heart. Can J Physiol Pharmacol 72:1101–1109
Saddik M, Gamble J, Witters LA, Lopaschuk GD (1993) Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 268:25836–25845
Dyck JR, Kudo N, Barr AJ, Davies SP, Hardie DG, Lopaschuk GD (1999) Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5′-AMP activated protein kinase. Eur J Biochem 262:184–190
Dyck JR, Lopaschuk GD (2002) Malonyl CoA control of fatty acid oxidation in the ischemic heart. J Mol Cell Cardiol 34:1099–1109
Sakamoto J, Barr RL, Kavanagh KM, Lopaschuk GD (2000) Contribution of malonyl-CoA decarboxylase to the high fatty acid oxidation rates seen in the diabetic heart. Am J Physiol Heart Circ Physiol 278:H1196–H1204
Gamble J, Lopaschuk GD (1997) Insulin inhibition of 5' adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism 46:1270–1274
Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD (1995) High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 270:17513–17520
Spiekerkoetter U, Sun B, Zytkovicz T, Wanders R, Strauss AW, Wendel U (2003) MS/MS-based newborn and family screening detects asymptomatic patients with very-long-chain acyl-CoA dehydrogenase deficiency. J Pediatr 143:335–342
Arnold GL, Van Hove J, Freedenberg D, Strauss A, Longo N, Burton B, Garganta C, Ficicioglu C, Cederbaum S, Harding C, Boles RG, Matern D, Chakraborty P, Feigenbaum A (2009) A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab 96:85–90
Marci M, Ajovalasit P (2009) Medium-chain acyl-CoA dehydrogenase deficiency in an infant with dilated cardiomyopathy. Cardiol Res Pract 2009:281389
Hale DE, Batshaw ML, Coates PM, Frerman FE, Goodman SI, Singh I, Stanley CA (1985) Long-chain acyl coenzyme A dehydrogenase deficiency: an inherited cause of nonketotic hypoglycemia. Pediatr Res 19:666–671
Turnbull DM, Bartlett K, Stevens DL, Alberti KG, Gibson GJ, Johnson MA, McCulloch AJ, Sherratt HS (1984) Short-chain acyl-CoA dehydrogenase deficiency associated with a lipid-storage myopathy and secondary carnitine deficiency. N Engl J Med 311:1232–1236
van Grunsven EG, van Berkel E, Ijlst L, Vreken P, de Klerk JB, Adamski J, Lemonde H, Clayton PT, Cuebas DA, Wanders RJ (1998) Peroxisomal D-hydroxyacyl-CoA dehydrogenase deficiency: resolution of the enzyme defect and its molecular basis in bifunctional protein deficiency. Proc Natl Acad Sci U S A 95:2128–2133
van Grunsven EG, van Berkel E, Mooijer PA, Watkins PA, Moser HW, Suzuki Y, Jiang LL, Hashimoto T, Hoefler G, Adamski J, Wanders RJ (1999) Peroxisomal bifunctional protein deficiency revisited: resolution of its true enzymatic and molecular basis. Am J Hum Genet 64:99–107
Eaton S, Bartlett K, Pourfarzam M (1996) Mammalian mitochondrial beta-oxidation. Biochem J 320(Pt 2):345–357
Tein I, De Vivo DC, Hale DE, Clarke JT, Zinman H, Laxer R, Shore A, DiMauro S (1991) Short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency in muscle: a new cause for recurrent myoglobinuria and encephalopathy. Ann Neurol 30:415–419
Kantor PF, Lucien A, Kozak R, Lopaschuk GD (2000) The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 86:580–588
Saudubray JM, Mitchell G, Bonnefont JP, Schwartz G, Nuttin C, Munnich A, Brivet M, Vassault A, Demaugre F, Rabier D (1992) Approach to the patient with a fatty acid oxidation disorder. Prog Clin Biol Res 375:271–288
Hale DE, Bennett MJ (1992) Fatty acid oxidation disorders: a new class of metabolic diseases. J Pediatr 121:1–11
Neely JR, Liebermeister H, Battersby EJ, Morgan HE (1967) Effect of pressure development on oxygen consumption by isolated rat heart. Am J Phys 212:804–814
Williamson JR, Ford C, Illingworth J, Safer B (1976) Coordination of citric acid cycle activity with electron transport flux. Circ Res 38:I39–I51
Jose AD, Stitt F (1969) Effects of hypoxia and metabolic inhibitors on the intrinsic heart rate and myocardial contractility in dogs. Circ Res 25:53–66
Patel MS, Korotchkina LG (2002) Pyruvate dehydrogenase complex as a marker of mitochondrial metabolism. Inhibition by 4-hydroxy-2-nonenal. Methods Mol Biol 186:255–263
Lissens W, De Meirleir L, Seneca S, Liebaers I, Brown GK, Brown RM, Ito M, Naito E, Kuroda Y, Kerr DS, Wexler ID, Patel MS, Robinson BH, Seyda A (2000) Mutations in the X-linked pyruvate dehydrogenase (E1) alpha subunit gene (PDHA1) in patients with a pyruvate dehydrogenase complex deficiency. Hum Mutat 15:209–219
Page B, Young R, Iyer V, Suzuki G, Lis M, Korotchkina L, Patel MS, Blumenthal KM, Fallavollita JA, Canty JM Jr (2008) Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ Res 102:103–112
Sidhu S, Gangasani A, Korotchkina LG, Suzuki G, Fallavollita JA, Canty JM Jr, Patel MS (2008) Tissue-specific pyruvate dehydrogenase complex deficiency causes cardiac hypertrophy and sudden death of weaned male mice. Am J Physiol Heart Circ Physiol 295:H946–H952
Shinde S, Kumar P, Mishra K, Patil N (2006) Defect in mitochondrial functions in damaged human mitral valve. Indian J Clin Biochem 21:156–160
Kalsi KK, Smolenski RT, Pritchard RD, Khaghani A, Seymour AM, Yacoub MH (1999) Energetics and function of the failing human heart with dilated or hypertrophic cardiomyopathy. Eur J Clin Investig 29:469–477
Benderdour M, Charron G, DeBlois D, Comte B, Des RC (2003) Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: an event that precedes hypertrophy development. J Biol Chem 278:45154–45159
Cooney GJ, Taegtmeyer H, Newsholme EA (1981) Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem J 200:701–703
Moreno-Sanchez R, Torres-Marquez ME, Devars S (1990) Substrate oxidation in the myocardium. Arch Inst Cardiol Mex 60:587–591
Johnson JD, Mehus JG, Tews K, Milavetz BI, Lambeth DO (1998) Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes. J Biol Chem 273:27580–27586
Johnson JD, Muhonen WW, Lambeth DO (1998) Characterization of the ATP- and GTP-specific succinyl-CoA synthetases in pigeon. The enzymes incorporate the same alpha-subunit. J Biol Chem 273:27573–27579
Elpeleg O, Miller C, Hershkovitz E, Bitner-Glindzicz M, Bondi-Rubinstein G, Rahman S, Pagnamenta A, Eshhar S, Saada A (2005) Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am J Hum Genet 76:1081–1086
Ostergaard E, Hansen FJ, Sorensen N, Duno M, Vissing J, Larsen PL, Faeroe O, Thorgrimsson S, Wibrand F, Christensen E, Schwartz M (2007) Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain 130:853–861
Carrozzo R, Dionisi-Vici C, Steuerwald U, Lucioli S, Deodato F, Di Giandomenico S, Bertini E, Franke B, Kluijtmans LA, Meschini MC, Rizzo C, Piemonte F, Rodenburg R, Santer R, Santorelli FM, van Rooij A, Vermunt-de Koning D, Morava E, Wevers RA (2007) SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 130:862–874
Morava E, Steuerwald U, Carrozzo R, Kluijtmans LA, Joensen F, Santer R, Dionisi-Vici C, Wevers RA (2009) Dystonia and deafness due to SUCLA2 defect; clinical course and biochemical markers in 16 children. Mitochondrion 9:438–442
Dupourque D, Kun E (1969) Malate dehydrogenases of ox kidney. 2. Two substrate kinetic and inhibition analyses. Eur J Biochem 7:247–252
Sharov VG, Goussev A, Lesch M, Goldstein S, Sabbah HN (1998) Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 30:1757–1762
Green DE, Tzagoloff A (1966) The mitochondrial electron transfer chain. Arch Biochem Biophys 116:293–304
Casademont J, Miro O (2002) Electron transport chain defects in heart failure. Heart Fail Rev 7:131–139
Smeitink JA, Loeffen JL, Triepels RH, Smeets RJ, Trijbels JM, van den Heuvel LP (1998) Nuclear genes of human complex I of the mitochondrial electron transport chain: state of the art. Hum Mol Genet 7:1573–1579
Robinson BH (1998) Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. Biochim Biophys Acta 1364:271–286
Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R (2013) Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18:239–250
Hoekstra AS, Bayley JP (2013) The role of complex II in disease. Biochim Biophys Acta 1827:543–551
Benit P, Lebon S, Rustin P (2009) Respiratory-chain diseases related to complex III deficiency. Biochim Biophys Acta 1793:181–185
Giordano FJ (2005) Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115:500–508
Maack C, Dabew ER, Hohl M, Schafers HJ, Bohm M (2009) Endogenous activation of mitochondrial KATP channels protects human failing myocardium from hydroxyl radical-induced stunning. Circ Res 105:811–817
Akar FG, Aon MA, Tomaselli GF, O'Rourke B (2005) The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115:3527–3535
Li JM, Shah AM (2004) Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287:R1014–R1030
Sinatra ST (2009) Metabolic cardiology: an integrative strategy in the treatment of congestive heart failure. Altern Ther Health Med 15:44–52
Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, Caceres V, Shi S, Kirk JA, Su J, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG (2012) Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest 122:291–302
Middleton E Jr (1998) Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol 439:175–182
Solanki I, Parihar P, Mansuri ML, Parihar MS (2015) Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr 6:64–72
de Groot H, Rauen U (1998) Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol 12:249–255
Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S (1995) Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155:381–386
Mueller M, Lukas B, Novak J, Simoncini T, Genazzani AR, Jungbauer A (2008) Oregano: a source for peroxisome proliferator-activated receptor gamma antagonists. J Agric Food Chem 56:11621–11630
Mueller M, Jungbauer A (2009) Peroxisome proliferator-activated receptor gamma is constitutively activated in yeast. Anal Biochem 385:365–367
Jungbauer A, Medjakovic S (2012) Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 71:227–239
Shin DW, Kim SN, Lee SM, Lee W, Song MJ, Park SM, Lee TR, Baik JH, Kim HK, Hong JH, Noh M (2009) (−)-Catechin promotes adipocyte differentiation in human bone marrow mesenchymal stem cells through PPAR gamma transactivation. Biochem Pharmacol 77:125–133
Kim B, Choi YE, Kim HS (2014) Eruca sativa and its flavonoid components, quercetin and isorhamnetin, improve skin barrier function by activation of peroxisome proliferator-activated receptor (PPAR)-alpha and suppression of inflammatory cytokines. Phytother Res 28:1359–1366
van Bilsen M, van Nieuwenhoven FA (2010) PPARs as therapeutic targets in cardiovascular disease. Expert Opin Ther Targets 14:1029–1045
Hennuyer N, Tailleux A, Torpier G, Mezdour H, Fruchart JC, Staels B, Fievet C (2005) PPARalpha, but not PPARgamma, activators decrease macrophage-laden atherosclerotic lesions in a nondiabetic mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol 25:1897–1902
Duez H, Chao YS, Hernandez M, Torpier G, Poulain P, Mundt S, Mallat Z, Teissier E, Burton CA, Tedgui A, Fruchart JC, Fievet C, Wright SD, Staels B (2002) Reduction of atherosclerosis by the peroxisome proliferator-activated receptor alpha agonist fenofibrate in mice. J Biol Chem 277:48051–48057
Whitman SC, Kurowska EM, Manthey JA, Daugherty A (2005) Nobiletin, a citrus flavonoid isolated from tangerines, selectively inhibits class A scavenger receptor-mediated metabolism of acetylated LDL by mouse macrophages. Atherosclerosis 178:25–32
Wei T, Xiong FF, Wang SD, Wang K, Zhang YY, Zhang QH (2014) Flavonoid ingredients of Ginkgo biloba leaf extract regulate lipid metabolism through Sp1-mediated carnitine palmitoyltranferase 1A up-regulation. J Biomed Sci 21:87
Baiges I, Palmfeldt J, Blade C, Gregersen N, Arola L (2010) Lipogenesis is decreased by grape seed proanthocyanidins according to liver proteomics of rats fed a high fat diet. Mol Cell Proteomics 9:1499–1513
Kamisoyama H, Honda K, Tominaga Y, Yokota S, Hasegawa S (2008) Investigation of the anti-obesity action of licorice flavonoid oil in diet-induced obese rats. Biosci Biotechnol Biochem 72:3225–3231
Watanabe N, Inagawa K, Shibata M, Osakabe N (2014) Flavan-3-ol fraction from cocoa powder promotes mitochondrial biogenesis in skeletal muscle in mice. Lipids Health Dis 13:64
Aoki F, Honda S, Kishida H, Kitano M, Arai N, Tanaka H, Yokota S, Nakagawa K, Asakura T, Nakai Y, Mae T (2007) Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci Biotechnol Biochem 71:206–214
Bronnikov GE, Kulagina TP, Aripovsky AV (2009) Dietary supplementation of old mice with flavonoid dihydroquercetin causes recovery of the mitochondrial enzyme activities in skeletal muscles. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology 3:453–458
Govindasamy C, Al-Numair KS, Veeramani C, Alsaif MA, Almajwal M (2015) Protective effect of kaempferol, a flavonoid compound, on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Progress in Nutrition, 17
Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD (2014) Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr 5:404–417
Jayachandran KS, Vasanthi HR, Rajamanickama GV (2010) Flavonoid rich fraction of Dioscorea bulbifera Linn. (Yam) enhances mitochondrial enzymes and antioxidant status and thereby protects heart from isoproterenol induced myocardial infarction. Curr Pharm Biotechnol 11:887–894
Sandhir R, Mehrotra A (2013) Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington's disease. Biochim Biophys Acta 1832:421–430
Moini H, Arroyo A, Vaya J, Packer L (1999) Bioflavonoid effects on the mitochondrial respiratory electron transport chain and cytochrome c redox state. Redox Rep 4:35–41
Barnes S, Peterson TG (1995) Biochemical targets of the isoflavone genistein in tumor cell lines. Proc Soc Exp Biol Med 208:103–108
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Yang CS, Kim S, Yang GY, Lee MJ, Liao J, Chung JY, Ho CT (1999) Inhibition of carcinogenesis by tea: bioavailability of tea polyphenols and mechanisms of actions. Proc Soc Exp Biol Med 220:213–217
Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83:456S–460S
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867
Baker RG, Hayden MS, Ghosh S (2011) NF-kappaB, inflammation, and metabolic disease. Cell Metab 13:11–22
Kreatsoulas C, Anand SS (2010) The impact of social determinants on cardiovascular disease. Can J Cardiol 26(Suppl C):8C–13C
Weisfeldt ML, Zieman SJ (2007) Advances in the prevention and treatment of cardiovascular disease. Health Aff (Millwood ) 26:25–37
Friesen JA, Rodwell VW (2004) The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol 5:248
Stancu C, Sima A (2001) Statins: mechanism of action and effects. J Cell Mol Med 5:378–387
Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N (2000) Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 130:2243–2250
Acknowledgements
The authors gratefully acknowledge the financial support from the Department of Science and Technology, New Delhi (DST-INSPIRE to Priyanka Parihar).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Parihar, P., Parihar, M.S. Metabolic enzymes dysregulation in heart failure: the prospective therapy. Heart Fail Rev 22, 109–121 (2017). https://doi.org/10.1007/s10741-016-9588-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9588-x