Abstract
Cajanus cajan (L.) Millsp. commonly known as pigeonpea, red gram or gungo pea is an important grain legume crop, particularly in rain-fed agricultural regions in the semi-arid tropics, including Asia, Africa and the Caribbean. This paper provides a baseline for the study of the domestication and early history of C. cajan, through reviewing its modern wild distribution, seed morphometrics of wild and domesticated populations, historical linguistics and the archaeological record. The distribution of wild populations, including published records and additional herbarium collections, suggest that the wild habitats of pigeonpea were at the interface of the forest-edge areas and more open savanna plains in eastern Peninsular India (e.g. Telangana, Chattisgarh, Odisha). Early archaeological finds presented here have been recovered from both the Southern peninsula and Odisha. Historical linguistic data suggests early differentiation into longer and shorter growing season varieties, namely arhar and tuar types, in prehistory. Pigeonpea had spread to Thailand more than 2000 years ago. Measurements of seeds from modern populations provide a baseline for studying domestication from archaeological seeds. Available measurements taken on archaeological Cajanus spp. suggest that all archaeological collections thus far fall into a domesticated Length:Width ratio, while they may also pick up the very end of the trend towards evolution of larger size (the end of the domestication episode) between 3700 and 3200 years BP. This suggests a trend over time indicating selection under domestication had begun before 3700 years ago and can be inferred to have started 5000–4500 years ago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cajanus cajan (L.) Millsp. has, until fairly recently, received relatively little research attention despite its importance in India and other countries worldwide. Colloquially known as the ‘orphan crop’ or ‘poor people’s meat’ due to its high protein content, Cajanus cajan (L.) Millspaugh (Syn.: Cajanus indicus Spreng.; Cajanus flavus DC.; Cytisus cajan L.) from the family Fabaceae, is more commonly known as pigeonpea. It is an important grain legume, particularly in rain-fed agricultural regions in the semi-arid tropics, as well as an excellent, high-protein cover/forage for livestock (Duke 1983; Pal et al. 2011; Randhawa 1958) which can be intercropped with sorghum and/or millets (Shetty and Rao 1981). The genus Cajanus has 32 species (Mallikarjuna et al. 2012, 411) with 18 occurring in India (Mallikarjuna et al. 2011). Despite some claims for an African origin (Watt 1889; Purseglove 1976) it has been convincingly demonstrated that the likely wild progenitor was Cajanus cajanifolius found today in eastern India (van der Maesen 1986), including the modern state of Odisha and adjacent states, an origin earlier suggested by Haines (1921-1925). This is reinforced by modern genetic data (Kassa et al. 2012). Today the largest producer of pigeonpea is India (Pal et al. 2016; Nwokolo 1996), but it is also found across large parts of Southeast Asia, Africa and the Caribbean where it is commonly known as congo pea, or gungo pea.
As a subsistence pulse crop C. cajan contains high levels of protein and amino acids such as methionine, lysine and tryptophan and is an important source of dietary vitamins and minerals particularly B vitamins and is therefore especially important for people living on subsistence diets (Oshodi et al. 2009). It is grown in large quantities in modern India (Fig. S1a), and shows increasing areas of cultivation (Fig. S1b). Pigeonpea seeds are found in a huge diversity of flavours (from bitter to sweet) and colours (from black to creamy white) (Upadhyaya et al. 2005). Pigeonpea is most commonly used to make ‘dhal’ (soaked dried, hulled, and split seeds) (Shinde et al. 2017) and in parts of Southeast Asia the seeds are used instead of soya bean to make tempe or tofu (Shurtleff and Aoyagi 2013; Owens et al. 2015; van der Maesen and Somaatmadja 1989) and noodles in Myanmar (APO 2003). Immature green pigeonpeas can also be harvested and cooked as a fresh vegetable, which is more common in the Caribbean and Southeast Asia. The leaves from this plant are considered an excellent fodder for cattle and the dry wood is considered a good fuel (Watt 1889). Thus, largely all parts of the pigeonpea plant are utilized and integrated into daily use. A number of potential medicinal uses have also been explored (e.g. Allen and Allen 1981; Al-Saeedi and Hossain 2015; Uchegbu and Ishiwu 2016).
Botanically, it is an annual or erect short-lived perennial shrub, measuring 1–2 m in height, with a variable habit. Modern day populations grow in a range of soil types and climates, throughout tropical and subtropical regions of the world from South Asia to Australia (Khoury et al. 2015; Kassa et al. 2012). It thrives with an annual rainfall of 600–1000 mm yet is also drought tolerant and can be grown in areas with < 300 mm rainfall (Kingwell-Banham and Fuller 2013). Hence, it is an important crop for small-scale farmers in semi-arid areas where rainfall is low or variable. C. cajan has an extensive habitat range throughout India growing at altitudes to over 1800 m (Watt 1889). Despite adaptation to versatile environmental conditions, crop productivity has remained stagnant for almost the last 5 decades at production levels of roughly 750 kg/ha (Fig. S1a) (Bohra et al. 2012).
Cultivated types can be grouped into two varieties. C. cajan (L.) Millsp., var. bicolor DC., Hindi arhar, a late maturing, large, bushy plant which normally takes between 6 and 11 months to reach maturity, and a short-season variety, C. cajan, var. flavus DC., Hindi tuvar, which can reach maturity more rapidly, within only 3–4 months (Kingwell-Banham and Fuller 2013). Whether or not these two varieties may be phylogenetic subspecies, they are useful varietal groups as they correspond to different cultivation regimes (season length) and different colloquial names (see below). Although the arhar types are regarded as more primitive and closer to the perennial ancestor (Smartt 1990), both varieties have a long history in excess of 2000 years, judging by historical linguistic evidence (see Supplement section), suggesting that this differentiation took place early in the evolution of this crop. C. cajan has one of the highest yields per area in comparison with other South Asian pulses (Fig. S1). In contrast, archaeologically, the recovery of any Cajanus sp. is quite low in comparison with other pulses from South Asian archaeological sites (Fuller and Harvey 2006; Harvey et al. 2006; Fuller and Murphy 2018; Murphy and Fuller 2016; Smartt 1990), suggesting that perhaps Cajanus use was more limited in the past and that it may have been domesticated later than some other legume taxa in India. Cajanus spp. are likewise found in very few sites in Southeast Asia compared to another South Asian pulse, Vigna radiata (L.) Wilczek, that is widely found (Castillo et al. 2016, 2018a).
Recent genetics, historical linguistics and evidence for origins
Cajanus cajanifolius (Haines) Maesen is accepted as the progenitor species of cultivated C. cajan (L.) Millsp. (van der Maesen 1986; Smartt 1990; Mallikarjuna et al. 2012; Sinha et al. 2015). Domesticated C. cajan possesses 75% less allelic diversity than the progenitor clade of wild Indian species, suggesting a severe “domestication bottleneck” (Saxena et al. 2014; Al-Saeedi and Hossain 2015). Hence, pigeonpea’s improvement is increasingly reliant on introgression with wild forms with their diversity of phenotypic traits, practice that would benefit from knowledge of its domestication history and early selection from the wild species (Kameswara Rao et al. 2003; Kassa et al. 2012; Pandey et al. 2008; Upadhyaya et al. 2007, 2013). This is especially important as habitat loss threatens wild Cajanus spp. populations (Khoury et al. 2015; Sahai and Rawat 2015).
The highest level of polymorphism in wild relatives and landraces were found within the states of Madhya Pradesh and Andhra Pradesh, leading Saxena et al. (2014) to infer domestication somewhere along India’s eastern coast (e.g. Andhra Pradesh), further south than the Odishan origins inferred by Fuller and Harvey (2006) on the basis of Van der Maesen’s (1986) wild distribution map. Archaeobotanical evidence should ultimately provide more solid evidence.
Historical linguistic evidence can also inform on the origins of pigeonpea. A compilation of names for pigeonpea across numerous languages in India and beyond is provided in Supplementary Information and Tables S1 and S2. Ancient linguistics in India early on differentiated the long growth cycle varieties (var. bicolor), from short-cycle varieties (var. flavus). We can infer two early roots for the long cycle forms, one from early Indic (Indo-European) and one from Dravidian. The first is the source of Hindi arhar, derived from ancient Prakrit adhai, with loan word names evident in some Austroasiatic (Munda) languages in eastern India as well as some Southeast Asia (e.g. Thai hae) and distant west African languages, like Togo and Hausa. An alternative source was the reconstructed Early Dravidian form *kar-unti (Southworth 2005), the source of various derivative names based on the element kan or gan, including some Southeast Asian names, such as in Burmese and Malay, as well as some southeastern African names, such as Malawi kardis. For short-cycle field crops there is a single widespread cognate set found in both the reconstructions of Early Dravidian (*tu-var-) and Old Indo-Aryan (*tubarī-). These shared terms suggest the evolution of short cycle varieties may have taken place near where these language families overlap geographically, namely around Odisha, Chattisgarh and/or northeast Andhra Pradesh, which is likely to be in or near zone for the domestication and early evolution of domesticated Cajanus cajan.
Materials and methods
Archaeobotany
The present study adopts a fourfold approach of examining three different lines of evidence. First, we surveyed herbarium specimens of likely wild progenitor populations held in herbarium collections from Royal Botanic Gardens, Kew and the Natural History Museum, London (NHM) (Table 1). These provided potential augmentation to the distribution of wild populations mapped by van der Maesen (1986) which can be combined with the geographic distribution of climatic conditions similar to where C. cajanifolius has been found. Second, we took measurements to provide an extensive morphometric baseline for seed size in modern domesticated and wild pigeonpea (Table 2), which provides a basis from which to infer the domesticated status of archaeological pigeonpea based upon seed measurements. Third, we recorded measurements of archaeological pigeonpea, both of specimens in our collections and those in the published literature (Table 3). These provide a time series of seed size data for regional populations, especially for the Deccan and South India, which allows us to trace the evolution of seed size as one aspect of the domestication syndrome in this species. Lastly, we provide an updated database on the archaeological occurrence of pigeonpea in time and space, allowing us to infer the region(s) in which it first occurred in the human diet and/or cultivation systems in prehistory (Table 3, Fig. 4).
Initially, herbarium collections for Cajanus were surveyed including those from South Asia and from Africa with the kind permission of the Natural History Museum, London (Fig. 1). This included specimens filed under genus Atylosia (synonymous to Cajanus). In addition, where seeds were visible on the herbarium specimen, or loose in attached pouches, these were measured, both for wild and cultivated populations, including wild populations from Africa; their seed metrics contribute to a baseline for the size range of wild seeds (Supplementary Table S4).
Seed measurements were also taken on modern crop populations of C. cajan from several reference collections including the UCL archaeobotany reference collection, augmented with additional germplasm obtained from the USDA, as well as from the Economic Botany collection of Royal Botanic Gardens, Kew (Table 3 and Supplementary Table S4). Archaeological specimens from South Asian and Southeast Asian sites were also measured from our collections, as well as some compiling of data available from published literature (Table 3 and Supplementary Table S5). Archaeobotanically, pigeonpea identification is aided by a distinctive apostrophe-shaped shoot bud (plumule) within the embryo that is often visible on the charred split cotyledon as an imprint (Fig. 2).
Archaeological examples of Cajanus cajan. a Example from Neolithic Sanganakallu, Karnataka, interior of cotyledon with plumule visible (from Fuller 1999). b Example from Chalcolithic Gopalpur, Odisha drawn by DQF, from Harvey et al. (2006); c Example from Early Historic Paithan, Maharashtra, with plumule highlighted (Photo by C). d Example from Terrace of the Leper King, Angkor Thom, Cambodia (Photo by CCC). (Color figure online)
Results
The known distribution of modern Cajanus reveals a limited latitudinal distribution of the ‘wild’ sister species and comparatively broad distribution of modern day domesticates of Cajanus (Fig. 3). Cajanus cajanifolius is clustered in eastern India in what is now the modern state of Odisha (formerly Orissa). What is notable is that whilst Cajanus spreads outside of its native habitat in South Asia into Southeast Asia it does not cross the ecological boundary of the Himalayas. In China several southern provinces have modern populations of Cajanus cajan, mentioned in floristic sources (Hu 2005; Ren and Gilbert 2010) and noted in our herbarium survey, but none of these appears to be cultivated. These occur as “wild” or free-growing populations, but near human disturbance. Our observations suggest that these have dehiscent pods, rather than domesticated type non-dehiscent pods. Other characteristics resemble C. cajan, suggesting that these populations should be regarded as feral, representing past “escapes” from cultivation. This then implies that at some time in the past pigeonpea was cultivated across parts of Southern China as far east as Taiwan, and that cultivation has been abandoned subsequently. The major transformations of agriculture allowed by the introduction of New World taxa, like maize and Phaseolus and Canavalia beans might have altered the attractiveness of Cajanus cultivars in some regions.
Map displaying all the known modern range of regular Cajanus cajan cultivation in the Old World, areas of inferred former cultivation based on feral populations in China and sites cultivation and/or feral population in Island Southeast Asia (blue circles beyond shaded area). The distribution of wild progenitors (yellow stars), augmented from van der Maesen 1986 by this study.
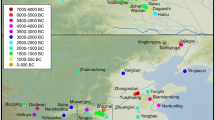
Archaeobotanical evidence for Cajanus is richest for India, with a few finds from mainland Southeast Asia (Fig. 4). As is evident, most of these finds lie outside the likely zones of domestication around the Southern Odisha, Northern Andhra, and eastern Maharashtra borders. In addition, the earliest finds to date come from the South Indian Neolithic at Piklihal and Sanganakallu, implying earlier cultivation and dispersal of this species prior to 1650 BC.
Map displaying all the known archaeological specimens of Cajanus cajan in relation to the modern wild distribution of Cajanus cajanifolius (after van der Maesen 1986, and this study). Archaeobotanical finds of Cajan cajan or cf. Cajanus cajan. Sites numbered: 1. Hallur, 2. Piklihal, 3. Kadebakele, 4. Sanganakallu, 5. Peddamudiyam, 6. Nevasa, 7. Paithan (2 phases), 8. Bhokardan, 9. Bhon, 10. Paturda, 11. Kholapur, 12. Tuljapur Garhi, 13. Kaundinyapur, 14. Bhagimohari, 15. Mahurzkari (2 phases), 16. Charda, 17. Golbai Sassan, 18. Gopalpur, 19. Vikrampura, 20. Wari Bateshwar, 21. Phu Khao Tong, 22. Khao Sam Kaeo, 23. Angkor Thom Terrace of the Leper King
A scatter plot of the length and width measurements of all the modern specimens, including wild species of Cajanus, shows a separation of the domesticated types from the wild progenitor (C. cajanifolius) and other congeneric wild taxa (Fig. 5). A great deal of intra-species variation is present within the domesticated C. cajan specimens. There are broadly two forms of domesticated seeds, those with a low Length/Width ratio, i.e. with small but “tall” seeds (lower left in Fig. 5 (L/W ratio 0.8–1) and those with large and long seeds (i.e. with L/W ratios > 1 and with width > 4). Although wild specimens we have been able to measure are limited, they all appear to have low L/W ratios that fall between 0.68 and 0.8- and with width of < 44 mm (Fig. 6). This indicates that seed L/W ratio appears be a useful way to determine whether seeds are domesticated or wild. In addition, seed Length appears to have increased significantly, on average, in domesticated C. cajan, whereas seed width may not have (Fig. 7). A t test of Width indicated no significant difference in the mean width, whereas a t-test of Length is significant (p = 8.05 × 10−11); a Kolmogorov–Smirnov test for equal distributions in the Length of C. cajan versus C. cajanifolius indicates significantly different distributions (p = .0001, including Monte Carlo permutations). This suggests that we would expect to see an increase in seed Length over time during the domestication process as well as an increase in L/W ratio.
It is well-known that archaeological seeds, preserved by charring, undergo shrinkage, and this is often estimated to be on the order to 10–20%, with 20% shrinkage used to estimate shrinkage in pulses (e.g. Fuller and Harvey 2006; Fuller and Murphy 2018) This leads to the inference that a minimum Length for charred domesticated specimens should be around 4 mm or more (based on the 25th percentile on modern material); whereas the upper end estimated from modern wild seeds is around 3 mm (based on the maximum in modern material). Although actual shrinkage will vary based on carbonization conditions in the past, which are difficult to estimate, this is unlikely to affect the ratios of grain dimensions and thus L/W ratios are suggested to be a useful method for determining domestication status while a time series of Length (and less likely width) may also provide addition support and document change that evolved during or after domestication.
Turning to the available archaeological finds, it is clear that pigeonpea was established outside its wild distribution by ca. 1500 BC (Fig. 4). Measurements indicate the L/W ratios fall within the expected domesticated range and do not show any significant changes through time among the archaeological materials (Fig. 8). This suggests that domestication took place prior to the available archaeological finds, i.e. before 1650 BC. When seed size data are plotted against time (Fig. 9) it is also clear that most of these fall in the > 3 mm length and therefore outside the predicted wild size. Nevertheless, the earliest specimens available, including one seed from Piklihal, Karnataka, fall below this size and could represent the very end of the trend towards size increase at the tail end of the domestication process (dashed line in Fig. 9). The limited sample size does not allow for this trend to be regarded as statistically significant however, and further archaeological finds are necessary for the domestication process to be studied in this pulse.
Plots of archaeological Cajanus cajan seed dimensions over time. Dashed line represents an interpretation of size trajectory indicated by site and phase assemblages plotted in terms of mean and standard deviation (lines), together with maximum (+) and minimum (−). When only individual specimens are available, they are plotted without lines
Conclusions
This review of the available evidence for pigeonpea (Cajanus cajan) suggests that it was domesticated in ancient India with a long history of use in South Asia. Evidence of extant wild populations, including herbarium specimens surveyed by the authors, suggests a wild distribution in the hills of the northeast peninsula and along the northern east coast of India, especially in the state of Odisha (as per van der Maesen 1986; Saxena et al. 2014), but plausibly extending southwards to Andhra Pradesh. A focal region in which to seek the earliest cultivation is perhaps near the borders of the modern states of Odisha, Telangana, Maharashtra, Andhra Pradesh and Chhattisgarh. These areas are largely under-studied archaeologically. At present, archaeological finds are earliest in the Southern peninsula (i.e. Sanganakallu, Karnataka), distant from wild habitats, and these are older than from Chalcolithic sites in coastal central Odisha. Both groups of finds are of similar size, and have L/W ratios and Lengths that place them with domesticated populations rather than wild populations. This indicates that the earlier cultivation and domestication process is not represented in archaeological finds available to date and places this process prior to 1650–1700 BC. We have suggested that the end of a trend towards increasing seed size might be represented among the earlier archaeological finds available, although more data are needed to assess whether this a true and statistically significant trend, and to determine when and where it began. Comparisons with the timing of domestication processes documented in other pulses (e.g. Fuller et al. 2014; Murphy and Fuller 2017) suggest that cultivation should have begun around 1000 years earlier than the currently documented end of the process; we thus infer cultivation was likely to have started 5000–4500 years ago.
This calls for further archaeobotanical data to clarify the contexts of initial cultivation and domestication. Domestication would have increased the yields of pigeonpea and made this an increasingly attractive pulse. Nevertheless, on no archaeological site yet sampled does C. cajan dominate the pulse component of the assemblage, suggesting that it was not as predominant in many ancient diets as it is in the present day.
There is no mention of pigeonpea among the plants encountered by a nineteenth century French naturalist in the Mekong valley (Thorel 2001). Despite its absence in the literature archaeological evidence, however, has recently attested to its presence in the fourteenth to fifteenth century in central Cambodia at Angkor Thom (Castillo et al. 2018a), with earlier Iron Age occurrences (4th–1st c BC) in southern Thailand (Castillo et al. 2016). Archaeobotanical evidence for cultivation in Medieval Cambodia (Castillo et al. 2018a) along with the presence of widespread feral populations in anthropogenic habitats (Fig. 3; Hu 2005; van der Maesen 1980, 257–258), suggest that it may have formerly been more widely cultivated in southern China, and probably also Island Southeast Asia. Traditional cultivation in parts of Burma, Thailand, Laos and the Malay Peninsula (Burkill 1966) suggest that its distribution in Southeast Asia may have become more restricted to upland and rainfed agricultural regions, rather than areas heavily committed to irrigated rice; although the lowland plains of Southeast Asia originally focused on rainfed rice with transitions to wet rice generally thought to occur in the early centuries AD (Castillo and Fuller 2010; Castillo et al. 2018b). Similarly, it has limited cultivation in the mountains of Oman (Gebauer et al. 2007), where it likely diffused alongside other crops from India and where it fits with summer rainfall cultivation. Some of the reduction in cultivation regions in recent centuries, such as across southern China, may be due to intensification of other crops like rice and introduction of new pulses such as Phaseolus and Canavalia from the New World.
The wild progenitor is also understudied. It often appears in anthropogenic habitats, as well as feral outside its likely origin. As with another native South Asian pulse, horsegram (Macrotyloma uniflorum) (Fuller and Murphy 2018), the native habitat for wild populations of Cajanus appears to be disappearing and this presents a critical issue as genetic interbreeding programs are needed to continue to improve the current domesticated species of pigeonpea for future use. Hence, looking to potential areas of wild progenitor stock for future botanical and genetic studies of both wild and domesticated pigeonpea populations in South Asia should be undertaken. Archaeobotanical evidence has the potential to shed light on how and where it was cultivated in the past, including regions where it is no longer a crop, but where it therefore has potential for future reintroduction and development.
References
Allen ON, Allen EK (1981) The Leguminosae A source book of characteristics, uses and nodulation. The University of Wisconsin Press, Madison
Al-Saeedi AH, Hossain MA (2015) Total phenols, total flavonoids contents and free radical scavenging activity of seeds crude extracts of pigeonpea traditionally used in Oman for the treatment of several chronic diseases. Asian Pac J Trop Dis 5(4):316–321. https://doi.org/10.1016/S2222-1808(14)60790-8
Asian Productivity Organisation (APO) (2003) Processing and utilization of legumes. Report of the APO Seminar on Processing and Utilization of Legumes, Japan, 9–14 Oct 2000. Tokyo, Japan
Bohra A, Rachit RK, Gnanesh BN, Kulbhushan S, Byregowda M, Rathore A, Kishor PB, Cook DR, Varshney RK (2012) An intra-specific consensus genetic map of pigeonpea [Cajanus Cajan (L.) Millspaugh] derived from six mapping populations. Theor Appl Genet 125(6):1325–1338. https://doi.org/10.1007/s00122-012-1916-5
Burkill IH (1966) A dictionary of the economic products of the Malay Peninsula; contributions by William Birtwistle. Published on behalf of the Government of Malaysia and Singapore by the Ministry of Agriculture and Co-operatives, Kuala Lumpur
Castillo CC, Fuller DQ (2010) Still too fragmentary and dependent upon chance? Advances in the study of early Southeast Asian archaeobotany. In: Bellina B, Bacus EA, Pryce O, Weissman Christie J (eds) 50 years of archaeology in Southeast Asia: essays in honour of Ian Glover. River Books, Bangkok, pp 91–111
Castillo CC, Polkinghorne M, Vincent B, Sur TB, Fuller DQ (2018a) Life goes on: archaeobotanical investigations of diet and ritual at Angkor Thom (fourteenth to fifteenth centuries CE). Holocene. https://doi.org/10.1177/0959683617752841
Castillo C, Higham C, Miller K, Chang N, Douka K, Higham T, Fuller DQ (2018b) Social responses to climate change in Iron Age northeast Thailand: new archaeobotanical evidence. Antiquity 92(365):1274–1291
Castillo CC, Bellina B, Fuller DQ (2016) Rice, beans and trade crops on the early maritime Silk Route in Southeast Asia. Antiquity 90(353):1255–1269. https://doi.org/10.15184/aqy.2016.175
Duke JA (1983) Cajanus cajan (L.) Millsp. Handbook of energy crops. Unpublished. www.hort.purdue.edu/newcrop/duke_energy/Cajanus_cajun.html. Accessed on 7 Jan 2015
Fuller DQ (1999) The origins of agriculture in south India: botanical and archaeological perspectives. Ph.D. dissertation, University of Cambridge
Fuller DQ, Harvey EL (2006) The archaeobotany of Indian pulses: identification, processing and evidence of cultivation. Environ Archaeol 11(2):219–246
Fuller DQ, Murphy C (2018) The origins and early dispersal of horsegram (Macrotyloma uniflorum), a major crop of ancient India. Genet Resour Crop Evol 65(1):285–305. https://doi.org/10.1007/s10722-017-0532-2
Fuller DQ, Denham T, Arroyo-Kalin M, Lucas L, Stevens CJ, Qin L, Allaby RG, Purugganan MD (2014) Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proc Natl Acad Sci 111(17):6147–6152. https://doi.org/10.1073/pnas.1308937110
Gebauer J, Luedeling E, Hammer K, Nagieb M, Buerkert A (2007) Mountain oases in northern Oman: an environment for evolution and in situ conservation of plant genetic resources. Genet Resour Crop Evol 54:465–481
Haines HH (1921–1925) The botany of Bihar and Orissa. Adlard & Sons, London
Harvey EL, Fuller DQ, Mohanty RK, Mohanta B (2006) Early agriculture in Orissa: some archaeobotanical results and field observations on the Neolithic. Man Environ 31(2):21–32
Hu S-Y (2005) Food plants of China. The Chinese University Press, Hong Kong
Kameswara Rao N, Reddy LJ, Bramel PJ (2003) Potential of wild species for genetic enhancement of some semi-arid food crops. Genet Resour Crop Evol 50(7):707–721. https://doi.org/10.1023/A:1025055018954
Kassa MT, Varma Penmetsa R, Carrasquilla-Garcia N, Sarma BK, Datta S, Upadhyaya HD, Varshney RK, von Wettberg EJB, Cook DR (2012) Genetic patterns of domestication in pigeonpea (Cajanus cajan (L.) Millsp.) and wild Cajanus relatives. PLOS 35:65. https://doi.org/10.1371/journal.pone.0039563
Khoury CK, Castaneda-Alvarez NP, Achicanoy HA, Sosa CC, Bernau V, Kassa MT, Norton SL et al (2015) Crop wild relatives of pigeonpea [Cajanus Cajan (L.) Millsp.]: distributions, ex situ conservation status, and potential genetic resources for abiotic stress tolerance. Biol Conserv 184:259–270. https://doi.org/10.1016/j.biocon.2015.01.032
Kingwell-Banham E, Fuller DQ (2013) Pigeonpea: origins and development. In: Smith C (ed) Encyclopedia of Global Archaeology. Springer, New York, pp 5941–5944
Mallikarjuna N, Kulbushan S, Lakshmi J, Varshney R, Srikanth S, Jadhav D (2012) Differences between Cajanus cajan (L.) Millspaugh and C. cajanifolius (Haimes) van der Maesen, the progenitor species of pigeonpea. Genet Resour Crop Evol 59:411–417. https://doi.org/10.1007/s10722-011-9691-8
Mallikarjuna N, Saxena KB, Jadhav DR (2011) Cajanus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, legume crops and forages. Springer, Heidelberg
Murphy C, Fuller DQ (2016) The transition to agricultural production in India: South Asian entanglements of domestication. In: Schug GR, Walimbe SR (eds) A companion to South Asia in the past. Blackwell Companions of Anthropology. Wiley Blackwell, Oxford, pp 344–357
Murphy C, Fuller DQ (2017) Seed coat thinning during horsegram (Macrotyloma uniflorum) domestication documented through synchrotron tomography of archaeological seeds. Sci Rep 7(1):5369. https://doi.org/10.1038/s41598-017-05244-w
Nwokolo E (1996) Pigeonpea (Cajanus cajan (L.) Millsp.). In: Nwokolo E, Smartt J (eds) Food and feed from legumes and oilseeds. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-0433-3_5
Oshodi AA, Olaofe O, Hall G (2009) Amino acid and mineral composition of pigeonpea (Cajanus cajan). Int J Food Sci Nutr 43(4):187–191. https://doi.org/10.3109/09637489309027541
Owens JD, Astuti M, Kuswanto KR (2015) Tempe and related products. In: Owens JD (ed) Indigenous fermented foods of Southeast Asia. Fermented foods and beverages series. CRC Press, Boca Raton, FL
Pal D, Mishra P, Sachan N, Ghosh AK (2011) Biological activities and medicinal properties of Cajanus Cajan (L) Millsp. J Adv Pharm Technol Res 2(4):207–214. https://doi.org/10.4103/2231-4040.90874
Pal G, Channanamchery R, Singh RK, Kethineni UB, Ram H, Prasad SR (2016) An economic analysis of pigeonpea seed production technology and its adoption behavior: Indian context. Sci World J. https://doi.org/10.1155/2016/7973638
Pandey A, Tomer AK, Bhandari DC, Pareek SK (2008) Towards collection of wild relatives of crop plants in India. Genet Resour Crop Evol 55(2):187–202. https://doi.org/10.1007/s10722-007-9227-4
Purseglove JW (1976) The origins and migrations of crops in tropical Africa. In: Harlan JR, De Wet JMJ, Stemler ABL (eds) Origins of African plant domestication. Mouton Publishers, The Hague
Randhawa MS (1958) Agriculture and animal husbandry in India. Indian Council of Agricultural Research, New Delhi
Ren S, Gilbert MG (2010). Flora of China, vol 10, pp 196, 228, 230. www.eFloras.org. Accessed 10 Jan 2018
Sahai K, Rawat KK (2015) A survey of the degrading population of Cajanus lineatus (Wight & Arn.) Maesen, in parts of the Western Ghats, India. Genet Resour Crop Evol 62(4):515–523. https://doi.org/10.1007/s10722-014-0176-4
Saxena RK, von Wettberg E, Upadhyaya HD, Sanchez V, Saxena K, Kimurto P, Varshney RK (2014) Genetic diversity and demographic history of Cajanus spp. Illustrated from genome-wide SNPs. PLoS ONE 9(2):e88568. https://doi.org/10.1371/journal.pone.0088568
Shetty SVR, Rao AN (1981) Weed-management studies in sorghum/pigeonpea and pearl millet/groundnut intercrop systems: some observations. In: Proceedings of the international workshop on intercropping, 10–13 Jan 1979, Patancheru, Hyderabad, India
Shinde YH, Amogha V, Pandit AB, Joshi JB (2017) Kinetics of cooking of unsoaked and pre-soaked split peas (Cajanus cajan). J Food Process Eng 40(5):e12527
Shurtleff W, Aoyagi A (2013) History of Tofu and Tofu Products (965 CE to 2013): extensively annotated Bibliography and Sourcebook. Soyinfo Center, Lafayette, CA
Sinha P, Singh VK, Suryanarayana V, Krishnamurthy L, Saxena RK, Varshney KK (2015) Evaluation and validation of housekeeping genes as reference for gene expression studies in pigeonpea (Cajanus cajan) under drought stress conditions. PLoS ONE 10(4):e0122847. https://doi.org/10.1371/journal.pone.0122847
Smartt J (1990) Grain legumes, evolution and genetic resources. Cambridge University Press, Cambridge
Southworth F (2005) Linguistic archaeology of South Asia. Routledge, London
Thorel C (2001) Agriculture and Ethnobotany of the Mekong Basin, the Mekong Exploration Commission Report (1866–1868) (trans: Tips WEJ), vol. 4. White Lotus Press, Bangkok
Uchegbu NN, Ishiwu CN (2016) Oxidative stress and hyperglycemia. Food Sci Nutr 4(5):772–777. https://doi.org/10.1002/fsn3.343
Upadhyaya HD, Reddy KN, Singh S, Gowda CLL (2013) Phenotypic diversity in Cajanus species and identification of promising sources for agronomic traits and seed protein content. Genet Resour Crop Evol 60(2):639–659. https://doi.org/10.1007/s10722-012-9864-0
Upadhyaya HD, Reddy KN, Gowda CLL, Singh S (2007) Phenotypic diversity in the pigeonpea (Cajanus cajan) core collection. Genet Resour Crop Evol 54(6):1167–1184. https://doi.org/10.1007/s10722-006-9008-5
Upadhyaya HD, Pundir RPS, Gowda CLL, Reddy KN, Singh S (2005) Geographical patterns of diversity for qualitative and quantitative traits in the pigeonpea germplasm collection. Plant Genet Resour 3(3):331–352. https://doi.org/10.1079/PGR200587
Van der Maesen LJG (1980) India is the native home of the pigeonpea. In: Arends JC, Boelema G, De Groot CT, Leeuwenberg AJM (eds) Liber Gratulatorius in Honorem H.C.D. De Wit. Miscellaneous Papers 19 (1980) Landbouwhogeschool, Wageningen, the Netherlands. H. Veenman & Zonen B.V., Wageningen, pp 257–262
van der Maesen LJG (1986) Cajanus DC. and Atylosia W & A. (Leguminosae): a revision of all taxa closely related to the pigeonpea, with notes on other related genera within the subtribe Cajaninae. Agricultural University Wageningen Papers 85–84, Agricultural University, Wageningen
van der Maesen LJG, Somaatmadja S (eds.) (1989) Plant resources of South-East Asia. No. 1. Pulses. Pudoc/Prosea, Wageningen, the Netherlands
Venkatasubbaiah PC, Kajale MD (1991) Biological remains from neolithic and early historic sites in Cuddapah District, Andhra Pradesh. Man Environ XVI(1):85–97
Watt G (1889) A dictionary of the economic products of India, vol II. Cosmo Publications Publishers, Delhi (2nd reprint 1972)
Westphal E (1974) Pulses of Ethiopia, their taxonomy and agricultural significance. Centre for Agricultural Publishing and Documentation, Wageningen
Zide AR, Zide NH (1976) Proto-Munda cultural vocabulary: evidence for early agriculture. Ocean Linguist Spec Publ 13:1295–1334
Websites
“Cajanus Cajan” Unpublished, 1983. https://www.hort.purdue.edu/newcrop/duke_energy/Cajanus_cajun.html. Accessed on 21 April 2016
FAOSTAT website, Food and Agriculture Organization of the United Nations. faostat.fao.org. Accessed on 09 Nov 2016
ICRISAT International Crops Research Institute for the Semi-Arid Tropics http://www.icrisat.org/newsroom/latest-news/happenings/happenings1492.htm#1. Accessed on 21 April 2016
JIRCAS (Japan International Research Center for Agricultural Sciences) 2010. Local Vegetables of Thailand. Website: www.jircas.affrc.go.jp/project/value_addition/Local_Vegetables_of_Thailand_home.html. Accessed on 27 Jan 2018
Royal Botanical Gardens Kew, Cajanus cajan (pigeonpea) http://www.kew.org/science-conservation/plants-fungi/cajanus-cajan-pigeon-pea. Accessed on 22 April 2016
USDA United States Department of Agriculture, Natural Resources Conservation Service http://plants.usda.gov/core/profile?symbol=CACA27. Accessed on 19 April 2016
Acknowledgements
CM, DQF and EKB carried research on Cajanus domestication as part of the Comparative Pathways to Agriculture Project (ComPAg) funded by a European Research Council advanced Grant (No. 323842). Research in Southeast Asia (by CCC) and Odisha (by CM, DQF and EKB) by was funded as part of the Early Rice Project through the Natural Environment Research Council (NERC), NE/N010957. Work in Vidarbha (by SN) was supported by Deccan College, Pune. Thanks to Dr. Gwilym Lewis, Head of the Legume team, Herbarium, Royal Botanic Gardens, Kew for his kind assistance and access to collections. Thanks to Dr. Mark Nesbitt for assistance with and access to the Economic Botany collection, Royal Botanic Gardens, Kew. Thanks to Jacek Wajer, Curator of the General Herbarium for access to and assistance with the herbarium collection at the Natural History Museum. Thanks to Lara Gonzalez Carretero, PhD student at UCL, Institute of Archaeology for her assistance with measuring Cajanus at Kew. Thanks to the United States Department of Agriculture (USDA) for providing modern seed samples of Cajanus cajan for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This research did not involve human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fuller, D.Q., Murphy, C., Kingwell-Banham, E. et al. Cajanus cajan (L.) Millsp. origins and domestication: the South and Southeast Asian archaeobotanical evidence. Genet Resour Crop Evol 66, 1175–1188 (2019). https://doi.org/10.1007/s10722-019-00774-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-019-00774-w