Abstract
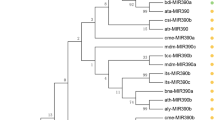
MiR390 is a conserved miRNA family and that has been confirmed to play pleiotropic roles in regulating the growth and development of plant species. Broccoli (B. oleracea L var. italica) is an important varietas of B. oleracea with abundant nutrients and cultivated worldwide. However, the mechanism underlying the miR390-mediated regulation of organ development in broccoli remains unknown. In the present study, bol-miR390a, a member of miR390 family, was identified in broccoli. Bol-miR390a displayed significantly differential expression patterns with the development of various broccoli organs. Ectopic overexpression of bol-miR390a accelerated the lateral organ development of transgenic lines in Arabidopsis. The lateral branches and roots of overexpressed bol-miR390a transgenic Arabidopsis were greater than those of the vector controls; thus, the biomass of 35S::bol-miR390a plants significantly increased relative to those of the vector controls. Moreover, the present results demonstrated that the expression levels of TRANS-ACTING SIRNA3 (TAS3)-derived siRNAs (tasiRNAs) displayed positive correlation with those of the bol-miR390a, whereas TAS3 and several auxin response factors (ARFs) including ARF2, ARF3, and ARF4, which were the potential targets of tasiRNAs, showed decreased expression trends in the overexpressed bol-miR390a transgenic plants compared with those in the vector controls. These results suggested that the bol-miR390a/TAS3/ARFs module might play important roles in the lateral organ development of broccoli. In summary, these findings confirmed that bol-miR390a is a crucial regulator in the lateral development of broccoli and suggested the potential application of bol-miR390a in breeding high-biomass crops and economic plants.






Similar content being viewed by others
References
Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouché N, Gasciolli V, Vaucheret H (2006) DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr Biol 16:927–932
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) MicroRNA-directed phasing during Trans-Acting siRNA biogenesis in plants. Cell 121:207–221
Armenta-Medina A, Lepe-Soltero D, Xiang D, Datla R, Abreu-Goodger C, Gillmor CS (2017) Arabidopsis thaliana miRNAs promote embryo pattern formation beginning in the zygote. Dev Biol 431:145–151
Axtell MJ, Jan C, Rajagopalan R, Bartel DP (2006) A two-hit trigger for siRNA biogenesis in plants. Cell 127:565–577
Axtell MJ, Snyder JA, Bartel DP (2007) Common functions for diverse small RNAs of land plants. Plant Cell 19:1750–1769
Axtell MJ, Westholm JO, Lai EC (2011) Vive la difference: biogenesis and evolution of microRNAs in plants and animals. Genome Biol 12:221
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Baskerville S, Bartel DP (2005) Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11:241–247
Bayat MR, Baluch N, Homayouni TS, Morgatskaya E, Kumar S, Kazemi P, Yeger H (2018) The role of Sulforaphane in cancer chemoprevention and health benefits: a mini-review. J Cell Commun Signal 12:91–101
Berezikov E, Plasterk RH (2005) Camels and zebrafish, viruses and cancer: a microRNA update. Hum Mol Genet 14:R183–R190
Boutet S, Vazquez F, Liu J, Beclin C, Fagard M, Gratias A, Morel JB, Crete P, Chen XM, Vaucheret H (2003) Arabidopsis HEN1: a genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr Biol 13:843–848
Bulgakov VP, Avramenko TV (2015) New opportunities for the regulation of secondary metabolism in plants: focus on microRNAs. Biotechnol Lett 37:1719–1727
Cho SH, Coruh C, Axtell MJ (2012) MiR156 and miR390 regulate tasiRNA accumulation and developmental timing in Physcomitrella patens. Plant Cell 24:4837–4849
Cullen BR (2006) Viruses and microRNAs. Nat Genet 38:S25–S30
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW (2005) AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132:4563–4574
Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, Carrington JC (2006) Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr Biol 16:939–944
Garcia D, Collier SA, Byrne ME, Martienssen RA (2006) Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol 16:933–938
Guo HS, Xie Q, Fei JF, Chua NH (2005) MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17:1376–1386
Guo RF, Deng YP, Huang ZK, Chen XD, Han XX, Lai ZX (2016) Identification of miRNAs affecting the establishment of brassica alboglabra seedling. Front Plant Sci 7:1760
Ha MJ, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15:509–524
Han MH, Goud S, Song L, Fedoroff N (2004) The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc Natl Acad Sci USA 101:1093–1098
He F, Xu CZ, Fu XK, Shen Y, Guo L, Leng M, Luo KM (2018) The MicroRNA390/TRANS-ACTING SHORT INTERFERING RNA3 module mediates lateral root growth under salt stress via the auxin pathway. Plant Physiol 177:775–791
Hobecker KV, Reynoso MA, Bustos-Sanmamed P, Wen JQ, Mysore KS, Crespi M, Blanco FA, Zanetti ME (2017) The MicroRNA390/TAS3 pathway mediates symbiotic nodulation and lateral root growth. Plant Physiol 174:2469–2486
Huang Y, Ji LJ, Huang QC, Vassylyev DG, Chen XM, Ma JB (2009) Structural insights into mechanisms of the small RNA methyltransferase HEN1. Nature 461:823–827
Huo XY, Wang C, Teng YB, Liu XY (2015) Identification of miRNAs associated with dark-induced senescence in Arabidopsis. BMC Plant Biol 15:266
Iwasaki M, Takahashi H, Iwakawa H, Nakagawa A, Ishikawa T, Tanaka H, Matsumura Y, Pekker I, Eshed Y, Vial-Pradel S, Ito T, Watanabe Y, Ueno Y, Fukazawa H, Kojima S, Machida Y, Machida C (2013) Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development 140:1958–1969
Jouannet V, Moreno AB, Elmayan T, Vaucheret H, Crespi MD, Maizel A (2012) Cytoplasmic Arabidopsis AGO7 accumulates in membrane-associated siRNA bodies and is required for ta-siRNA biogenesis. EMBO J 31:1704–1713
Kenesi E, Carbonell A, Lózsa R, Vértessy B, Lakatos L (2017) A viral suppressor of RNA silencing inhibits ARGONAUTE 1 function by precluding target RNA binding to pre-assembled RISC. Nucleic Acids Res 45:7736–7750
Kurihara Y, Watanabe Y (2004) Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci USA 101:12753–12758
Laubinger S, Sachsenberg T, Zeller G, Busch W, Lohmann JU, Rätsch G, Weigel D (2008) Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc Natl Acad Sci USA 105:8795–8800
Lee Y, Kim M, Han JJ, Yeom KH, Lee S, Baek SH, Kim VN (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23:4051–4060
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Li H, Wang Y, Wu M, Li LH, Jin C, Zhang QL, Chen CB, Song WQ, Wang CG (2017) Small RNA sequencing reveals differential miRNA expression in the early development of broccoli (Brassica oleracea var. italica) pollen. Front Plant Sci 8:404
Li H, Zhang QL, Li LH, Yuan JY, Wang Y, Wu M, Han ZP, Liu M, Chen CB, Song WQ, Wang CG (2018) Ectopic overexpression of bol-miR171b increases chlorophyll content and results in sterility in broccoli (Brassica oleracea L var. italica). J Agric Food Chem 66:9588–9597
Lim PO, Lee IC, Kim JY, Kim HJ, Ryu JS, Woo HR, Nam HG (2010) Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J Exp Bot 61:1419–1430
Lin YL, Lai ZX (2013) Comparative analysis reveals dynamic changes in miRNAs and their targets and expression during somatic embryogenesis in longan (Dimocarpus longan Lour.). PLoS ONE 8:e0060337
Liu Q, Chen YQ (2009) Insights into the mechanism of plant development: interactions of miRNAs pathway with phytohormone response. Biochem Biophys Res Commun 384:1–5
Liu Q, Yan QQ, Liu Y, Hong F, Sun ZF, Shi LL, Huang Y, Fang YD (2013) Complementation of HYPONASTIC LEAVES1 by double-strand RNA-binding domains of DICER-LIKE1 in nuclear dicing bodies. Plant Physiol 163:108–117
Mallory AC, Vaucheret H (2006) Functions of microRNAs and related small RNAs in plants. Nat Genet 38:S31–S36
Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang GL, Zamore PD, Barton MK, Bartel DP (2004) MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5’ region. EMBO J 23:3356–3364
Marin E, Jouannet V, Herz A, Lokerse AS, Weijers D, Vaucheret H, Nussaume L, Crespi MD, Maizel A (2010) MiR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 22:1104–1117
Palatnik JF, Allen E, Wu XL, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425:257–263
Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS (2005) Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA 102:3691–3696
Pasquinelli AE (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13:271–282
Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI (2000) Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408:86–89
Ramachandran V, Chen XM (2008) Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science 321:1490–1492
Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403:901–906
Rogers K, Chen XM (2013) Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 25:2383–2399
Rubio-Somoza I, Weigel D (2011) MicroRNA networks and developmental plasticity in plants. Trends Plant Sci 16:258–264
Sarkar Das S, Yadav S, Singh A, Gautam V, Sarkar AK, Nandi AK, Karmakar P, Majee M, Sanan-Mishra N (2018) Expression dynamics of miRNAs and their targets in seed germination conditions reveals miRNA-ta-siRNA crosstalk as regulator of seed germination. Sci Rep 8:1233
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of microRNAs on the plant transcriptome. Dev Cell 8:517–527
Shivaraj SM, Singh A (2016) Sequence variation in Brassica AP2 and analysis of interaction of AP2-miR172 regulatory module. Plant Cell Tiss Org 125:191–206
Shivaraj SM, Jain A, Singh A (2018) Highly preserved roles of Brassica MIR172 in polyploid Brassicas: ectopic expression of variants of Brassica MIR172 accelerates floral transition. Mol Genet Genom 293:1121–1138
Spanudakis E, Jackson S (2014) The role of microRNAs in the control of flowering time. J Exp Bot 65:365–380
Sun GL (2012) MicroRNAs and their diverse functions in plants. Plant Mol Biol 80:17–36
Talmor-Neiman M, Stav R, Klipcan L, Buxdorf K, Baulcombe DC, Arazi T (2006) Identification of trans-acting siRNAs in moss and an RNA-dependent RNA polymerase required for their biogenesis. Plant J 48:511–521
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Unver T, Namuth-Covert DM, Budak H (2009) Review of current methodological approaches for characterizing microRNAs in plants. Int J Plant Genom 2009:262463
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136:669–687
Wang L, Mai YX, ZhangYC LQ, Yang HQ (2010) MicroRNA171c-targeted SCL6-II, SCL6-III, and SCL6-IV genes regulate shoot branching in Arabidopsis. Mol Plant 3:794–806
Xia R, Xu J, Meyers BC (2017) The emergence, evolution, and diversification of the miR390-TAS3-ARF pathway in land plants. Plant Cell 29:1232–1247
Xie ZX, Khanna K, Ruan SL (2010) Expression of microRNAs and its regulation in plants. Semin Cell Dev Biol 21:790–797
Xing LJ, Zhu M, Zhang M, Li WZ, Jiang HY, Zou JJ, Wang L, Xu MY (2017) High-throughput sequencing of small RNA transcriptomes in maize kernel identifies miRNAs involved in embryo and endosperm development. Genes 8:385
Yang TX, Wang YY, Teotia S, Zhang ZH, Tang GL (2018) The making of leaves: how small RNA networks modulate leaf development. Front Plant Sci 9:824
Yoon EK, Kim JW, Yang JH, Kim SH, Lim J, Lee WS (2014) A molecular framework for the differential responses of primary and lateral roots to auxin in Arabidopsis thaliana. J Plant Biol 57:274–281
Yu B, Yang ZY, Li JJ, Minakhina S, Yang MC, Padgett RW, Steward R, Chen XM (2005) Methylation as a crucial step in plant microRNA biogenesis. Science 307:932–935
Zhang BH, Wang QG, Pan XP (2007) MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol 210:279–289
Zhang JH, Zhang SG, Han SY, Wu T, Li XM, Li WF, Qi LW (2012) Genome-wide identification of microRNAs in larch and stage-specific modulation of 11 conserved microRNAs and their targets during somatic embryogenesis. Planta 236:647–657
Zhang SX, Liu YH, Yu B (2015) New insights into pri-miRNA processing and accumulation in plants. Wiley Interdiscip Rev RNA 6:533–545
Zhang L, Yao L, Zhang N, Yang JW, Zhu X, Tang X, Calderón-Urrea A, Si HJ (2018) Lateral root development in Potato is mediated by Stu-mi164 regulation of NAC transcription factor. Front Plant Sci 9:383
Zhou GK, Kubo M, Zhong RQ, Demura T, Ye ZH (2007) Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development in Arabidopsis. Plant Cell Physiol 48:391–404
Zhou CN, Han L, Fu CX, Wen JQ, Cheng XF, Nakashima J, Ma JY, Tang YH, Tan Y, Tadege M, Mysore KS, Xia GM, Wang ZY (2013) The trans-acting short interfering RNA3 pathway and NO APICAL MERISTEM antagonistically regulate leaf margin development and lateral organ separation, as revealed by analysis of an argonaute7/lobed leaflet1 mutant in Medicago truncatula. Plant Cell 25:4845–4862
Zhou L, Quan SW, Xu H, Ma L, Niu JX (2018) Identification and expression of miRNAs related to female flower induction in Walnut (Juglans regia L.). Molecules 23:1202
Acknowledgements
This work was funded by grants from the Natural Science Foundation of China (Grant Nos. No. 31872115 and No. 31470669), the National science and technology major special project of transgenes (Grant No. 2018ZX08020003-001-003), the Science and Technology Foundation of Tianjin, China (Grant No. 18ZXZYNC00160) and the State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University, China (Grant No. NFUZD2015).
Author information
Authors and Affiliations
Contributions
LH identified the function assay of bol-miR390a; YW and PL constructed the expression vectors; YJ and YY conducted the genetic transformation; JY and LL conducted the qRT-PCR assay; XH and CC identified the targets of bol-miR390a; WS and ML conducted the phenotypic assay; LH, HL and CW designed the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, L., Wang, Y., Jia, Y. et al. Ectopic overexpression of bol-miR390a from broccoli (B. oleracea L var. italica) increases lateral branches in Arabidopsis. Plant Growth Regul 92, 547–558 (2020). https://doi.org/10.1007/s10725-020-00657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-020-00657-6