Abstract
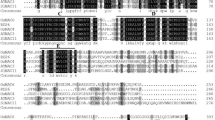
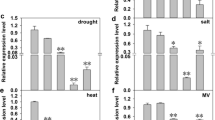
HY5, a bZIP transcription factor, plays a key role in the light- and cold-stress signaling pathways. In this study, the tomato HY5 gene (SlHY5) was isolated and characterized with respect to its function in cold-stress tolerance. The expression level of SlHY5 was similar in the root, stem, leaf, flower, and fruit of tomato. SlHY5 was upregulated gradually during cold stress and was induced in response to NaCl and the plant hormone abscisic acid. Overexpression of SlHY5 in tomato markedly increased cold stress tolerance. In addition, the SlHY5-overexpressing lines exhibited enhanced expression of genes related to antioxidant defense systems (SOD and CAT) and anthocyanin biosynthesis (CHS, CHI, and F3H) under cold stress compared to the wild-type plants, which suggested that overexpression of SlHY5 alleviated oxidative damage and lipid peroxidation, relieved membrane injuries induced by cold stress, enhanced anthocyanin accumulation, and thereby enhanced the cold-stress tolerance of SlHY5-overexpressing tomato plants. Furthermore, SlHY5 was involved in the regulation of several cold-induced genes, including PR1, CYSb, LEA, Osmotin, and ICE1, suggesting that constitutive overexpression of SlHY5 in tomato modulates the expression of other stress-responsive genes, thereby imparting cold tolerance. In conclusion, these results indicate that SlHY5 enhances plant tolerance to cold stress and that it may be used to facilitate the enhancement of stress tolerance in tomato.






Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- qRT-PCR:
-
Quantitative real-time PCR
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- H2O2 :
-
The hydrogen peroxide
- O2− :
-
Superoxide anion
- DAB:
-
Diaminobenzidine
- NBT:
-
Nitroblue tetra-zolium
- SOD:
-
Superoxide dismutase
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- POD:
-
Peroxidase
- PR1:
-
Pathogenesis-related proteins 1
- CYSb:
-
Cystatin b gene
- LEA:
-
Late embryogenesis abundant
- ICE1:
-
Inducer of CBF expression 1
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Barrero-Gil J, Huertas R, Rambla JL, Granell A, Salinas J (2016) Tomato plants increase their tolerance to low temperature in a chilling acclimation process entailing comprehensive transcriptional and metabolic adjustments. Plant, Cell Environ 39:2303–2318
Campos PS, Quartin V, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J Plant Physiol 160:283–292
Catalá R, Medina J, Salinas J (2011) Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc Natl Acad Sci USA 108:16475–16480
Chen D, Xu G, Tang W, Jing Y, Ji Q, Fei Z, Lin R (2013) Antagonistic basic helix-loop-helix/bZIP transcription factors form transcriptional modules that integrate light and reactive oxygen species signaling in Arabidopsis. Plant Cell 25:1657–1673
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17:1043–1054
Chinnusamy V, Zhu JK, Sunkar R (2010) Gene regulation during cold stress acclimation in plants. Methods Mol Biol 639:39–55
Danquah A, de Zelicourt A, Colcombet J, Hirt H (2014) The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32:40–52
Franklin KA, Whitelam GC (2007) Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat Genet 39:1410–1413
Fujikawa S, Takabe K (1996) Formation of multiplex lamellae by equilibrium slow freezing of cortical parenchyma cells of mulberry and its possible relationship to freezing tolerance. Protoplasma 190:189–203
Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9:1353–1365
Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20:1203–1207
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gong HJ, Chen KM, Zhao ZG, Chen GC, Zhou WJ (2008) Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol Plant 52:592–596
Hakim Ullah A, Hussain A, Shaban M, Khan AH, Alariqi M, Gul S, Jun Z, Lin S, Li J, Jin S, Munis MFH (2018) Osmotin: a plant defense tool against biotic and abiotic stresses. Plant Physiol Biochem 123:149–159
Harvaux M, Kloppstech K (2001) The protective functions of carotenoid and avonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 213:953–966
Heidarvand L, Amiri RM (2010) What happens in plant molecular responses to cold stress? Acta Physiol Plant 32:419–431
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27:297–300
Horváth E (2009) Protoplast isolation from Solanum lycopersicum L. leaf tissues and their response to short-term NaCl treatment. Acta Biol Szeged 53:83–86
Hsieh TH, Lee JT, Chang YY, Chan MT (2002a) Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiol 130:618–626
Hsieh TH, Lee JT, Yang PT, Chiu LH, Charng YY, Wang YC, Chan MT (2002b) Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol 129:1086–1094
Huang X, Ouyang X, Yang P, Lau OS, Li G, Li J, Chen H, Deng XW (2012) Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 24:4590–4606
Jongsma M, Koornneef M, Zabel P, Hille J (1987) Tomato protoplast DNA transformation: physical linkage and recombination of exogenous DNA sequences. Plant Mol Biol 8:383–394
Lau OS, Deng XW (2010) Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol 13:571–577
Laxa M, Liebthal M, Telman W, Chibani K, Dietz KJ (2019) The role of the plant antioxidant system in drought tolerance. Antioxidants 8:94
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749
Lindemose S, O’Shea C, Jensen MK, Skriver K (2013) Structure, function and networks of transcription factors involved in abiotic stress responses. Int J Mol Sci 14:5842–5878
Liu H, Ouyang B, Zhang J, Wang T, Li H, Zhang Y, Yu C, Ye Z (2012) Differential modulation of photosynthesis, signaling, and transcriptional regulation between tolerant and sensitive tomato genotypes under cold stress. PLoS ONE 7:e50785
Loyola R, Herrera D, Mas A, Wong DCJ, Holl J, Cavallini E, Amato A, Azuma A, Ziegler T, Aquea F (2016) The photomorphogenic factors UV-B RECEPTOR 1, ELONGATED HYPOCOTYL 5, and HY5 HOMOLOGUE are part of the UV-B signalling pathway in grapevine and mediate flavonol accumulation in response to the environment. J Exp Bot 67:5429–5445
Martinez M, Gómez-Cabellos S, Giménez MJ, Barro F, Diaz I, Diaz-Mendoza M (2019) Plant proteases: from key enzymes in germination to allies for fighting human gluten-related disorders. Front Plant Sci 10:1–8
Nylander M, Heino P, Helenius E, Palva ET, Ronne H, Welin BV (2001) The low-temperature- and salt-induced RCI2A gene of Arabidopsis complements the sodium sensitivity caused by a deletion of the homologous yeast gene SNA1. Plant Mol Biol 45:341–352
Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405:462–466
Park EJ, Jekni Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen THH (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40:474–487
Patade VY, Khatri D, Kumari M, Grover A, Gupta SM, Ahmed Z (2013) Cold tolerance in Osmotin transgenic tomato (Solanum lycopersicum L.) is associated with modulation in transcript abundance of stress responsive genes. SpringerPlus 2:117
Puyang X, An M, Han L, Zhang X (2015) Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poa pratensis L.) cultivars. Ecotoxicol Environ Saf 117:96–106
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Shi Y, Zhang Y, Yao H, Wu J, Sun H, Gong H (2014) Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol Biochem 78:27–36
Shin DH, Choi M, Kim K, Bang G, Cho M, Choi SB, Choi G, Park YI (2013) HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett 587:1543–1547
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Sivankalyani V, Geetha M, Subramanyam K, Girija S (2015) Ectopic expression of Arabidopsis RCI2A gene contributes to cold tolerance in tomato. Transgenic Res 24:237–251
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Ann Rev Plant Physiol 35:543–584
Sun HJ, Uchii S, Watanabe S, Ezura H (2006) A highly efficient transformation protocol for micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol 47:426–431
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, beta-lains and carotenoids. Plant J 54:733–749
Thomashow MF (1998) Role of cold-responsive genes in plant freezing tolerance. Plant Physiol 118:1–8
Thomashow MF (2010) Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol 154:571–577
Uemura M, Tominaga Y, Nakagawara C, Shigematsu S, Minami A, Kawamura Y (2006) Responses of the plasma membrane to low temperatures. Physiol Plant 126:81–89
Wang H, Ma LG, Li JM, Zhao HY, Deng XW (2001) Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294:154–158
Wang W, Zhao P, Zhou X, Xiong H, Sun M (2015) Genome-wide identification and characterization of cystatin family genes in rice (Oryza sativa L.). Plant Cell Rep 34:1579–1592
Wang WL, Wang X, Zhang J, Huang M, Cai J, Zhou Q, Dai TB, Jiang D (2020) Salicylic acid and cold priming induce late-spring freezing tolerance by maintaining cellular redox homeostasis and protecting photosynthetic apparatus in wheat. Plant Growth Regul 90:1093–1121
Weiss J, Egea-Cortines M (2009) Transcriptomic analysis of cold response in tomato fruits identifies dehydrin as a marker of cold stress. J Appl Genet 50:311–319
Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27:581–590
Xu L, Pan R, Shabala L, Shabala S, Zhang WY (2019) Temperature influences waterlogging stress-induced damage in Arabidopsis through the regulation of photosynthesis and hypoxia-related genes. Plant Growth Regul 89:143–152
Xue GP (2002) Characterisation of the DNA-binding profile of barley HvCBF1 using an enzymatic method for rapid, quantitative and high-throughput analysis of the DNA-binding activity. Nucleic Acids Res 30:e77
Xue GP (2003) The DNA-binding activity of an AP2 transcriptional activator HvCBF2 involved in regulation of low-temperature responsive genes in barley is modulated by temperature. Plant J 33:373–383
Yaday SK (2010) Cold stress tolerance mechanisms in plants. A review. Agron Sustain Dev 30:515–527
Yang BC, Song ZH, Li CN, Jiang JH, Zhou YY, Wang RP, Wang Q, Ni C, Liang Q, Chen HD (2018) RSM1, an Arabidopsis MYB protein, interacts with HY5/HYH to modulate seed germination and seedling development in response to abscisic acid and salinity. PLoS Genet 12:e1007839
Yin Y, Ma QP, Zhu ZX, Cui QY, Chen CS, Chen X, Fang WP, Li XH (2016) Functional analysis of CsCBF3 transcription factor in tea plant (Camellia sinensis) under cold stress. Plant Growth Regul 80:335–343
Zhang X, Liu S, Takano T (2008) Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol 68:131–143
Zhang Y, Zheng S, Liu Z, Wang L, Bi Y (2011) Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedling. J Plant Physiol 168:367–374
Zheng X, Tian S (2006) Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem 96:519–523
Acknowledgements
This study is supported by Shaanxi Natural Science Foundation of China (2018JQ3056).
Author information
Authors and Affiliations
Contributions
JG analyzed the results and wrote the paper. SF revised the manuscript. NH and TZ conducted most of the experiments. HS conducted experiments of subcellular localization of SlHY5. YZ analyzed the original data. HG conceived the idea for the project, and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, N., Fan, S., Zhang, T. et al. SlHY5 is a necessary regulator of the cold acclimation response in tomato. Plant Growth Regul 91, 1–12 (2020). https://doi.org/10.1007/s10725-020-00583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-020-00583-7