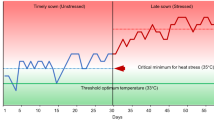
Abstract
Rice varieties are generally bred for higher yield but may possess genomic regions conferring tolerance to abiotic stresses. Climate change driven heat stress during reproductive stage of the crop affects spikelet fertility and yield. Though genetic regions associated with heat stress tolerance have been identified in rice, but response of rice varieties and allelic phenotypic effect favoring spikelet fertility during heat stress has not been comprehensively studied. Hence, the present study aimed at assessing the response of 198 rice varieties during the dry season (2016) followed by validation of selected 67 varieties in the second dry season (2017) through staggered sowing for high temperature. The analysis showed mean spikelet sterility of 21.82% and 33.81% for the first and second sowing, respectively. Further, average difference in spikelet sterility for unit increase in maximum temperature during the flowering period was observed to be 7.93%. Employment of nine heat stress associated markers for genetic analysis identified four sub-populations in the 67 varieties inferred through neighbor-joining phylogenetic tree and sub-structure analysis. Marker-trait association analysis showed two markers namely RM205, RM242 were significantly associated with spikelet sterility with phenotypic variance (R2) of 7.7% and 6.0%, respectively. Allelic phenotypic effect of favorable alleles for both the markers reduced spikelet sterility by 14.49% compared to mean spikelet sterility (33.81%). Furthermore, four rice varieties showed spikelet sterility < 15%. Thus, predominantly moderate tolerance to susceptible response was observed for rice varieties in this study. Besides, favorable allele of RM205, RM242 could be effectively used for improving tolerance in rice varieties to heat stress.








Similar content being viewed by others
Data availability statement
All the data has been given in supplementary information.
References
Bernier J, Kumar A, Ramaiah V, Spaner D, Atlin G (2007) A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Sci 47(2):507–516
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinform 23(19):2633–2635
Dai P, Miao Y, He S, Pan Z, Jia Y, Cai Y, Du X (2019) Identifying favorable alleles for improving key agronomic traits in upland cotton. BMC Plant Biol 19(1):138
Das S, Krishnan P, Nayak M, Ramakrishnan B (2014) High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ Expt Bot 101:36–46
Dixit S, Singh A, Cruz MTS, Maturan PT, Amante M, Kumar A (2014) Multiple major QTL lead to stable yield performance of rice cultivars across varying drought intensities. BMC Genom 15(1):16
Earl DA (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conser Genet Res 4:359–361
Frederiks TM, Christopher JT, Harvey GL, Sutherland MW, Borrell AK (2012) Current and emerging screening methods to identify post-head-emergence frost adaptation in wheat and barley. J Expt Bot 63(15):5405–5416
Fujimori S, Hasegawa T, Rogelj J, Su X, Havlik P, Krey V, Riahi K (2018) Inclusive climate change mitigation and food security policy under 1.5° C climate goal. Environ Res Lett 18:7.
Hakata M, Wada H, Masumoto-Kubo C, Tanaka R, Sato H, Morita S (2017) Development of a new heat tolerance assay system for rice spikelet sterility. Plant Met 13(1):34
IRRI I, (2002) Standard evaluation system for rice. International Rice Research Institute, Los Baños, pp 1–45
Ishimaru T, Hirabayashi H, Sasaki K, Ye C, Kobayashi A (2016a) Breeding efforts to mitigate damage by heat stress to spikelet sterility and grain quality. Plant Prod Sci 19(1):12–21
Ishimaru T, Xaiyalath S, Nallathambi J, Sathishraj R, Yoshimoto M, Phoudalay L, Muthurajan R (2016b) Quantifying rice spikelet sterility in potential heat-vulnerable regions: field surveys in Laos and southern India. Field Crops Res 190:3–9
Jagadish SVK, Craufurd PQ, Wheeler TR (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.). J Expt Bot 58:1627–1635
Jagadish SVK, Murty MVR, Quick WP (2015) Rice responses to rising temperatures–challenges, perspectives and future directions. Plant Cell Environ 38(9):1686–1698
Jha UC, Bohra A, Singh NP (2014) Heat stress in crop plants: its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed 133(6):679–701
Johnson NC, Xi SP, Kosaka Y, Li X (2018) Increasing occurrence of cold and warm extremes during the recent global warming slowdown. Nat commun 9(1):1724
Krishnan P, Ramakrishnan B, Raja Reddy K, Reddy VR (2011) High-temperature effects on rice growth, yield, and grain quality. Adv Agron 111:87
Kumar A, Dixit S, Ram T, Yadaw RB, Mishra KK, Mandal NP (2014) Breeding high-yielding drought-tolerant rice: genetic variations and conventional and molecular approaches. J Expt Bot 65(21):6265–6278
Lynn J (2018) Communicating the IPCC: Challenges and Opportunities. Handbook of Climate Change Communication 3:131–143. Springer, Cham.
Mahantashivayogayy AK, Lakkund BS, Ramesha MS, Ibrahum M, Reddy BM, Gurupradas G, Pramesh, (2016) Screening rice genetic resources for heat tolerance. Ecoscan 10(1–2):203–206
Manigbas NL, Lambio LAF, Luvina B, Cardenas CC (2014) Germplasm innovation of heat tolerance in rice for irrigated lowland conditions in the Philippines. Rice Sci 21(3):162–169
Maruyama A, Weerakoon WMW, Wakiyama Y, Ohba K (2013) Effects of increasing temperatures on spikelet fertility in different rice cultivars based on temperature gradient chamber experiments. J Agron Crop Sci 199:416–423
Milne I, Shaw P, Stephen G, Bayer M, Cardle L, Thomas WT, Marshall D (2010) Flapjack—graphical genotype visualization. Bioinform 26(24):3133–3134
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8(19):4321–4326
Perrier X, Flori A, Bonnot F (2003) Data analysis methods. In: Hamon P, Seguin M, Perrier X, Glaszmann JC (eds) Genetic diversity of cultivated tropical plants. Enfield, Science Publishers, Montpellier, pp 43–76
Pradhan SK, Barik SR, Sahoo A, Mohapatra S, Nayak DK, Mahender A, Pandit E (2016) Population structure, genetic diversity and molecular marker-trait association analysis for high temperature stress tolerance in rice. PLoS ONE 11:e0160027
Prasad PVV, Boote KJ, Allen LH, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res 95:398–411
Prasanth VV, Basava KR, Babu MS, Venkata Tripura VGN, Devi SR, Mangrauthia SK, Sarla N (2016) Field level evaluation of rice introgression lines for heat tolerance and validation of markers linked to spikelet fertility. Physiol Mol Bio Plants 22(2):179–192
Pritchard JK, Wen W, Falush D (2003) Documentation for structure software: Version 2.
Reig-Valiente JL, Viruel J, Sales E, Marqués L, Terol J, Gut M, Domingo C (2016) Genetic diversity and population structure of rice varieties cultivated in temperate regions. Rice 9(1):58
Shanmugavadivel PS, Prakash C, Ramkumar MK, Tiwari R, Mohapatra T, Singh NK (2017) High Resolution Mapping of QTLs for Heat Tolerance in Rice Using a 5K SNP Array. Rice 10:28
Singh N, Choudhury DR, Tiwari G, Singh AK, Kumar S, Srinivasan K, Singh R (2016) Genetic diversity trend in Indian rice varieties: an analysis using SSR markers. BMC Gen 17(1):127
Teixeira EI, Fischer G, van Velthuizen H, Walter C, Ewert F (2013) Global hot-spots of heat stress on agricultural crops due to climate change. Agrl Meteorol 170:206–215
Tenorio FA, Ye C, Redoña E, Sierra S, Laza M, Argayoso MA (2013) Screening rice genetic resources for heat tolerance. SABRAO J Breed Genet 45(3):371–381
Tian X, Matsui T, Li S, Yoshimoto M, Kobayasi K, Hasegawa T (2010) Heat-Induced Floret Sterility of Hybrid Rice (Oryza sativa L.) Cultivars under Humid and Low Wind Conditionsin the Field of Jianghan Basin, China. Plant Prod Sci 13(3):243–251.
Torres RO, Henry A (2016) Yield stability of selected rice breeding lines and donors across conditions of mild to moderately severe drought stress. Field Crops Res 220:37–45
Vivitha P, Raveendran M, Vijayalakshmi D (2017) Introgression of QTLs controlling spikelet fertility maintains membrane integrity and grain yield in improved white Ponni derived progenies exposed to heat stress. Rice Sci 24(1):32–40
Wei H, Liu J, Wang Y, Huang N, Zhang X, Wang L, Zhong X (2012) A dominant major locus in chromosome 9 of rice (Oryza sativa L.) confers tolerance to 48 C high temperature at seedling stage. J Hered 104(2):287–294
Welch JR, Vincent JR, Auffhammer M, Moya PF, Dobermann A, Dawe D (2010) Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc Natl Acad Sci 107:14562–14567
Williams LJ, Abdi H (2010) Fisher’s least significant difference (LSD) test. Encyclopedia Res Des 218:840–853
Wossen T, Berger T, Haile MG, Troost C (2018) Impacts of climate variability and food price volatility on household income and food security of farm households in East and West Africa. Agri Sys 163:7–15
Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Peng S (2016) Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci Rep 6:34978
Ye C, Argayoso MA, Redoña ED, Sierra SN, Laza MA, Dilla CJ, Diaz GQ (2012) Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed 131(1):33–41
Ye C, Tenorio FA, Redoña ED, Morales-Cortezano PS, Cabrega GA, Jagadish KS, Gregorio GB (2015) Fine-mapping and validating qHTSF4. 1 to increase spikelet fertility under heat stress at flowering in rice. Theor Appl Genet 128(8):1507–1517
Yun-Ying CAO, Hua D, Yang L-N, Wang Z-Q, Zhou S-C, Yang J-C (2008) Effect of heat stress during meiosis on grain yield of rice cultivars differing in heat tolerance and its physiological mechanism. Acta Agron Sinica 34(12):2134–2142
Funding
We sincerely acknowledge the Indian Council of Agricultural Research (ICAR) for providing funds for conducting the research. We equally acknowledge Dr. Himanshu Pathak, Director, ICAR- NRRI, Cuttack for supporting the research and providing the necessary facilities for conducting the research.
Author information
Authors and Affiliations
Contributions
PC, UN conceptualized the experiment and performed the analysis. HNS provided the materials for the study. PC and SS wrote the manuscript. CB and NB did the phenotypic analysis. MP, DBN did the genotyping analysis. AK, JLK did the statistical analysis.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Informed consent
All the authors has read and approved the manuscript for submission and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10722_2021_1106_MOESM1_ESM.tif
Supplementary Fig.1 Distribution of varieties based on spikelet sterility (%) in the first year of study. Sixty-two varieties and five check varieties (ADT43, Dular, Annapurna, IR36 and IR50) were taken for second year of study. (TIF 611 kb)
10722_2021_1106_MOESM3_ESM.tif
Supplementary Fig.3 Mean spikelet sterility % of favorable and non-favorable alleles for the markers RM242 and RM205 (TIF 3728 kb)
Rights and permissions
About this article
Cite this article
Chidambaranathan, P., Balasubramaniasai, C., Behura, N. et al. Effects of high temperature on spikelet sterility in rice (Oryza sativa L.): association between molecular markers and allelic phenotypic effect in field condition. Genet Resour Crop Evol 68, 1923–1935 (2021). https://doi.org/10.1007/s10722-021-01106-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-021-01106-7