Abstract
Salinity responses of Populus alba L. clone ‘Marte’ were evaluated using a split-root system to simulate the heterogeneous soil conditions in the field and to assess the salinity tolerance of clone ‘Marte’. During the 26 days of experiment, we measured morpho-physiological parameters as well as the mineral content in substrate and plant organs in split-root plants watered with no salt on both sides of the system (0/0 mM - S1), salt on one side (0/100 mM – S2) and salt on both sides (100/100 mM – S3). Shoot elongation, stem diameter increase, and number of new leaves were not significantly different among and within each salt treatment. Leaf relative chlorophyll content and chlorophyll a fluorescence did not show any difference, while there was a decrease of 85% in terms of net photosynthesis (Pn) and 88% in stomatal conductance (gs) in S3 plants compared to S1. Na, absorbed by one side of the 0/100 mM plants, was equally distributed in all aerial organs and reached a concentration in-between the other two groups, as a confirmation of the physiological results obtained. An increase in Mn concentrations in woody cutting, stems and leaves of S2 and S3 system compared to S1 group was observed. Moreover, S3 plants showed an increase of K concentration in leaves. In ‘Marte’ poplar, Na increment was only translated in a reduction of photosyntetic performances, and the increase of some key mineral elements at leaf level such as K and Mn suggested an attempt to overcome the stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinization is a global issue and has a tremendous impact on plant growth and health (Ran et al. 2021). According to recent estimations, around 1 Bha of the total land area consists of saline or sodic soils (Daliakopoulos et al. 2016; Shokat and Großkinsky 2019; Kumar et al. 2021). This surface represents the 7% of land area and 33% of irrigated lands (Chele et al. 2021). Europe contributes with 30–50 Mha of lands affected by salinization (Daliakopoulos et al. 2016; Shokat and Großkinsky 2019).
Plants have to cope with salinity, usually characterized by a first rapid “osmotic phase”, followed by a slower “ionic phase” in which salt accumulation produce toxic effects (Munns and Tester 2008). Under salt stress, ion/nutrient imbalances, metabolic disorders and oxidative stress are reduced (or avoided) in tolerant species by changes at morphological, physiological and molecular level through complex adaptive strategies (Kibria and Hoque 2019; Sun et al. 2020).
In salinized soil, the mineral nutrient bioavailability is altered and plants, as sessile organisms, try to cope with this condition by increasing root proliferation for exploring new areas. The study of mineral nutrient translocation and imbalances inside a plant grown under salinity is a very important aspect in describing plant response to salt, due to the role of ions in growth and in signaling/activation of biochemical response mechanisms. It is postulated that salt excess can destroy the balance of ion distribution between plant and soil, leading to nutrients imbalance and cell metabolism disorders (Zhao et al. 2021); moreover, salinity inhibits the uptake of essential macro-elements, such as N, K, Ca, Mg, as well as micro-nutrients such as Fe, Cu, Zn, Mn and B (Wu et al. 2013). During salt stress, plants respond to nutritional imbalances by adapting ion transport and achieving a new ionic homeostasis. Usually, salinity stress leads to K loss in plant cells and an increase of Ca in the cytosol (Zhao et al. 2021). The reduction in the uptake of other mineral nutrients such as Mn, and in some cases Mg deficiency, are also observed (Karimi et al. 2005). In plants, the photosynthetic system is usually damaged by salinity, and net photosynthetic rate is decreased in poplar trees too. The reduction of CO2 assimilation and stomatal conductance of 48.3% and 48.5% was measured in Populus euphratica under a 300 mM NaCl treatment and it was associated to significant decrease in chlorophyll fluorescence (expressed as Fv/Fm value) indicating a damage in the photosynthetic system (Zhao et al. 2017).
An interesting approach to study plant responses to environmental factors is the split-root system (SRS). It consists in exposing two separate portions of a single root system to non-uniform situations (Larrainzar et al. 2014). The main advantage of SRS is to apply a contrasting growth condition to roots that share a common aerial part simulating both horizontal and vertical soil heterogeneity (Saiz-Fernández et al. 2021). Thus, it is possible to test long-distance root-shoot signals, to determine compensatory effects for adjusting imbalances induced by soil contamination, and to discriminate local regulation mechanisms in aerial parts induced by root signaling (Hirota et al. 2004). This approach has been used in different plant species and limiting conditions to evaluate the effect of a partial stress exposure (Agapit et al. 2020; Saiz-Fernández et al. 2021). For example, split-root system has been used to study the regulation of Fe-deficiency response in cucumber (De Nisi et al. 2012) and tomato (Coppa et al. 2018; Celletti et al. 2020) and salinity response in Atriplex nummularia (Bazihizina et al. 2009, 2012), cotton (Kong et al. 2012), alfalfa (Xiong et al. 2018) and sorghum (Zhang et al. 2019a). In poplar, split-root system was exploited to study hormonal responses in plants under water scarcity (Marino et al. 2017) and to study zinc response in comparison with willow (Palm et al. 2021).
Poplar trees (Populus spp.) play an important economic and environmental role in many regions of the world (Mao et al. 2008). They are considered as a model for woody plants, and their response to salinity has been investigated in previous years at morphological, physiological and molecular level (Zhang et al. 2019b). Different species and clones of Populus genus have demonstrated a different degree of sensitivity or tolerance towards excess salt. Populus euphratica, for example, is considered the most tolerant poplar species (Chen and Polle 2010) and Populus alba has shown a moderate degree of salt tolerance with an intra-specific variability among different clones and geographic provenances (Sixto et al. 2006; Beritognolo et al. 2007; Zhang et al. 2019b). Some relatively salt resistant clones have shown to be superior to Populus nigra in terms of Na exclusion capacity, confining the element in roots more effectively and maintaining a higher K/Na ratio in leaves (Mao et al. 2010). However, this exclusion is not exempt from a quick translocation to the aerial part, whose consequences are a rapid stomatal closure and low transpiration (Beritognolo et al. 2011). Even if specific “salt-stress” studies are not available, Populus alba L. clone ‘Marte’ is indicated for growing in salty soils (Salvati et al. 2007) and showed a plasticity in contrasting water deficit greater than other clones (Traversari et al. 2018).
Considering that very limited studies are available on poplar response to heterogeneous salinity (Feng et al. 2018), we performed an experiment on Populus alba L. clone ‘Marte’ response to salt excess using a split-root system. The main questions we wanted to answer were: i) How morpho- and physiological parameters are altered in ‘Marte’ clone, in particular when plants are exposed to a heterogeneous salt supply? ii) Do the half-roots grown in Na-free condition accelerate Na uptake in the other half-roots watered with salt, compared with both treated half-roots? iii) Is Na itself transferred from the half-roots supplied with salt to the opposite untreated half-roots? iv) Do other elements such as Ca, K, Mg, Mn, Zn, Fe and Cu change their distribution inside the plant for counteract Na induced imbalance in mineral nutrition?
Materials and methods
Plant growth, experimental design and treatment
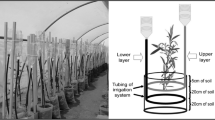
Plants of Populus alba L. clone ‘Marte’ were grown outdoor in 3.6 L pots containing a combination of peat, sand and perlite (50/40/10 v/v/v, pH 6.8) at the Crop Science Research Center of Pisa – Italy (43°43’46.3"N 10°25’23.3"E). Two years old poplar woody cuttings were provided by Alasia Franco Vivai, Savigliano (CN, Italy). Each woody cutting was rooted in perlite in a nursery and homogeneously rooted cuttings, uniform in size, were selected for the experiment. The root apparatus of each plant was divided in two homogeneous parts and separated into two compartments (split-root system). Plants were watered at field capacity with distilled water every two days and 10 g of a universal granular fertilizer (GRANVERDE, Cifo Srl, Bologna, Italy) were provided at the beginning of the growth period. This fertilizer was a NPK 16-10-18 containing Mg 2%. Two new shoots were selected for each plant and only plants whose shoots were grown on opposite sides, corresponding to the two halves of the root system, were used for the experiment (Fig. 1). Plants (n = 12) reached a height of 52.5 ± 13.7 cm (average height of all shoots) after one month of growth. They were divided in three homogeneous groups of treatment (n = 4 for each group) and arranged in a completely randomized design. One group of plants (System 1, S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), the second group (System 2, S2) with distilled water on one side and water containing 100 mM NaCl on the other side (0/100 mM NaCl), and the third group (System 3, S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Plants were watered every 2–3 days for 26 days and, at the end of the experiment, each pot received a volume of solution (with or without salt) of 6.25 L, equivalent to an amount of 36.5 g NaCl in the salt-containing solution. During the treatment, weather data (temperature, relative air humidity and rainfalls) were collected from the closest weather station (43°42’16’’N 10°23’53’’E).
Schematic representation of the split-root system in Populus alba clone ‘Marte’. The split-root system distinguishes local (roots) versus systemic Na-signalling (apical and basal shoots). In this ‘split-root’ design, roots of a single plant are ‘split’ in half and each half is exposed to: homogeneous conditions as S1 (control plants are also ‘split’, but without Na supply) and S3 (both root systems are grown under 100 mM NaCl) or heterogeneous Na-environment as S2 (100 mM NaCl on only one side)
Morphological and physiological measurements
For each plant, shoot height and stem diameter were measured at the beginning and at the end of the experiment. The height of each shoot was measured from the apex to the insertion of stem on main woody cutting. Stem diameter was measured with a digital caliper 2 cm above the insertion. The number of leaves was counted at the beginning and the end of experiment for each shoot. The new leaves developed during the experiment resulted as difference between number of leaves at the beginning and number of leaves at the end of the experiment. The number of fallen leaves was evaluated on the basis of the presence of scars along the shoot at the end of the experiment.
Leaf relative chlorophyll content was monitored using a SPAD meter (SPAD502 Plus Chlorophyll Meter, Spectrum, Plainfield, IL, USA) on both shoots of each plant on five consecutives fully expanded leaves. For fluorescence and gas exchange measurements, a median fully expanded leaf for each shoot of each plant was selected.
Chlorophyll a fluorescence was measured in order to evaluate photosystem II efficiency (PSII) using a portable pulse-modulated chlorophyll fluorometer (Hansatech FM2, Hansatech, Inc., UK). Measures were taken on dark-adapted leaves (1 h) exposed to a saturating light pulse (8000 µmol photon m− 2 s− 1; 700 ms). PSII efficiency was determined as Fv/Fm ratio. Fv represents the difference between the maximum fluorescence (Fm) and the minimum fluorescence yield measured in dark-adapted state (F0).
Instantaneous gas exchanges and net photosynthesis were measured at the beginning and at the end of the treatment between 10:00 and 12:00 am with a portable photosynthesis system (CIRAS-2, PP Systems International, MA, USA) equipped with a leaf chamber. Photosynthetic rate (Pn, µmol CO2 m− 2 s− 1), stomatal conductance (gs, mmol m− 2 s− 1) and transpiration (E, mmol H2O m− 2 s− 1) were measured operating at 100 ml min− 1 flow rate, ambient temperature, 410 ± 10 ppm CO2 and a photosynthetic flux density of 1000 µmol m− 2 s− 1.
Plant and substrate sampling
A sample of substrate (10 g) was collected from each pot at the beginning and at the end of the experiment to evaluate mineral content. Roots, woody cutting, leaves, and stem were sampled at harvesting according to the scheme in Fig. 1. Leaves and stem were collected by dividing in half each shoot (apical and basal portion) and dried for mineral content analysis or Relative Water Content (RWC) determination. A stem portion (1 cm) was excised from basal and apical part to determine the fresh weight (FW). It was immersed in water overnight under vacuum, weighted to determine the turgor weight (TW) and dried 48 h at 105 °C to determine the dry weight (DW). Apical and basal leaves were collected, immersed in water overnight, weighted to determine the TW and dried 48 h at 105 °C to determine the DW. RWC was calculated from the equation:
Mineral analysis of plant and substrate samples
Plant and substrate samples were dried at 70 °C until their weight remained constant. Approximately 0.3 g of leaves, shoot (leaves or stem) and roots and 0.5 g of substrate samples were ground and added to 8 and 10 ml of 65% nitric acid, respectively, in 70 ml teflon vessels. The mixtures were digested using the COOLPEX Smart Microwave Reaction System (Yiyao Instrument Technology Development Co., Ltd., Shanghai, China). Final samples were diluted in milliQ water and analyzed for sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), zinc (Zn), iron (Fe), copper (Cu) and manganese (Mn) concentration through atomic emission spectroscopy (4210 MP-AES, Agilent). Calibration curves were obtained measuring a multi-element standard control in a 5% HNO3 (v/v) solution. Instrumental parameters (nebulizer flow and viewing position) were optimized for each group of samples. Results were expressed on a dry mass basis (mg kg− 1 DW). We used two analytical reference standards (WEPAL IPE, Wageningen University) with certified concentrations as controls: Daucus carota L. leaf (1.06 × 104 ± 1.01 x 103 mg kg− 1 of Na) and shoot (852 ± 126.7 mg kg− 1 of Na).
Statistical analysis
All data reported are presented as mean + standard deviation (sd). Statistical analysis was carried out using RStudio software (RStudio, PBC, Boston, MA). Statistical significance was tested by Student’s t-test and by one-way ANOVA analysis with Tukey’s Multiple Comparison Test at p < 0.05. GraphPad Prism version 8.0.1 software for Windows (GraphPad Software, San Diego, California USA) was used for figures. A multivariate statistical technique, the Principal Component Analysis (PCA), was used to investigate and reveal the correlations between variables (mineral elements) within the measurements. Only PCA with eigenvalues > 1, explaining more than a single parameter alone, were extracted. The Varimax rotation was applied on the obtained factor (the analysis was performed using NCSS 2004 Statistical Analysis System Software).
Results
In this study, salinity response of Populus alba L. clone ‘Marte’ was evaluated using a split-root system. This approach was exploited in order to simulate the heterogeneous conditions of soils and assess experimentally the salinity tolerance of clone ‘Marte’ evaluating the effects of a partial stress exposure. During the 26 days of treatment, we measured several morpho- and physiological parameters and, at the end, we analyzed the mineral content in plant organs and in the substrate. Over the entire experiment, plants were grown under the average temperature (Tavg) of 24.6 °C and the average minimum (Tmin) and maximum (Tmax) of 19.0 and 30.0 °C respectively. The average relative humidity (RHavg) was 76.4% and the average minimum (RHmin) and maximum (RHmax) were 48.4% and 98.7% (Fig. S1).
Morphological measures
At the end of the trial, no differences were observed between the three poplar groups in terms of shoot elongation (Fig. 2A), stem diameter increase (Fig. 2B) and number of new leaves (Fig. 2C). The S3 plants showed symptoms of scorching on leaves, but no relevant variations were observed in terms of shoot height and diameter or number of new/fallen leaves compared to the S1 and S2 group.
Plant growth at the end of the experiment measured on both shoots of each Populus alba clone ‘Marte’ plant. (A) Shoot height, (B) stem diameter and (C) number of new leaves. System 1 (S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), System 2 (S2) with distilled water on one side and 100 mM NaCl on the other side (0/100 mM NaCl) and System 3 (S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Each bar is the mean + sd (n = 4). Data were analyzed with one-way ANOVA among each system followed by Tukey’s Multiple Comparison Test (P < 0.05). Moreover, t-test analysis was performed between the two sides of each system. P-values of t-test and ANOVA are reported. Different letters denote significant different systems
Physiological measures
Relative chlorophyll content was measured weekly on five consecutives fully expanded leaves, and no relevant differences were observed between groups (Table 1). Considering the PSII photosystems through fluorescence measurements, data demonstrated that poplar plants with partially and fully treated root systems with 100 mM NaCl did not show a specific variation on dark-adapted maximum efficiency values. Fv/Fm, in fact, was very close to the generally observed value of 0.84 for non-stressed plants (Table 1). On the contrary, the salt treatment had an evident effect on photosyntetic parameters (Fig. 3A-B). At the end of the experiment, both net photosynthesis (Pn,) and stomatal conductance (gs,) showed an overall decrease of 85% and 88%, respectively, in S3 plants compared to S1 group. In S2 group (0/100 mM NaCl) the decrease was of 30% (Pn) and 40% (gs) showing the highest variability in the CO2 assimilation and stomatal conductance reduction. A similar decrease of RWC was recorded in S2 and S1 plants, while there was a significant reduction of 5% and 9%, respectively, in apical and basal leaves RWC of S3 group (Table 2). No differences in stem RWC were observed between groups/sides (Table 2).
Gas exchange parameters measured at the end of the experiment on both shoots of each Populus alba clone ‘Marte’ plant. (A) Net photosynthesis (Pn), (B) stomatal conductance (gs) and (C) transpiration (E). System 1 (S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), System 2 (S2) with distilled water on one side and 100 mM NaCl on the other side (0/100 mM NaCl) and System 3 (S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Each bar is the mean + sd (n = 4). Data were analyzed with one-way ANOVA among each system followed by Tukey’s Multiple Comparison Test (P < 0.05). Moreover, t-test analysis was performed between the two sides of each system. P-values of t-test and ANOVA are reported. Different letters denote significant different systems
Mineral analysis of plant material and substrate samples
Substrate composition was homogeneous between systems and sides at the beginning of the treatment period (data not shown). At the end, as expected, we assessed an increase of Na in treated pots. In particular, the amount of Na was 12 and 14 times higher in treated pots of S2 plants and all pots of S3 group, respectively, compared to S1 group (Fig. 4). Analyses of root, woody cutting, stem and leaf ion concentrations revealed that the application of 100 mM NaCl to one portion of the root system (S2) led to an increased Na accumulation in woody cutting, stem and leaves (Fig. 5), but generally did not change the accumulation of other macro- and micronutrients at root level (Figs. 6 and 7 and Fig. S2-6). Moreover, the treated roots of S2 plants reached the similar concentration of Na measured in roots of S3 group. Na translocation in stems and basal leaves of S2 plants reached an average concentration between S1 and S3 plants with no differences between sides, indicating that sodium was equally distributed in all the aerial part (Fig. 5). Same tendency, even not significant in the comparison between S1 and S2 plants, was observed in apical leaves. Potassium concentrations showed no differences in roots (Fig. 6). Excluding woody cutting, for all other aerial organs S2 and S1 group were identical, while S3 plants showed a decrease in K uptake in stems combined with an increase of K concentration in leaves compared to control group. No differences between sides inside each system were observed. Interestingly, we observed an increase in Mn concentrations in woody cutting, stems and leaves of S2 and S3 system compared to S1 group (Fig. 7). The results for all the other macro- (Ca, Mg) and micronutrients (Zn, Fe, Cu) are shown in Fig S2-6. For these elements, even if the differences between treatments, particularly in the comparison with S1 plants, were not so evident as in the case of Na, K and Mn, we observed: (a) reduction of Ca concentration in the roots of S3 plants, combined to a significant increase in stems and an increase in basal leaves, which was significant for the S2 group; (b) increase of Mg in the basal leaves of the S2 plants; (c) increase of Zn in basal and apical leaves of both S2 and S3 plants; (d) slight increase in Cu concentrations in leaves and apical stems of S2 and S3 plants. A PCA was carried out with the aim of studying the relationship among all the mineral elements (Na, Cu, Zn, Fe, Mn, Mg, Ca and K) in different split-root systems (Fig. 8; Table 3). In Fig. S7, PCA scree plots are reported. The first two principal components (PC) explained almost 69.1% of variability in leaves, 65.7% of the variability in stems and 54.4% of the variability in roots. Data in Fig. 8 indicate that the S2 system showed an intermediate response between the non-treated (S1) and the full-treated (S3) group in all organs analyzed.
Na concentration of substrate sampled at the end of the experiment from each Populus alba clone ‘Marte’ plant. System 1 (S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), System 2 (S2) with distilled water on one side and 100 mM NaCl on the other side (0/100 mM NaCl) and System 3 (S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Each bar is the mean + sd (n = 4). Data were analyzed with one-way ANOVA among each system followed by Tukey’s Multiple Comparison Test (P < 0.05). Moreover, t-test analysis was performed between the two sides of each system. P-values of t-test and ANOVA are reported. Different letters denote significant different systems
Sodium (Na) concentration in Populus alba clone ‘Marte’ organs after 26 days of treatment. System 1 (S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), System 2 (S2) with distilled water on one side and 100 mM NaCl on the other side (0/100 mM NaCl) and System 3 (S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Each bar is the mean + sd (n = 4). Data were analyzed with one-way ANOVA among each system followed by Tukey’s Multiple Comparison Test (P < 0.05). Moreover, t-test analysis was performed between the two sides of each system. P-values of ANOVA and only P < 0.05 for t-test are reported. Different letters denote significant different systems
Potassium (K) concentration in Populus alba clone ‘Marte’ organs after 26 days of treatment. System 1 (S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), System 2 (S2) with distilled water on one side and 100 mM NaCl on the other side (0/100 mM NaCl) and System 3 (S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Each bar is the mean + sd (n = 4). Data were analyzed with one-way ANOVA among each system followed by Tukey’s Multiple Comparison Test (P < 0.05). Moreover, t-test analysis was performed between the two sides of each system. P-values of ANOVA and only P < 0.05 for t-test are reported. Different letters denote significant different systems
Manganese (Mn) concentration in Populus alba clone ‘Marte’ organs after 26 days of treatment. System 1 (S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), System 2 (S2) with distilled water on one side and 100 mM NaCl on the other side (0/100 mM NaCl) and System 3 (S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Each bar is the mean + sd (n = 4). Data were analyzed with one-way ANOVA among each system followed by Tukey’s Multiple Comparison Test (P < 0.05). Moreover, t-test analysis was performed between the two sides of each system. P-values of ANOVA and only P < 0.05 for t-test are reported. Different letters denote significant different systems
Biplot representation of Principal Component Analysis (PCA) based on measured variables in organs of Populus alba clone ‘Marte’. System 1 (S1) was watered with distilled water on both sides of the system (0/0 mM NaCl), System 2 (S2) with distilled water on one side and 100 mM NaCl on the other side (0/100 mM NaCl) and System 3 (S3) with 100 mM NaCl on both sides of the system (100/100 mM NaCl). Each ellipse contains 80% of the individuals grouped by systems. (A) Leaves: PC1 and PC2 represent almost 69.1% of variability, (B) stem: PC1 and PC2 represent 65.7% of the variability and (C) roots: PC1 and PC2 represent 54.4% of the variability
Discussion
Many studies on plant response to salinity have been conducted in previous years, and most of them were performed under uniform salinity treatments. Considering that salts distribution in soil can be variable, even at single plant level, studies under non-uniform stress conditions are of interest (Feng et al. 2018). For this reason, in this work we studied Populus alba L. clone ‘Marte’ response to heterogeneous salinity using a split-root system. We selected this clone because it is indicated for growing in salty soils (Salvati et al. 2007). Moreover, we selected 100 mM NaCl as our salinity condition because, taking into account different experimental conditions, it emerged from previous studies as a suitable concentration for assessing the tolerance (or sensitivity) of P. alba clones (Beritognolo et al. 2007, 2011; Mao et al. 2008, 2010). As reported by Zhang et al. (2019b) and Sixto et al. (2005), Populus alba has a wide variation of salinity tolerance within the species. ‘Guadalquivir F-21–38′, ‘Guadalquivir F-21–39′, and ‘Guadalquivir F-21–40′ clones are considered salt tolerant, while other clones have a common salt sensitivity. Considering the different performances between clones, P. alba could be used as a model species to understand the mechanisms of salt stress response.
In the split-root system study reported here, a stimulus was applied to one side of the roots and the response of the other side (not directly exposed to the stimulus) was analyzed. When NaCl was applied to one side of roots (S2 system), the untreated portion did not show a response in terms of Na accumulation, suggesting that long-distance transport mechanisms are not involved in this response, but only a local response is visible on the root side directly exposed to the stress. On the contrary, a systemic response has been observed when Na was analyzed moving to the apical part of the plant. This is a clear indication that local and systemic responses under salt stress coexist as part of the efficient interaction between roots and shoots.
Even if the S2 system showed an intermediate response between the non-treated and the full-treated group, both groups treated with salt were able to maintain a water use efficiency comparable to control (data not shown). All these results suggest that Populus alba L. clone ‘Marte’ can tolerate a prolonged exposure to 100 mM NaCl without altering growth parameters or producing severe damages to the photosynthetic machinery. This is the first experimental confirmation of the relative tolerance of this clone to salinity. Taking into account some different experimental conditions, our observations are in line with previous results obtained in other Populus alba clones identified as salt-tolerant (Sixto et al. 2006; Mao et al. 2008; Beritognolo et al. 2011). However, Feng et al. (2018) did not observe a compensatory effect in Populus x euramericana, a salt-sensitive poplar, under heterogeneous salinity. In this context, a future evaluation of root morphology, total and fine roots biomass and root water use is required.
In terms of mineral elements, we evidenced an accumulation of Na in all stressed roots. The accumulation was not significantly different between the stressed root of the half-treated group (S2) and both roots of the full-treated plants (S3) and the non-stressed root of the half-treated group was not significantly different from the control group. Na accumulation in roots evidenced a strong exclusion activity for this clone (Zhang et al. 2019b), but not enough to impede translocation. In full-stressed plants (S3), average concentrations reached in aerial organs were higher than concentrations reached in roots. In half-stressed plants we observed intermediate concentrations between control and full-stressed plants. Na, absorbed by half of the root system, was equally distributed in all aerial organs. This also explain the higher variability observed in this group.
Abiotic stresses such as Na exposure may decrease fixation of CO2 by affecting components such as the aperture of stomata through which CO2 and H2O gases are exchanged. Photosynthetic gas exchange of grey poplar leaves is highly sensitive to salt stress and the application of 75 mM NaCl reduces net assimilation without visibly damaging the leaves (Teuber et al. 2008). In our experimental condition, Na translocation pattern was correlated with the CO2 assimilation values obtained, even if we did not observe damages in the photosyntetic machinery. A possible explanation could be that, at the end of the treatment, Na was starting to produce a toxic effect but, until that moment, its dose-dependent effect was mainly related to a reduction of stomatal conductance. Indeed, it is known that plant response to salinity occurs in two phases: (a) osmotic phase and (b) ionic phase (Munns and Tester 2008). Usually, the osmotic stress has an immediate effect on growth rates compared to ionic stress. In sensitive species, the ionic effect becomes dominant over the osmotic one due to the lack of the ability to control Na transport (Munns and Tester 2008). Under salinity, the Na accumulation in plant cells is determined by the ion-exchange activity of Na efflux and influx membrane pumps.
Potassium did not change in roots between different groups but a significant reduction in stems of full-stressed plants, combined with an increase in leaves was observed. Under salinity, K concentration tends to decrease in poplar roots and stems and to increase in leaves. The increment of foliar K depends on the competition with Na: K absorption is limited in roots due to salinity and the amount previously accumulated is translocated to the above-organs (Chen and Polle 2010). Leaf increment of K could be involved in the promotion of chlorophyll synthesis in plant cells, regulation of stomata opening and activation of many enzymes involved in photosynthesis. Potassium deficiency can reduce leaf stomatal conductance, the activity of electron transfer and photosynthetic phosphorylation, and reduce the activity of the key photosynthetic enzyme Rubisco, thereby reducing the net photosynthetic rate and causing crop yield reduction (Du et al. 2019). Our results could indicate an attempt of the plant to contrast the salt stress. Shoot K reduction is also associated to the formation of smaller vessels (Chen and Polle 2010).
Both half- and full-stressed plants showed an increase in stem manganese (Mn) concentration and a tendency of increase in leaves, even if not significant. Mn is an important trace element, involved in many essential metabolic processes (Rahman et al. 2016; Alejandro et al. 2020). The increase that we observed in half- and full-treated plants could be explained by the activation of processes for contrasting salt-induced damages and oxidative stress effects (Gonzalez et al. 1998; Liu et al. 2017). Even if less pronounced, we also observed an increase in S2 and S3 plants in Zn and Cu concentrations of leaves and apical stems. For Zn, the variation in leaves was more evident in S3 plants, while for Cu, the increase in leaves and apical stems was more pronounced in the S2 group. In general, the changes observed in plants partially or fully exposed to salt could be attributed, as in the case of Mn, to the role of these elements in reducing the oxidative damage caused by salinity (Ravet and Pilon 2013). A decrease in Ca concentration in roots of full-stressed plants, combined to an increase in stem and basal leaves, as well as an increase of Mg in basal leaves were also observed. The reduction of these two elements has been observed in several salt-sensitive woody plants grown under salinity (Chen and Polle 2010; Sharmin et al. 2021) and, for this reason, the results we obtained in above-ground organs could be another indication of the salinity tolerance of clone ‘Marte’.
Conclusion
Our results provided important insights into the response of Populus alba L. clone ‘Marte’ to a heterogeneous saline environment. The study highlighted the importance of investigating the response of plants to non-uniform environmental conditions, giving a contribute to the development of stress-tolerant poplar clones. In fact, Populus alba L. clone ‘Marte’ can tolerate a prolonged exposure to 100 mM NaCl. When this concentration is applied to a portion of the root system, the presence of a Na-free side does not accelerate Na uptake by the salt-treated half. In addition, Na is not transferred from the half-roots supplied with salt to the opposite untreated half-roots. Na supply does not change plant growth, but reduces photosynthesis in a dose-dependent manner, because Na is distributed in leaves following the order: system S3 > system S2 > system S1. Moreover, mineral elements such as Ca, K, Mn, Mg, Fe and Cu change their distribution inside the plant in order to balance the mineral nutrition disorder induced by Na. Our findings opened questions that should be further studied in future. As previously mentioned, a future evaluation of root morphology, total and fine roots biomass and root water use is required in order to identify the eventual presence of compensatory mechanisms between the two sides of the root system. Moreover, other long-distance response mechanisms and salt/oxidative stress markers should be investigated. The split-root system revealed an extremely useful approach. As all other approaches, this system presents advantages and limitations. In practical terms, studies based on this technique can overcome problems related to specific experimental designs. For example, in a drought experiment in which the supply of a compound in a water-based solution form is required, possibly avoiding plant rehydration, the split-root system can be an interesting opportunity: the water solution containing the compound can be applied to a portion of the root system and, when the compound is absorbed, the portion is cut, thus limiting the rehydration process. Main limitations are related to the setting of the system, which is time consuming. In addition, the proper time for the cut could vary according to the plant species, and alterations produced by the system itself could influence the outcome of the experiment (Saiz-Fernández et al. 2021). However, the possibility to perform studies simulating realistic stress conditions, as in the case of our study, make the advantages greater than limitations.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Agapit C, Gigon A, Girin T, Leitao L, Blouin M (2020) Split-root system optimization based on the survival, growth and development of the model Poaceae Brachypodium distachyon. Physiol Plant 168(1):227–236. https://doi.org/10.1111/ppl.12971
Alejandro S, Höller S, Meier B, Peiter E (2020) Manganese in plants: from Acquisition to subcellular allocation. Front Plant Sci 11. https://doi.org/10.3389/fpls.2020.00300
Bazihizina N, Colmer TD, Barrett-Lennard EG (2009) Response to non-uniform salinity in the root zone of the halophyte Atriplex nummularia: growth, photosynthesis, water relations and tissue ion concentrations. Ann Bot 104(4):737–745. https://doi.org/10.1093/aob/mcp151
Bazihizina N, Barrett-Lennard EG, Colmer TD (2012) Plant responses to heterogeneous salinity: growth of the halophyte Atriplex nummularia is determined by the root-weighted mean salinity of the root zone. J Exp Bot 63(18):6347–6358. https://doi.org/10.1093/jxb/ers302
Beritognolo I, Piazzai M, Benucci S, Kuzminsky E, Sabatti M, Scarascia Mugnozza G et al (2007) Functional characterization of three italian Populus alba L. genotypes under salinity stress. Trees 21(4):465–477. https://doi.org/10.1007/s00468-007-0139-x
Beritognolo I, Harfouche A, Brilli F, Prosperini G, Gaudet M, Brosché M et al (2011) Comparative study of transcriptional and physiological responses to salinity stress in two contrasting Populus alba L. genotypes. Tree Physiol 31(12):1335–1355. https://doi.org/10.1093/treephys/tpr083
Celletti S, Pii Y, Valentinuzzi F, Tiziani R, Fontanella MC, Beone GM et al (2020) Physiological responses to Fe deficiency in split-root tomato plants: possible roles of auxin and ethylene? Agronomy 10(7). https://doi.org/10.3390/agronomy10071000
Chele KH, Tinte MM, Piater LA, Dubery IA, Tugizimana F (2021) Soil salinity, a serious environmental issue and plant responses: a metabolomics perspective. Metabolites 11(11). https://doi.org/10.3390/metabo11110724
Chen S, Polle A (2010) Salinity tolerance of Populus. Plant Biol 12(2):317–333. https://doi.org/10.1111/j.1438-8677.2009.00301.x
Coppa E, Celletti S, Pii Y, Mimmo T, Cesco S, Astolfi S (2018) Revisiting Fe/S interplay in tomato: a split-root approach to study the systemic and local responses. Plant Sci 276:134–142. https://doi.org/10.1016/j.plantsci.2018.08.015
Daliakopoulos IN, Tsanis IK, Koutroulis A, Kourgialas NN, Varouchakis AE, Karatzas GP et al (2016) The threat of soil salinity: a european scale review. Sci Total Environ 573:727–739. https://doi.org/10.1016/j.scitotenv.2016.08.177
De Nisi P, Vigani G, Dell’Orto M, Zocchi G (2012) Application of the split root technique to study iron uptake in cucumber plants. Plant Physiol Biochem 57:168–174. https://doi.org/10.1016/j.plaphy.2012.05.022
Du Q, Zhao X, Xia L, Jiang C, Wang X, Han Y, Wang J, Yu H (2019) Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L). J Integr Agric 18:395–406. https://doi.org/10.1016/S2095-3119(18)61953-7
Feng XH, An P, Guo K, Li XG, Liu XJ (2018) Differences in responses of moderately salt-tolerant and salt-sensitive tree species to heterogeneous salinity. Biol Plant 62(3):589–594. https://doi.org/10.1007/s10535-018-0768-5
Gonzalez A, Steffen KL, Lynch JP (1998) Light and excess manganese. Implications for oxidative stress in common bean. Plant Physiol 118(2):493–504. https://doi.org/10.1104/pp.118.2.493
Hirota I, Sakuratani T, Sato T, Higuchi H, Nawata E (2004) A split-root apparatus for examining the effects of hydraulic lift by trees on the water status of neighbouring crops. Agrofor Syst 60:181–187. https://doi.org/10.1023/B:AGFO.0000013293.77907.64
Karimi G, Ghorbanli M, Heidari H et al (2005) The effects of NaCl on growth, water relations, osmolytes and ion content in Kochia prostrata. Biol Plant 49:301–304. https://doi.org/10.1007/s10535-005-1304-y
Kibria MG, Hoque MdA (2019) A review on plant responses to Soil Salinity and Amelioration strategies. Open J Soil Sci 9:219–231. https://doi.org/10.4236/ojss.2019.911013
Kong X, Luo Z, Dong H, Eneji AE, Li W (2012) Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J Exp Bot 63(5):2105–2116. https://doi.org/10.1093/jxb/err420
Kumar S, Li G, Yang J, Huang X, Ji Q, Liu Z et al (2021) Effect of salt stress on growth, physiological parameters, and ionic concentration of Water Dropwort (Oenanthe javanica) Cultivars. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.660409
Larrainzar E, Gil-Quintana E, Arrese-Igor C, González EM, Marino D (2014) Split-root systems applied to the study of the legume-rhizobial symbiosis: what have we learned? J Integr Plant Biol 56:1118–1124. https://doi.org/10.1111/jipb.12231
Liu J, Wu L, Chen D, Li M, Wei CB (2017) Soil quality assessment of different Camellia oleifera stands in mid-subtropical China. Appl Soil Ecol 113:29–35. https://doi.org/10.1016/j.apsoil.2017.01.010
Mao H, Iwanaga F, Yamanaka N, Yamamoto F (2008) Growth, photosynthesis, and ion distribution in hydroponically cultured Populus alba L. cuttings grown under various salinity concentrations. Landsc Ecol Eng 4(2):75–82. https://doi.org/10.1007/s11355-008-0042-7
Mao H, Okada Y, Michimata S, Wang W, Iwanaga F, Yamanaka N et al (2010) Responses to nonaeration and/or salinity stress in hydroponically cultured Populus nigra and Populus alba cuttings. Landsc Ecol Eng 6(1):11–21. https://doi.org/10.1007/s11355-009-0073-8
Marino G, Brunetti C, Tattini M, Romano A, Biasioli F, Tognetti R et al (2017) Dissecting the role of isoprene and stress-related hormones (ABA and ethylene) in Populus nigra exposed to unequal root zone water stress. Tree Physiol 37(12):1637–1647. https://doi.org/10.1093/treephys/tpx083
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Palm E, Guidi Nissim W, Mancuso S, Azzarello E (2021) Split-root investigation of the physiological response to heterogeneous elevated zn exposure in poplar and willow. Environ Exp Bot 183. https://doi.org/10.1016/j.envexpbot.2020.104347
Rahman A, Hossain MS, Mahmud JA, Nahar K, Hasanuzzaman M, Fujita M (2016) Manganese-induced salt stress tolerance in rice seedlings: regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol Mol Biol Plants 22(3):291–306. https://doi.org/10.1007/s12298-016-0371-1
Ran X, Wang X, Gao X, Liang H, Liu B, Huang X (2021) Effects of salt stress on the photosynthetic physiology and mineral ion absorption and distribution in white willow (Salix alba L). PLoS ONE 16. https://doi.org/10.1371/journal.pone.0260086
Ravet K, Pilon M (2013) Copper and iron homeostasis in plants: the challenges of oxidative stress. Antioxid Redox Signal 19(9):919–932. https://doi.org/10.1089/ars.2012.5084
Saiz-Fernández I, Černý M, Skalák J, Brzobohatý B (2021) Split-root systems: detailed methodology, alternative applications, and implications at leaf proteome level. Plant Methods 17(1). https://doi.org/10.1186/s13007-020-00706-1
Salvati R, Chirici G, Corona P (2007) Modello di valutazione dell’attitudine fisica del territorio per la realizzazione di impianti cedui da biomassa in Italia. L’Italia Forestale e Montana 5:399–410
Sharmin S, Lipka U, Polle A, Eckert C (2021) The influence of transpiration on foliar accumulation of salt and nutrients under salinity in poplar (Populus × canescens). PLoS ONE 16(6):e0253228. https://doi.org/10.1371/journal.pone.0253228
Shokat S, Großkinsky DK (2019) Tackling salinity in sustainable agriculture-what developing countries may learn from approaches of the developed world. Sustain (Switzerland) 11(17). https://doi.org/10.3390/su11174558
Sixto H, Grau JM, Alba N, Alía R (2005) Response to sodium chloride in different species and clones of genus Populus L. For Int J For Res 78(1):93–104. https://doi.org/10.1093/forestry/cpi009
Sixto H, Aranda I, Grau JM (2006) Assessment of salt tolerance in Populus alba clones using chlorophyll fluorescence. Photosynthetica 44(2):169–173. https://doi.org/10.1007/s11099-006-0002-0
Sun F, Yu H, Qu J, Cao Y, Ding L, Feng W, Khalid MHB, Li W, Fu F (2020) Maize ZmBES1/BZR1-5 decreases ABA sensitivity and confers tolerance to osmotic stress in transgenic Arabidopsis. Int J Mol Sci 21(3):996. https://doi.org/10.3390/ijms21030996
Teuber M, Zimmer I, Kreuzwieser J, Ache P, Polle A, Rennenberg H, Schnitzler J-P (2008) VOC emissions of Grey poplar leaves as affected by salt stress and different N sources. Plant Biol 10:86–96. https://doi.org/10.1111/j.1438-8677.2007.00015.x
Traversari S, Francini A, Traversi ML, Emiliani G, Sorce C, Sebastiani L et al (2018) Can sugar metabolism in the cambial region explain the water deficit tolerance in poplar? J Exp Bot 69(16):4083–4097. https://doi.org/10.1093/jxb/ery195
Wu D, Shen Q, Cai S, Chen ZH, Dai F, Zhang G (2013) Ionomic responses and correlations between elements and metabolites under salt stress in wild and cultivated barley. Plant Cell Physiol 54(12):1976–1988. https://doi.org/10.1093/pcp/pct134
Xiong X, Liu N, Wei YQ, Bi YX, Luo JC, Xu RX et al (2018) Effects of non-uniform root zone salinity on growth, ion regulation, and antioxidant defense system in two alfalfa cultivars. Plant Physiol Biochem 132:434–444. https://doi.org/10.1016/j.plaphy.2018.09.028
Zhang H, Wang R, Wang H, Liu B, Xu M, Guan Y et al (2019a) Heterogeneous root zone salinity mitigates salt injury to Sorghum bicolor (L.) Moench in a split-root system. PLoS ONE 14(12). https://doi.org/10.1371/journal.pone.0227020
Zhang X, Liu L, Chen B, Qin Z, Xiao Y, Zhang Y et al (2019b) Progress in understanding the physiological and molecular responses of Populus to salt stress. Int J Mol Sci 20(6). https://doi.org/10.3390/ijms20061312
Zhao CY, Si JH, Feng Q, Deo RC, Yu TF, Li PD (2017) Physiological response to salinity stress and tolerance mechanics of Populus euphratica. Environ Monit Assess 189:533. https://doi.org/10.1007/s10661-017-6257-z
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021) Regulation of plant responses to salt stress. Int J Mol Sci 22(9). https://doi.org/10.3390/ijms22094609
Funding
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them. Financial support was provided by PiAnta project granted (CUP J54I19000770002) by Regione Toscana, POR FESR 2014–2020 for part of the equipment used (4210 MP-AES Agilent Technologie, LIO5P digital Bioclass). Financial support was provided by Agrobioscience PhD program at Scuola Superiore Sant’Anna of Pisa to LDM.
Open access funding provided by Scuola Superiore Sant’Anna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
LS and AF conceived the idea and drafted the manuscript. LDM performed physiological and morphological measurements, mineral elements analysis, statistical analysis and drafted the manuscript. AF, AG and LS reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
All authors have given approval for publication.
Additional information
Communicated by Abhijeet Shankar Kashyap.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Della Maggiora, L., Francini, A., Giovannelli, A. et al. Assessment of the salinity tolerance, response mechanisms and nutritional imbalance to heterogeneous salt supply in Populus alba L. clone ‘Marte’ using a split-root system. Plant Growth Regul 101, 251–265 (2023). https://doi.org/10.1007/s10725-023-01017-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01017-w