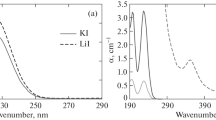
The main difficulties of making an accurate determination of the redox state of glass are noted. The specific effect of tin on the valence equilibrium of iron in the surface layers of float-glass is shown. The spectrophotometric method of determining heterovalent forms of iron in commercial silicate glasses, which is applicable for flat and hollow articles, is described. The method is distinguished by high speed and accessibility.

Similar content being viewed by others
References
S. V. Mulevanov, B. Z. Bliskovskii, V. I. Korovushkin, and A. I. Smirnova, “Valence and coordination state of iron in multicomponent glasses with pyroxene composition,” Fiz. Khim. Stekla, 12(6), 722 – 726.
S. V. Mulevanov, N. I. Min’ko, and S. A. Kemenov, “Effect of phosphorus on the structural-chemical and optical properties of sheet construction glass,” Vestn. Belgorod. Gosudar. Tekhnol. Univ. im. V. G. Shukhova, No. 10, 207 – 209 (2005).
A. B. Atkarskaya, “Effect of the oxidation–reduction potential on the proneness of glass to form bubbles,” Steklo Keram., No. 4, 3 – 8 (2010); A. B. Atkarskaya, “Effect of the oxidation–reduction potential on the proneness of glass to form bubbles,” Glass Ceram., 67(3 – 4), 99 – 104 (2010).
A. B. Atkarskaya and M. I. Zaitseva, “Redox equilibrium of iron in silicate glasses,” Steklo Keram., No. 10, 5 – 8 (2005); A. B. Atkarskaya and M. I. Zaitseva, “Redox equilibrium of iron in silicate glasses,” Glass Ceram., 62(9 – 10), 304 – 307 (2005).
V. V. Sakovich, L. A. Bobrova, A. M. Lazareva, and T. V. Sharykhina, “Monitoring chemical composition of medical glass by means of atomic emission spectroscopy,” Steklo Keram., No. 4, 7 – 9 (2006);. V. V. Sakovich, L. A. Bobrova, A. M. Lazareva, and T. V. Sharykhina, “Monitoring chemical composition of medical glass using atomic emission spectroscopy,” Glass Ceram., 63(3 – 4), 110 – 112 (2006).
M. Fialin, C. Wagner, and N. Metrich, “Fe3+/ΣFe vs. FeLα peak energy for minerals and glasses: Recent advances with the electron microprobe,” Am. Mineral., 86, 456 – 465 (2001).
J. A. Howell, J. R. Hellmann, and C. L. Muhlstein, “Nanomechanical properties of commercial float glass,” J. Non-Cryst. Solids, 354(17), 1891 – 1899 (2008).
A.-M. Flank, P. Lagarde, J. Jupille, and H. Montigaud, “Redox profile of the glass surface,” J. Non-Cryst. Solids, 357, 3200 – 3206 (2011).
R. Klement, J. Kraxner, and M. Liška, “Spectroscopic analysis of iron doped glasses with composition close to the E-glass: a preliminary study,” Ceramics – Silikaty, 53(3), 180 – 183 (2009).
Yu. A. Guloyan, “Conditions for producing amber and brown glass,” Steklo Keram., No. 10, 3 – 5 (2005); Yu. A. Guloyan, “Conditions for producing amber and brown glass,” Glass Ceram., 62(9 – 10), 301 – 303 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 5, pp. 6 – 9, May, 2014.
Rights and permissions
About this article
Cite this article
Mulevanov, S.V., Nartsev, V.M. Spectrophotometric Determination of the Redox State of Glass. Glass Ceram 71, 148–151 (2014). https://doi.org/10.1007/s10717-014-9639-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-014-9639-5