Abstract
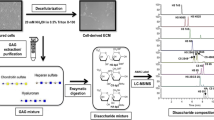
Glycosaminoglycans (GAGs) are major components of cartilage extracellular matrix (ECM), which play an important role in tissue homeostasis not only by providing mechanical load resistance, but also as signaling mediators of key cellular processes such as adhesion, migration, proliferation and differentiation. Specific GAG types as well as their disaccharide sulfation patterns can be predictive of the tissue maturation level but also of disease states such as osteoarthritis. In this work, we used a highly sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to perform a comparative study in terms of temporal changes in GAG and disaccharide composition between tissues generated from human bone marrow- and synovial-derived mesenchymal stem/stromal cells (hBMSC/hSMSC) after chondrogenic differentiation under normoxic (21% O2) and hypoxic (5% O2) micromass cultures. The chondrogenic differentiation of hBMSC/hSMSC cultured under different oxygen tensions was assessed through aggregate size measurement, chondrogenic gene expression analysis and histological/immunofluorescence staining in comparison to human chondrocytes. For all the studied conditions, the compositional analysis demonstrated a notable increase in the average relative percentage of chondroitin sulfate (CS), the main GAG in cartilage composition, throughout MSC chondrogenic differentiation. Additionally, hypoxic culture conditions resulted in significantly different average GAG and CS disaccharide percentage compositions compared to the normoxic ones. However, such effect was considerably more evident for hBMSC-derived chondrogenic aggregates. In summary, the GAG profiles described here may provide new insights for the prediction of cartilage tissue differentiation/disease states and to characterize the quality of MSC-generated chondrocytes obtained under different oxygen tension culture conditions.








Similar content being viewed by others
References
Richter, D.L., Schenck, R.C., Wascher, D.C., Treme, G.: Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health. 8, 153–160 (2016). https://doi.org/10.1177/1941738115611350
Darling, E.M., Athanasiou, K.A.: Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23, 425–432 (2005). https://doi.org/10.1016/j.orthres.2004.08.008
Rackwitz, L., Djouad, F., Janjanin, S., Nöth, U., Tuan, R.S.: Functional cartilage repair capacity of de-differentiated, chondrocyte- and mesenchymal stem cell-laden hydrogels in vitro. Osteoarthr. Cartil. 22, 1148–1157 (2014). https://doi.org/10.1016/j.joca.2014.05.019
Huang, Y.Z.S., Xie, H.Q., Silini, A., Parolini, O., Zhang, Y., Deng, L., Huang, Y.C.: Mesenchymal stem/progenitor cells derived from articular cartilage. Synovial Membrane and Synovial Fluid for Cartilage Regeneration: Current Status and Future Perspectives. Stem Cell Rev. Reports. 13, 575–586 (2017). https://doi.org/10.1007/s12015-017-9753-1
Li, C.Y., Wu, X.Y., Tong, J.B., Yang, X.X., Zhao, J.L., Zheng, Q.F., Zhao, G.B., Ma, Z.J.: Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 6, 55 (2015). https://doi.org/10.1186/s13287-015-0066-5
Tan, A.R., Hung, C.T.: Concise review: Mesenchymal stem cells for functional cartilage tissue engineering: taking cues from chondrocyte-based constructs. Stem Cells Transl. Med. 6, 1295–1303 (2017). https://doi.org/10.1002/sctm.16-0271
Yoshimura, H., Muneta, T., Nimura, A., Yokoyama, A., Koga, H., Sekiya, I.: Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 327, 449–462 (2007). https://doi.org/10.1007/s00441-006-0308-z
Bernardo, M.E., Emons, J.A.M., Karperien, M., Nauta, A.J., Willemze, R., Roelofs, H., Romeo, S., Marchini, A., Rappold, G.A., Vukicevic, S., Locatelli, F., Fibbe, W.E.: Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect. Tissue Res. 48, 132–140 (2007). https://doi.org/10.1080/03008200701228464
Sakaguchi, Y., Sekiya, I., Yagishita, K., Muneta, T.: Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52, 2521–2529 (2005). https://doi.org/10.1002/art.21212
Shirasawa, S., Sekiya, I., Sakaguchi, Y., Yagishita, K., Ichinose, S., Muneta, T.: In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem. 97, 84–97 (2006). https://doi.org/10.1002/jcb.20546
Fan, J., Varshney, R.R., Ren, L., Cai, D., Wang, D.A.: Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng. Part B. Rev. 15, 75–86 (2009). https://doi.org/10.1089/ten.teb.2008.0586
Ogata, Y., Mabuchi, Y., Yoshida, M., Suto, E.G., Suzuki, N., Muneta, T., Sekiya, I., Akazawa, C.: Purified human synovium mesenchymal stem cells as a good resource for cartilage regeneration. PLoS One. 10, e0129096 (2015). https://doi.org/10.1371/journal.pone.0129096
Zhang, L., Su, P., Xu, C., Yang, J., Yu, W., Huang, D.: Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol. Lett. 32, 1339–1346 (2010). https://doi.org/10.1007/s10529-010-0293-x
Zhou, S., Cui, Z., Urban, J.P.G.: Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 50, 3915–3924 (2004). https://doi.org/10.1002/art.20675
Adesida, A.B., Mulet-Sierra, A., Jomha, N.M.: Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 3, 9 (2012). https://doi.org/10.1186/scrt10022385573
Leijten, J., Georgi, N., Moreira Teixeira, L., van Blitterswijk, C.A., Post, J.N., Karperien, M.: Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc. Natl. Acad. Sci. 111, 13954–13959 (2014). https://doi.org/10.1073/pnas.1410977111
Bae, H.C., Park, H.J., Wang, S.Y., Yang, H.R., Lee, M.C., Han, H.-S.: Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater. Res. 22, 1–8 (2018). https://doi.org/10.1186/s40824-018-0134-x
Weyers, A., Linhardt, R.J.: Neoproteoglycans in tissue engineering. FEBS J. 280, 2511–2522 (2013). https://doi.org/10.1111/febs.12187
Gasimli, L., Linhardt, R.J., Dordick, J.S.: Proteoglycans in stem cells. Biotechnol. Appl. Biochem. 59, 65–76 (2012). https://doi.org/10.1002/bab.1002
Uygun, B.E., Stojsih, S.E., Matthew, H.W.T.: Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng. Part A. 15, 3499–3512 (2009). https://doi.org/10.1089/ten.TEA.2008.0405
Plaas, A.H.K., West, L.A., Wong-Palms, S., Nelson, F.R.T.: Glycosaminoglycan sulfation in human osteoarthritis: disease-related alterations at the non-reducing termini of chondroitin and dermatan sulfate. J. Biol. Chem. 273, 12642–12649 (1998). https://doi.org/10.1074/jbc.273.20.12642
Chanalaris, A., Clarke, H., Guimond, S.E., Vincent, T.L., Turnbull, J.E., Troeberg, L.: Heparan sulfate proteoglycan synthesis is Dysregulated in human osteoarthritic cartilage. Am. J. Pathol. 189, 632–647 (2019). https://doi.org/10.1016/j.ajpath.2018.11.011
Veraldi, N., Parra, A., Urso, E., Cosentino, C., Locatelli, M., Corsini, S., Pedrini, E., Naggi, A., Bisio, A., Sangiorgi, L.: Structural features of heparan sulfate from multiple osteochondromas and chondrosarcomas. Molecules. 23, 3277 (2018). https://doi.org/10.3390/molecules23123277
Wan, S., Borland, S., Richardson, S.M., Merry, C.L.R., Saiani, A., Gough, J.E.: Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 46, 29–40 (2016). https://doi.org/10.1016/j.actbio.2016.09.033
Mouw, J.K., Case, N.D., Guldberg, R.E., Plaas, A.H.K., Levenston, M.E.: Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthr. Cartil. 13, 828–836 (2005). https://doi.org/10.1016/j.joca.2005.04.020
Li, G., Li, L., Tian, F., Zhang, L., Xue, C., Linhardt, R.J.: Glycosaminoglycanomics of cultured cells using a rapid and sensitive LC-MS/MS approach. ACS Chem. Biol. 10, 1303–1310 (2015)
Liu, X., Krishnamoorthy, D., Lin, L., Xue, P., Zhang, F., Chi, L., Linhardt, R.J., Iatridis, J.C.: A method for characterising human intervertebral disc glycosaminoglycan disaccharides using liquid chromatography-mass spectrometry with multiple reaction monitoring. Eur. Cell. Mater. 35, 117–131 (2018). https://doi.org/10.22203/eCM.v035a09
Sun, X., Li, L., Overdier, K.H., Ammons, L.A., Douglas, I.S., Burlew, C.C., Zhang, F., Schmidt, E.P., Chi, L., Linhardt, R.J.: Analysis of Total human urinary glycosaminoglycan disaccharides by liquid chromatography-tandem mass spectrometry. Anal. Chem. 87, 6220–6227 (2015). https://doi.org/10.1021/acs.analchem.5b00913
Silva, J.C., Carvalho, M.S., Han, X., Xia, K., Mikael, P.E., Cabral, J.M.S., Ferreira, F.C., Linhardt, R.J.: Compositional and structural analysis of glycosaminoglycans in cell-derived extracellular matrices. Glycoconj. J. 36, 141–154 (2019). https://doi.org/10.1007/s10719-019-09858-2
Gasimli, L., Hickey, A.M., Yang, B., Li, G., Dela Rosa, M., Nairn, A.V., Kulik, M.J., Dordick, J.S., Moremen, K.W., Dalton, S., Linhardt, R.J.: Changes in glycosaminoglycan structure on differentiation of human embryonic stem cells towards mesoderm and endoderm lineages. Biochim. Biophys. Acta - Gen. Subj. 1840, 1993–2003 (2014). https://doi.org/10.1016/j.bbagen.2014.01.007
Mikael, P.E., Willard, C., Koyee, A., Barlao, C.-G., Liu, X., Han, X., Ouyang, Y., Xia, K., Linhardt, R.J., Dordick, J.S.: Remodeling of Glycosaminoglycans during differentiation of adult human bone Mesenchymal stromal cells toward hepatocytes. Stem Cells Dev. 28, 278–289 (2019). https://doi.org/10.1089/scd.2018.0197
Dos Santos, F., Andrade, P.Z., Boura, J.S., Abecasis, M.M., da Silva, C.L., Cabral, J.M.S.: Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 223, 27–35 (2010). https://doi.org/10.1002/jcp.21987
Santhagunam, A., Dos Santos, F., Madeira, C., Salgueiro, J.B., Cabral, J.M.S.: Isolation and ex vivo expansion of synovial mesenchymal stromal cells for cartilage repair. Cytotherapy. 16, 440–453 (2013). https://doi.org/10.1016/j.jcyt.2013.10.010
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., Horwitz, E.: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8, 315–317 (2006). https://doi.org/10.1080/14653240600855905
Nagase, T., Muneta, T., Ju, Y.J., Hara, K., Morito, T., Koga, H., Nimura, A., Mochizuki, T., Sekiya, I.: Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis Rheum. 58, 1389–1398 (2008). https://doi.org/10.1002/art.23418
Ferro, T., Santhagunam, A., Madeira, C., Salgueiro, J.B., da Silva, C.L., Cabral, J.M.S.: Successful isolation and ex vivo expansion of human mesenchymal stem/stromal cells obtained from different synovial tissue-derived (biopsy) samples. J. Cell. Physiol. 234, 3973–3984 (2019). https://doi.org/10.1002/jcp.27202
Markway, B.D., Tan, G.K., Brooke, G., Hudson, J.E., Cooper-White, J.J., Doran, M.R.: Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 19, 29–42 (2010). https://doi.org/10.3727/096368909X478560
Lafont, J.E., Talma, S., Hopfgarten, C., Murphy, C.L.: Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol. Chem. 283, 4778–4786 (2008). https://doi.org/10.1074/jbc.M707729200
Gawlitta, D., van Rijen, M.H.P., Schrijver, E.J.M., Alblas, J., Dhert, W.J.A.: Hypoxia impedes hypertrophic Chondrogenesis of human multipotent stromal cells. Tissue Eng. Part A. 18, 1957–1966 (2012). https://doi.org/10.1089/ten.tea.2011.0657
Shang, J., Liu, H., Li, J., Zhou, Y.: Roles of hypoxia during the Chondrogenic differentiation of Mesenchymal stem cells. Curr. Stem Cell Res. Ther. 9, 141–147 (2014). https://doi.org/10.2174/1574888x09666131230142459
Cicione, C., Muiños-López, E., Hermida-Gómez, T., Fuentes-Boquete, I., Díaz-Prado, S., Blanco, F.J.: Effects of Severe Hypoxia on Bone Marrow Mesenchymal Stem Cells Differentiation Potential. Stem Cells Int. 232896 (2013). https://doi.org/10.1155/2013/232896
Segawa, Y., Muneta, T., Makino, H., Nimura, A., Mochizuki, T., Ju, Y.J., Ezura, Y., Umezawa, A., Sekiya, I.: Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J. Orthop. Res. 27, 435–441 (2009). https://doi.org/10.1002/jor.20786
Osago, H., Kobayashi-Miura, M., Hamasaki, Y., Hara, N., Hiyoshi, M., Tsuchiya, M.: Complete solubilization of cartilage using the heat-stable protease thermolysin for comprehensive GAG analysis. Anal. Biochem. 548, 115–118 (2018). https://doi.org/10.1016/j.ab.2018.02.028
Lauder, R.M., Huckerby, T.N., Brown, G.M., Bayliss, M.T., Nieduszynski, I.A.: Age-related changes in the sulphation of the chondroitin sulphate linkage region from human articular cartilage aggrecan. Biochem. J. 358, 523–528 (2001). https://doi.org/10.1042/0264-6021:3580523
Sharma, A., Rees, D., Roberts, S., Kuiper, N.J.: A case study: glycosaminoglycan profiles of autologous chondrocyte implantation (ACI) tissue improve as the tissue matures. Knee. 24, 149–157 (2017). https://doi.org/10.1016/j.knee.2016.10.002
Hitchcock, A.M., Yates, K.E., Costello, C.E., Zaia, J.: Comparative glycomics of connective tissue glycosaminoglycans. Proteomics. 8, 1384–1397 (2008). https://doi.org/10.1002/pmic.200700787
Hitchcock, A.M., Yates, K.E., Shortkroff, S., Costello, C.E., Zaia, J.: Optimized extraction of glycosaminoglycans from normal and osteoarthritic cartilage for glycomics profiling. Glycobiology. 17, 25–35 (2007). https://doi.org/10.1093/glycob/cwl046
Lin, T.-S., Hsieh, C.-H., Kuo, C., Juang, Y.-P., Hsieh, Y.S., Chiang, H., Hung, S.-C., Jiang, C.-C., Liang, P.-H.: Sulfation pattern of chondroitin sulfate in human osteoarthritis cartilages reveals a lower level of Chondroitin-4-sulfate. Carbohydr. Polym. 229, 115496 (2020). https://doi.org/10.1016/j.carbpol.2019.115496
Wang, Q.G., Hughes, N., Cartmell, S.H., Kuiper, N.J.: The composition of hydrogels for cartilage tissue engineering can influence glycosaminoglycan profiles. Eur. Cells Mater. 19, 86–95 (2010). https://doi.org/10.22203/eCM.v019a09
Acknowledgements
This study was financed by Center for Biotechnology and Interdisciplinary Studies-Rensselaer Polytechnic Institute funds and by the National Institutes of Health though the Grant # DK111958. This work was also supported by funding received by iBB-Institute for Bioengineering and Biosciences through Programa Operacional Regional de Lisboa 2020 (Project N. 007317), through the EU COMPETE Program and from FCT-Portuguese Foundation for Science and Technology (Programme grant UID/BIO/04565/2020) and project PRECISE-Accelerating progress toward the new era of precision medicine (PAC-PRECISE-LISBOA-01-0145-FEDER-016394, SAICTPAC/0021/2015). João C. Silva is also grateful to FCT for financial support through the scholarship SFRH/BD/105771/2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This work does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 462 kb)
Rights and permissions
About this article
Cite this article
Silva, J.C., Han, X., Silva, T.P. et al. Glycosaminoglycan remodeling during chondrogenic differentiation of human bone marrow−/synovial-derived mesenchymal stem/stromal cells under normoxia and hypoxia. Glycoconj J 37, 345–360 (2020). https://doi.org/10.1007/s10719-020-09911-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-020-09911-5