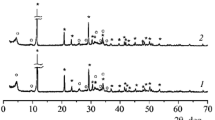
Powder mixtures prepared by mechanical activation from synthetic hydrated acidic calcium phosphates Ca(H2PO4)2 · H2O and CaHPO4 · 2H2O were used to obtain resorbable ceramic in the system Ca (PO3)2–Ca2P2O7. The phase composition of the ceramic after firing in the interval 700 – 1000°C was represented by biocompatible and bioresorbable phases: calcium polyphosphate Ca(PO3)2, tromelite Ca4P6O19, and calcium pyrophosphate Ca2P2O7. The obtained materials can be used to fabricate resorbable implants for regenerative treatment of defects of bone tissue.










Similar content being viewed by others
References
T. V. Safronova and V. I. Putlyaev, “Powder systems for calcium phosphate ceramics,” Inorg. Mater., 53, 17 – 26 (2017).
G. MacLennan and C. A. Beevers, “The crystal structure of monocalcium phosphate monohydrate, Ca(H2PO4)2 · H2O,” Acta Cryst., 9(2), 187 – 190 (1956).
B. Boonchom and C. Danvirutai, “The morphology and thermal behavior of Calcium dihydrogen phosphate monohydrate (Ca(H2PO4)2 · H2O) obtained by a rapid precipitation route at ambient temperature in different media,” J. Optoelectron. Biomed. Mater., 1, 115 – 123 (2009).
L. E. Jackson and A. J. Wright, “A new synthetic route to calcium polyphosphates,” Key Eng. Mater., 284, 71 – 74 (2005).
J. Trommer, M. Schneider, H. Worzala, and A. N. Fitch, “Structure determination of CaH2P2O7 from in situ powder diffraction data,” Mater. Sci. Forum, 321, 374 – 379 (2000).
E. H. Brown, W. E. Brown, J. R. Lehr, et al., “Calcium ammonium pyrophosphates,” J. Phys. Chem., 62(3), 366 – 367 (1958).
Y. V. Subbarao and R. Ellis, “Reaction products of polyphosphates and orthophosphates with soils and influence on uptake of phosphorus by plants,” Soil Sci. Soc. Am. J., 39(6), 1085 – 1088 (1975).
E. H. Brown, J. R. Lehr, J. P. Smith, and A. W. Frazier, “Fertilizer Materials, Preparation and Characterization of Some Calcium Pyrophosphates,” J. Agricult. Food Chem., 11(3), 214 – 222 (1963).
D. Zobel and N. Ba, “Untersuchungen zur Phosphitpyrolyse; Reaktionen beim Erhitzen von CaH2(HPO3)2 · H2O in Abwesenheit von Sauerstoff,” Z. Chem., 9(11), 433 (1969).
T. V. Safronova, E. A. Mukhin, V. I. Putlyaev, et al., “Amorphous calcium phosphate powder synthesized from calcium acetate and polyphosphoric acid for bioceramics application,” Ceram. Int., 43, 1310 – 1317 (2017).
H. A. Höppe, “Synthesis, crystal structure, and vibrational spectra of Ca4P6O19 (trömelite) – a catena – hexaphosphate,” Zeitschrift für Anorganische und Allgemeine Chemie, 631(6 – 7), 1272 – 1276 (2005).
T. V. Safronova, V. I. Putlyaev, M. A. Shekhirev, and A. V. Kuznetsov, “Composite ceramic containing a bioresorbable phase,” Steklo Keram., No. 3, 31 – 35 (2007); T. V. Safronova, V. I. Putlyaev, M. A. Shekhirev, and A. V. Kuznetsov, “Composite ceramic containing a bioresorbable phase,” Glass Ceram., 64(3 – 4), 102 – 106 (2007).
T. V. Safronova, A. V. Kuznetsov, S. A. Korneychuk, et al., “Calcium phosphate powders synthesized from solutions with \( \left[{\mathrm{Ca}}^{2+}\right]/\left[{\mathrm{PO}}_4^{3-}\right] \) = 1 for bioresorbable ceramics,” Cent. Eur. J. Chem., 7(2), 184 – 191 (2009).
T. V. Safronova, S. A. Kurbatova, T. B. Shatalova, et al, “Calcium pyrophosphate powder synthesized from pyrophosphoric acid and calcium acetate for obtaining bioceramic,” Materialovedenie, 7(7), 41 – 48 (2016).
T. V. Safronova, V. I. Putlyaev, S. A. Kurbatova, et al., “Properties of amorphous calcium pyrophosphate powder synthesized with the use of ion-exchange for obtaining bioceramic,” Neorg. Mater., 51(11), 1269 – 1276 (2015).
T. V. Safronova, V. I. Putlayev, K. A. Bessonov, and V. K. Ivanov, “Ceramics based on calcium pyrophosphate nanopowders,” Proc. Appl. Ceram., 7(1), 9 – 14 (2013).
Soorya Kabekkodu (ed.), ICDD (2010). PDF-4+ 2010 (Database), International Centre for Diffraction Data, Newtown Square, PA, USA(2010); URL: http://www.icdd.com/products/pdf2.htm.
The equipment used in this work was acquired using the resources of the program of advancement of Moscow University. This research was supported by RFFI (grants Nos. 16-53-00154, 16-08-01172) and BRFFI (grant No. Kh16R-030).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 7, pp. 37 – 44, July, 2018.
Rights and permissions
About this article
Cite this article
Safronova, T.V., Putlyaev, V.I., Knot’ko, A.V. et al. Calcium Phosphate Ceramic in the System Ca(PO3)2–Ca2P2O7 Based on Powder Mixtures Containing Calcium Hydrophosphate. Glass Ceram 75, 279–286 (2018). https://doi.org/10.1007/s10717-018-0072-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-018-0072-z