Abstract
Technologies have been developed for the in-situ treatment of urine patches deposited by grazing livestock to mitigate nitrogen (N) losses using N transformation inhibitors. For this mitigation to be effective, close contact between the applied inhibitors and the N in the urine patch is required (similar to N-fertilisers coated with inhibitors). This research aimed to determine the proportions of urine-N that mixed with inhibitor at or exceeding the threshold concentration (inhibitor concentration at which the nitrification rate is reduced by at least 40%) when inhibitors were applied to simulated urine patches at 4, 24 and 48 h after synthetic urine application. Three commonly used nitrification inhibitors (NIs) [dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP), and 2-chloro-6-(trichloromethyl) pyridine (nitrapyrin)] were applied at 40 mL of inhibitor per urine-patch at two different concentrations. The field studies were undertaken in two dairy-grazed pasture soils with contrasting drainage. Large proportions of applied NIs (38%–59% DCD, 27%–58% DMPP, and 31%–58% nitrapyrin) were retained in the pasture canopy. In most cases, the inhibitor threshold concentration was present only within the top 0–20 mm of the soil, with only 16%–40% of the urine-N present. In some cases, the proportions of urine-N intercepted was 12%–15% higher when inhibitors were applied 4 h after urine application compared to delayed application of 24 and 48 h after urine application. Our results revealed that a substantial proportion of N in the urine-patch remained out of the reach of the inhibitor solution. This is possibly due to the small volume (40 mL per 2 L urine patch, 1:50) of the inhibitors applied, with up to 59% of inhibitor solution retained in the pasture canopy. The time delays (4 to 48 h) between the urine deposition and the inhibitor application could have also contributed to this poor physical mixing between inhibitor and urine. Increasing the volume of water applied with the inhibitor and assessing the effect of rainfall/irrigation on increasing urine-N and inhibitor mixing warrants further consideration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In New Zealand (NZ), agriculture is responsible for about 50% of total national greenhouse gas (GHG) emissions, and nitrous oxide (N2O, a potent GHG) contributes c. 11% of national emissions (MfE 2022). Livestock excreta deposited in livestock-grazed pastures are the major contributors (c. 60%) to total N2O emitted, with urine patches accounting for most of these emissions (MfE 2022). Mitigating N2O emissions from urine deposited on grazed pastures will help limit the negative impacts of GHG on the environment and achieve NZ’s international commitment to reduce net GHG emissions by 30% below 2005 levels by 2030 under the Paris Agreement (MfE 2022).
Nitrification inhibitors (NIs) are compounds that slow down the rate of nitrification [transformation of ammonium (NH4+) to nitrate (NO3−)] in soils. The use of NIs with nitrogen (N) fertilisers has been widely investigated and has shown extensive benefits in reducing N2O emissions, by 44% as reviewed in Qiao et al. (2015). As mentioned earlier, in NZ's grazed pasture systems a significant portion of N2O emissions originates from urine patches. To mitigate these emissions, NZ farmers initiated the blanket application of NI Dicyandiamide (DCD) on dairy cattle grazed pastures mostly at 10 kg ha−1 (Di and Cameron 2006; Di et al. 2010) and observed an average reduction in emissions of 57% (Di and Cameron 2016). To account for the effect of DCD on mitigating N2O emissions, the NI technology was incorporated into NZ's GHG inventory reporting (Clough et al. 2007). However, the blanket application of DCD to entire paddocks resulted in the discovery of low levels of DCD residues in milk products which led to its voluntary withdrawal from use in NZ (MPI 2013). Targeted treatment of urine patches with inhibitors avoids the need for blanket application of NIs across the entire paddock and can reduce the risk of NIs entering the food chain via grazing livestock. This may also provide a potential for cost-saving as NIs are not applied to areas unaffected by urine deposition. Cattle urine patches cover only 6% to 12% of the total grazed area in a single grazing event (Moir et al. 2011; Maire et al. 2018).
Technologies are available for detecting urine patches (based on electrical conductivity using ground-based Spikey® technology), which allows for precise and targeted application of the inhibitor specifically to the identified patches (Jolly et al. 2021). In practice, this means there would be a delay from a few hours up to several days between the grazing event and the NI application. During this period, urine-N could move down the soil profile. This downward movement may result in physical separation between the urine-N and the NI, potentially reducing the effectiveness of the NI. Reducing N losses from urine patches using NIs (and other N transformation inhibitors) requires close contact between the inhibitors and the urine (Adhikari et al. 2020; Rodriguez et al. 2021). Among various biophysical and biochemical factors, the characteristics of the inhibitor, soil type, and timing and concentration of the applied inhibitors have a key role in influencing the co-location (physical contact) of inhibitors and urine, and thereby the inhibitor efficacy to reduce N2O emissions (Giltrap et al. 2022). However, in most previous studies quantifying reductions in N2O with the use of NIs, the inhibitor was applied shortly after the uniform application of urine in the chamber surface area (often followed by additional irrigation of 5–10 mm of water). This ensured good mixing between the inhibitor and the urine, and thereby enhanced inhibitor effectiveness as evidenced in Saggar et al. (2013). However, it is not practically feasible to apply inhibitors immediately after a grazing event, resulting in poor physical mixing between the inhibitor and the deposited urine when the inhibitor application is delayed.
Only a few studies have considered the effects of time delay between the urine deposition and the inhibitor application on efficacy of the inhibitors. For example, Zaman and Nguyen (2012) observed that a 5-day delay between a urine application and DCD resulted in reductions of 18%–59% in the efficacy of NI in mitigating N2O emissions from evenly applied urine. Furthermore, as indicated in the above paragraph, the reductions reported in most of the previous NZ and overseas studies were achieved with uniform application of urine across the entire chamber surface area to create evenly wetted patch areas. However, naturally deposited urine may travel deeper into the soil compared with evenly applied urine before the NIs are applied, which further reduces the physical contact between urine-N and NIs upon application, and thereby the efficacy of NIs. This is evidenced in a previous unpublished study (S Saggar Pers. Com.) where the application of DCD, 3,4-dimethylpyrazole phosphate (DMPP), and 2-chloro-6-(trichloromethyl) pyridine (nitrapyrin) 4 h after natural urine patch simulation failed to achieve any statistically significant effect on N2O emissions reduction. This situation prompted the need for further research to understand the physical mixing between the inhibitor and the proportion of urine-N within naturally deposited urine patches and assess the impact of the timing of NI application following urine deposition in inhibitor urine mixing and to what extent this limits on-farm effectiveness of the inhibitor. This information is essential for estimating optimal inhibitor application rates/timing to maximise the effectiveness at reducing N2O emissions, while reducing the amount of NI released into the environment (and thereby reducing the risk of unintended adverse effects on grazing livestock and humans).
The objective of the research reported here was to determine the proportion of urine-N captured by at least the threshold concentration (inhibitor concentration at which the nitrification rate is reduced by at least 40%) of three key NIs (i.e. DCD, DMPP, and nitrapyrin) in two dairy-grazed pasture soils. This study is part of a major research project to enhance our understanding of the degree of physical mixing expected between the naturally deposited cattle urine and inhibitors in the soil. The goal is to accurately assess the potential for emission reduction by assessing the proportion of urine-N captured by amendments, such as NIs.
Materials and methods
Experimental set up and treatments
Field experiments were conducted during spring/summer 2021 at two typical dairy farms in NZ: Massey University No. 4 Dairy Farm, Palmerston North and AgResearch’s Ruakura dairy farm; hereafter referred to as ‘Manawatū farm’ and ‘Waikato farm’, respectively. The pasture consisted predominantly of a mixture of perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.) at both farms. The poorly drained soil at the Manawatū farm is Tokomaru silt-loam, classified as Argillic-fragic Perch-gley Pallic soil and the well-drained soil at the Waikato farm is Horotiu silt loam, classified as Typic orthic allophanic in the NZ soil classification system (Hewitt 2010), and termed as Alfisol and Andosol, respectively (FAO–UNESCO 1998). The selected areas for the experiments were fenced off for at least 10 weeks before the start of the experiments to avoid interference from uneven deposition of fresh dung, urine inputs from livestock grazing, and N-fertiliser application.
Field plots were established in a randomised complete block design with 4 replicates of each treatment. There were 6 treatments in each soil including 2 application rates (0.5 and 1 g patch−1 for DCD, 0.3 and 0.6 g patch−1 for DMPP, and 0.3 and 0.6 g patch−1 for nitrapyrin) and 3 application times (4, 24 and 48 h after urine application) for each inhibitor. The application rates of inhibitors were selected based on a previous review (Adhikari et al. 2021a) and a laboratory study with the same soils (Chibuike et al. 2022). One inhibitor was investigated at a time to facilitate manageability of sample numbers (24 urine patches), and subsequent inhibitors investigated as soon as practicable. The field work was completed in 12, and 22 days at Manawatū and Waikato farms, respectively. The daily air temperature, soil temperature (at 100 mm depth) and wind run for the days of treatment application and sample collection at both farm sites are presented in Supplementary material (Table S1).
Individual treatment plots (1 × 1 m), with 0.5 m2 circular sampling area, were separated by a distance of at least 0.5 m. An additional 2 m buffer zone was established to separate each inhibitor block. A week before the trial begins, four soil samples (0–100 mm depth, each representing 7–8 cores outside the plot and bulked) were collected to determine basic soil properties such as pH, total carbon (C), total nitrogen (N), mineral-N (NH4+ and NO3−), base saturation, and cation exchange capacity (CEC). The Manawatū soil had a pH of 5.3, contained 14.9 mEq 100 g−1 CEC, 5.6 mg kg–1 NH4+ and 6.1 mg kg–1 NO3−, and had 4.5% total C, 0.4% total N, and 82.7% base saturation. The Waikato soil had a pH of 5.7, contained 23.3 mEq 100 g−1 CEC, 3.5 mg kg–1 NH4+ and 6.5 mg kg–1 NO3−, and had 6.8% total C, 0.6% total N, and 41.3% base saturation. Intact soil cores were also collected from outside of the plot area before the trial began to measure bulk density of the site soils at 0–20, 20–50, 50–100, 100–150 mm depths. The bulk density of Manawatū soil at different depths ranged between 1.1 Mg m−3 and 1.2 Mg m−3, while it ranged between 0.8 Mg m−3 and 0.9 Mg m−3 for Waikato soil.
Preparation of synthetic urine
Synthetic urine with a total N content of 6 g L−1 was prepared using a modification of the method described by de Klein et al. (2003) and adjusting chemicals in proportion to total N content. Potassium bromide (KBr) was replaced with potassium chloride (KCl) at an equivalent rate of K, since bromide, a tracer (de Klein et al. 2003) was not needed in our study.
Application of synthetic urine and inhibitors
The pasture in the experimental areas was cut to 50 mm height on the day before urine application to simulate grazing. We poured 2 L of synthetic urine (average urine volume per urination event for dairy cattle; Selbie et al. 2015) onto the central point of the 0.5 m2 circular sampling area from a height of approximately 1.2 m and allowed it to spread naturally (to simulate natural cattle urine deposition). Cylindrical gas chamber bases with 0.5 m2 (800 mm diameter) were used to enclose the sampling area. The chamber/sampling area was covered during the period between urine application and inhibitor application when there was rain forecast to have consistent conditions for each treatment by avoiding any influence of rain on the movement of urine down into the soil profile. This allowed us to properly assess the effect of timing of inhibitors’ application on the proportion of urine-N that was captured by at least the threshold concentrations of inhibitors. Urine was applied in the morning and inhibitors were applied 4, 24, and 48 h after urine application using a Spikey® spot-spray unit, calibrated for delivering 40 mL of inhibitor solution to an area of 400 mm wide stripe across the chamber diameter (equivalent to 1,307 L ha−1). The volume of inhibitor used in this study was based on the previous NZ practice of the blanket application of 10 kg DCD ha−1 to entire paddocks.
Pasture sampling
To determine the proportion of each inhibitor retained by the pasture canopy following application of inhibitors, pasture samples were collected within 2 h of inhibitor application, immediately before soil sampling. A 200 mm diameter ring was centrally located on each sampling area and sampled by cutting pasture to ground level. All pasture samples collected from the 200 mm ring area was transferred to 200 mL plastic containers to avoid any potential loss of inhibitor before analysis.
Soil sampling
Thirteen soil cores (150 mm depth) were collected from each patch over a 600 mm diameter area centred to the middle of urine patch [along 4 transects radiating from the centre of the patch (1 from the centre of the urine patch and 3 each from 4 sections (at 100 mm, 200 mm and 300 mm distance from centre) within 2 h of inhibitor application immediately after pasture sampling. A wooden stick marked at 100, 200 and 300 mm was used to locate the soil sampling location. Each soil core was cut to 4 Sects. (0–20, 20–50, 50–100, 100–150 mm), bulked by plot, sieved to 4 mm removing plant materials including roots. Sieved samples were mixed thoroughly and subsampled for analysis of inhibitor, urea, mineral-N and moisture content. Inhibitor analyses were either performed immediately or samples were frozen if it was not feasible to analyse them immediately.
In this study, the soil sampling time (2 h after inhibitor application) was selected based on the results of a mini field experiment conducted in the Manawatū soil (by applying higher rate of DCD 4 h after natural urine patch simulation) which showed no influence of sampling time (2, 4, 24, and 48 h after DCD application) on the proportion of inhibitor distributed at different depths (Table S2). This suggests that time window of soil sampling (2 h) deployed in our study is sufficient for evaluating NIs downward movement in the soil profile in the absence of rain. To determine total N and mineral-N, soil was extracted with 0.5 M K2SO4 (1:5, wt/v) and analysis was performed using oxidative combustion-infrared and colorimetric methods, respectively (Mulvaney 1996). Urea as organic N is calculated by the difference between total N from urine and mineral-N from urine. Estimating the total urine-N recovered in the soil as a proportion of applied N is difficult due to uncertainty in the urine patch area and the possibility of some samples being collected from areas without urine due to the irregular shape of the urine patch (Selbie et al. 2015). Therefore, the distribution of urine-N in soil profile is presented as proportion of the total urine-N detected 4 h after urine application.
Inhibitor analysis
Measurement of DCD was performed using the methods described in Schwarzer and Haselwandter (1996) and Kim et al. (2012). The concentrations of DCD in the acidified extracts were determined on an UltiMate 3000 HPLC system (ThermoFisher Scientific, NZ) using a ROA-organic acid H + column (150 × 4.6 mm) and Diode-Array Detection (DAD) technique. DMPP and nitrapyrin extraction and analysis were carried out following methods described in Adhikari et al. (2021b), using HPLC–DAD and gas chromatography-mass spectrometry (GC–MS) techniques, respectively. Two of the 13 soil cores collected were from outside the inhibitor spray zone (inhibitor was applied to an area of 400 mm wide stripe across the chamber diameter), and this was accounted for when calculating the inhibitor mass kg−1 soil.
Determining proportion of urine-N captured by threshold concentrations of inhibitors
For the purposes of this study, the ‘threshold concentration’ of an inhibitor is defined as the inhibitor concentration in the soil (in mg kg−1 soil) at which the inhibitor efficiency in reducing nitrification rate under laboratory conditions is at least 40% (Chibuike et al. 2022). Values for threshold concentrations for each soil and inhibitor were based on a previous laboratory study (Chibuike et al. 2022). According to the results of that (ongoing) study, the threshold concentrations of inhibitors were 2.5 mg kg−1 (Manawatū soil) and 3.4 mg kg−1 (Waikato soil) for DCD; 13 mg kg−1 for DMPP (Waikato soil), and 5 mg kg−1 (Manawatū soil); and 6.8 mg kg−1 (Waikato soil) for nitrapyrin. The Manawatū soil did not achieve the targeted reduction in nitrification with up to 9.9 mg DMPP kg−1 (the greatest concentration of DMPP tested), and thus threshold concentration was not reported by Chibuike et al. (2022). To assess the effect of the timing of inhibitor application on the interception of urine-N by effective inhibitor concentrations, we examined the proportion of urea-N and ammonium-N across soil depths where inhibitor concentrations were at least at the threshold concentrations found by Chibuike et al. (2022). Due to the destructive nature of the NI and urine-N measurements, it was not possible to simultaneously measure N2O emissions in this study.
Statistical methods
The data for distribution of urine-N in soil, fresh pasture biomass at the time of inhibitor application, proportions of applied inhibitor intercepted by pasture canopy, and total proportions of applied inhibitors recovered in soil were analysed using an analysis of variance (ANOVA), and treatment means were compared using Fisher's least significant difference (LSD) Test. A linear regression was used to determine the relationship between the fresh pasture biomass and proportion of applied inhibitor intercepted by pasture canopy. Shapiro-Wilk and Bartlett's tests were used to check the normality and homogeneity of variance of the data, respectively, and appropriate transformations were performed, when necessary, to meet these requirements. All analyses were conducted using the Genstat statistical software (Genstat 64-bit Release 21.1, VSN International Ltd., P < 0.05 was used).
Results
Inhibitor intercepted by pasture canopy
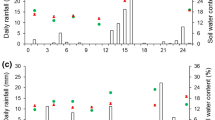
The proportions of the NIs intercepted by the pasture canopy following their applications at 4, 24 and 48 h after urine deposition, and fresh pasture biomass at the time of inhibitor application are presented in Table S3 (Supplementary material). The proportion of applied inhibitor intercepted by pasture canopy ranged between 38 and 59% for DCD, 27% and 58% for DMPP, and 31% and 58% for nitrapyrin. Figure 1 shows the relationship between fresh pasture biomass and the proportion of applied inhibitor intercepted by pasture canopy. There was a strong positive relationship between fresh pasture biomass standing and the proportion of inhibitor intercepted by the pasture canopy (R2 = 0.87). The relationship showed that inhibitor pasture canopy interception increased by about 5% with each 1000 kg ha−1 increase in fresh pasture biomass.
Relationship between fresh pasture biomass at the time of inhibitor application and proportion of applied inhibitor intercepted by pasture canopy (combined inhibitor types and study sites) * P < 0.001. Points represent individual replicates of each treatment. Standard error of mean values for fresh pasture biomass and % applied inhibitor intercepted by pasture canopy are 0.3 t ha−1 and 1.3%, respectively
Urine-N distribution in the soil profile
The distribution of urine-N in the soil profile as proportions of the total urine-N detected 4 h after urine application is presented in Table 1. The N data presented are the combined averages for both rates of inhibitor application as both inhibitor rates were investigated at the same time and the inhibitor rate was unlikely to have influenced the urine-N distribution as soil sampling was performed immediately after inhibitor application on each occasion. In general, total urine-N measured in both soils (at 0–150 mm depth) were less at 24 h and 48 h compared to 4 h. Similarly, the proportions of urine-N detected in all different depths at 24 h and 48 h were lower or similar compared with 4 h. The N distribution along the soil profile was not distinctly influenced by soil type in this study. In both soils the proportions of urine-N decreased with depth, but there was still about 10% (average) of the N in the lowest measured depth range (100–150 mm). In the Manawatū soil, the proportion of urine-N ranged between 19 and 42% at 0–20 mm, 19% and 35% at 20–50 mm, 12% and 29% at 50–100 mm, and 9% and 27% at 100–150 mm. Similarly, urine-N in the Waikato soil ranged between 27 and 39% at 0–20 mm, 21% and 33% at 20–50 mm, 19% and 30% at 50–100 mm, and 7% and 13% at 100–150 mm.
The amounts of different urine-N species recovered as a proportion of the total urine-N detected 4 h after urine application in DCD, DMPP and Nitrapyrin treatments are shown in Supplementary material (Figs. S1, S2, and S3, respectively). In all cases most of the N was in the form of NH4+.
Inhibitor distribution in the soil profile
The proportions of applied inhibitors recovered in the Manawatū and Waikato soils are presented in Fig. 2. Overall, total recoveries of DCD in the 150 mm of the soil (Manawatū, 29% to 42%; and Waikato, 29% to 44%) were higher than those for DMPP (Manawatū, 20% to 38%; and Waikato, 17% to 34%) and nitrapyrin (Manawatū, 21% to 35%; and Waikato, 18% to 36%). There was no distinct effect of soil and inhibitor types in downward movement of inhibitors along the soil profile. In all cases most of the inhibitors remained in the top 20 mm of the soil. In the Manawatū soil, the inhibitors measured at 0–20 mm as a proportion of the total amount recovered were between 83 and 93% for DCD; 92% and 94% for DMPP; and 87% and 92% for nitrapyrin. Similarly, the proportions of the inhibitors in the top 20 mm of the Waikato soil ranged from 79%–89%, 87%– 92%, and 75%–88% of total amount recovered for DCD, DMPP, and nitrapyrin, respectively.
The proportion of applied inhibitors recovered in the Manawatū and Waikato soils by depth (mean ± s.e.m.; n = 4) for different inhibitor treatments. Error bars represent the combined s.e.m. across four quantities for different depths. a) DCD = dicyandiamide; b) DMPP = 3,4-dimethylpyrazole phosphate; c) nitrapyrin = 2-chloro-6-(trichloromethyl) pyridine. Among the various treatments, the only difference in total proportion of applied inhibitors recovered in soil was observed with the lower rate of nitrapyrin (0.3 g nitrapyrin patch−1) applied to Waikato soil (4 h vs. 24 h, Fisher’s LSD Test, P < 0.05)
The concentration of NIs at different depths for Manawatū and Waikato soils are presented in Tables S4 and S5 (Supplementary material), respectively. Overall, doubling the inhibitor concentration without altering the volume resulted in higher concentrations of inhibitors in the soils. However, this increase did not lead to reach the threshold concentrations [2.5 mg kg−1 (Manawatū soil) and 3.4 mg kg−1 (Waikato soil) for DCD; 13 mg kg−1 for DMPP (Waikato soil), and 5 mg kg−1 (Manawatū soil); and 6.8 mg kg−1 (Waikato soil) for nitrapyrin] reported in Chibuike et al. (2022) in any of the deeper soil layers below 20 mm soil depth. One exception was the higher rate of DCD at the Waikato site where threshold concentrations were also achieved in the 20–50 mm depth.
Proportion of urine-N captured by threshold concentrations of inhibitors
The proportions of the ammoniacal-and urea-N measured in soil depths where the threshold concentrations of inhibitors were achieved (top 20 mm except for the higher rate of DCD applied to Waikato soil where N present in 20–50 mm depth was also included) were used to assess the proportion of the applied urine-N intercepted by inhibitors (Table 2). The proportion of urine-N captured by a threshold concentration of DMPP at the Manawatū soil is not reported as the threshold concentration for DMPP has not yet been established for that soil. The proportions of urine-N intercepted by the threshold concentrations of inhibitors in the Manawatū soil ranged from 24%– 40% for DCD, and 16%–23% for nitrapyrin. Similarly, in the Waikato soil, the proportions of urine-N intercepted by the threshold concentrations of inhibitors ranged from 31%–67% for DCD, 23%–40% for DMPP, and 21%–40% for nitrapyrin. In general, the proportion of urine-N intercepted by the threshold concentrations of inhibitors was less than 50% and was not affected by inhibitor application rates. In one case (higher rate of DCD at the Waikato site) an effective inhibitor concentration was reached to 50 mm and so this proportion increased to 63% on average showing the effect of inhibitor rate on the physical mixing between the inhibitor and urine-N. Results from this study also showed that a higher proportion of urine-N was intercepted by inhibitors when inhibitors were applied 4 h after urine application compared to 24 or 48 h after urine application. However, statistically significant differences between timing of inhibitor application were observed only for the treatments with the lower rate of DCD in the Manawatū soil (37% at 4 h vs 24% to 26% at 24 or 48 h, respectively); and for both rates of DMPP treatments in the Waikato soil (39% to 40% at 4 h vs 23% to 35% at 24 to 48 h, only the difference between 4 and 48 h was significant for the lower rate treatment).
Discussion
Proportion of inhibitors captured by pasture canopy
The substantial retention of applied NIs in pasture canopy is consistent with a previous field study by Giltrap et al. (2022) who reported a comparable range of 20 to 40% of inhibitor’s interception in pasture canopy. The variations in pasture canopy interception in our study can be directly linked with differences in fresh pasture biomass at the time of inhibitor application, with a strong positive relationship between the two factors (Fig. 1). The increase in inhibitor canopy interception with increasing pasture biomass is due to the fact that the volume of inhibitor solution retained is positively corelated with the available surface area. It should be noted that a given pasture biomass can retain only up to a certain volume. Therefore, increasing the inhibitor volume without changing the application amount in terms of grams of inhibitor per urine patch could reduce inhibitor interception by the canopy. The differences in fresh pasture biomass at the time of inhibitor application (5–14 t ha−1) were associated with both variations in the sampling time [i.e., time delay between the urine patch deposition and inhibitor application or pasture sampling (4, 24, and 48 h)] and natural spatial variation in pasture coverage within dairy grazed pastures. Therefore, the effect of sampling time on the proportion of inhibitor intercepted by the pasture canopy could not be assessed accurately in this study.
Physical mixing of inhibitor with urine-N
The inhibitor threshold concentrations were achieved only in the top 0–20 mm of the soil with either rate of inhibitors in almost all cases. This corresponded to only 16%–40% of the urine-N being intercepted by threshold concentrations of inhibitors, suggesting that the inhibitor effectiveness at reducing N2O emissions is likely to be limited due to poor co-location of inhibitor and urine-N. This is particularly true when there is no post application of irrigation/rainfall within the first week of urine application which is when most nitrification occurs. This could explain why previous unpublished study (S Saggar Pers. Com.) did not find an effect of NIs (DCD, DMPP, and nitrapyrin) on N2O emissions under similar conditions where inhibitors were applied 4 h after natural urine patch simulation. In the one case (a higher rate of DCD at the Waikato site) where there was an effective inhibitor concentration reached down to 50 mm, the proportion of urine-N co-located with the inhibitor threshold concentration increased to 58%–67%. The poor physical mixing between urine-N and the inhibitors at threshold concentrations is mainly associated with: 1) a high proportion of inhibitors captured on the plant canopy (27%–59%), limiting the amount that makes it to the soil (only 41% –73%); 2) the fact that any inhibitor that did reach the soil was not transported far down to the soil profile compared to the urine transport. This is probably due to the relatively low volume (as opposed to mass) of the inhibitor applied (40 mL) compared to the urine volume (2 L), and downward movement of urine-N along the soil profile during the period (4 to 48 h) between the urine deposition and inhibitor application. A similar result for DCD was reported in a previous field study by Giltrap et al. (2022) who measured 20%–40% of the applied inhibitor in plant canopy, and effective concentrations of inhibitors only in the top 20 mm of the pasture soils.
The results from this study suggest that, in the absence of rainfall/irrigation, a substantial proportion of the urine-N is not initially mixed with effective concentrations of the inhibitors when there is some time (4 – 48 h) delay between the urine deposition and the inhibitor application. This limits the inhibitor effectiveness at mitigating N2O emissions when there is no post application of irrigation/rainfall within the first week of urine application (the period when most of the nitrification occurs). However, the reduction in N2O emissions is not simply proportional to the reduction in nitrification rate. For instance, Giltrap et al. (2010) reported that a 50% reduction in N2O emissions with the use of inhibitor was best simulated by a reduction of 60% in nitrification. It should be noted that concentrations of NIs lower than the threshold concentrations can still inhibit some nitrification activity and thereby reduce N2O emissions. Furthermore, the inability of the inhibitors to fully intercept urine-N may not necessarily be reflected in a proportionately lower reduction in N2O emissions. This is because the N2O production rate varies with soil depth due to greater chances of complete denitrification of N2O to N2 in the deeper soil (longer residence time). Thus, the impact of the inability of the inhibitor to physically intercept urine-N in the deeper soil layers will be less in terms of emissions reduction when the greater proportion of total N2O emissions originate from upper soil layers. This aspect must be investigated to fully understand the potential impacts of physical co-location between the inhibitor and urine on emissions reductions.
Potential contribution of increasing inhibitor volume and rainfall/irrigation on urine-N and inhibitor mixing
In this study, the amount of inhibitor applied (g inhibitor urine patch−1) did not appear to be the limiting factor, but rather the low volume of inhibitor carrier applied relative to the urine volume (1:50, 40 mL of inhibitor to the 2L of urine patch). Increasing the volume of inhibitor carrier (simply by adding more water which does not change the amount of inhibitors) or post application of irrigation/rainfall can be an effective approach for increasing urine-N interception by the inhibitors and achieving at least threshold concentrations of inhibitors within a larger soil volume. There are two possible mechanisms by which this could enhance the physical interaction between urine and inhibitors: i) reducing the proportion of inhibitor captured by pasture canopy; ii) increasing the inhibitor’s ability to travel deeper into the soil profile. It is estimated that achieving inhibitor concentrations at or above the threshold to the 50 mm soil depth would increase the physical interaction between urine-N and the inhibitor to between 34 and 76%. However, it should be noted that post application of rainfall/irrigation can also move down the urine in the soil profile if N is still present in urea form. Thus, for achieving optimal mixing of urine-N and inhibitors, research for critical information on the ideal timing and amount of rainfall/irrigation is warranted.
Proportion of urine-N intercepted by a threshold concentration of inhibitors as affected by timing of inhibitor application after urine
The higher proportion of urine-N intercepted by a threshold concentration of inhibitor when inhibitors were applied 4 h after urine compared to 24 and 48 h after urine (in some cases), suggests the possibility of greater benefits when inhibitors are applied within a few hours of urine deposition. The lower interception of urine-N by inhibitors at 24 h and 48 h compared with 4 h is possibly attributed to gaseous N losses occurring between 4 and 24 h or 48 h after urine application [such as ammonia (NH3) and N2O emissions] and N uptake by plants. The NZ National GHG Inventory (MfE 2022) uses an average loss via NH3 volatilisation from urine in NZ pasture soil of 10% of the N deposited, of which about 50% is emitted within the first 24 h (Adhikari et al. 2020; Rodriguez et al. 2021). Although the urine patches were covered during the period between urine application and inhibitor application to avoid any influence of rain, variations in other climatic conditions such as soil and air temperatures could have resulted in differences on amount of these gaseous N losses occurred among the inhibitor treatments in the present study as all the inhibitors were not investigated at the same time.
Conclusions
Increasing the proportion of urine N intercepted by threshold concentrations of inhibitors is required for effective mitigation of N2O emissions with the use of NIs. This is less likely to occur with the application of 40 mL inhibitor per urine patch at 4 to 48 h after urine deposition as poor physical mixing between the inhibitor and urine was observed under these conditions. There is the possibility of greater mixing between the inhibitor and urine when inhibitors are applied within few hours of urine deposition compared to delayed application at 24 or 48 h, as evidenced in some cases with the current study. This study has highlighted that the volume of inhibitor carrier applied relative to urine volume (40 mL:2 L, 1:50) can affect effective mixing of urine-N with inhibitors. A question remains: could increasing the volume of the inhibitor carrier enhance the proportion of urine-N intercepted by inhibitors, reduce pasture canopy interception of diluted inhibitor concentration, and thus increase the efficacy of inhibitor in reducing N2O emissions? Future research on optimizing the ratio of inhibitor to urine volume and determining the ideal inhibitor concentrations to improve their efficiency in mitigating on-farm N2O emissions is warranted. It is also essential to investigate how rainfall/irrigation timing and amount affect the transport of inhibitors into the soil, ensuring that they reach the critical zones where they can most effectively reduce N2O emissions.
References
Adhikari KP, Saggar S, Hanly JA, Guinto DF (2020) Urease inhibitors reduced ammonia emissions from cattle urine applied to pasture soil. Nutr Cycl Agroecosyst 117:317–335. https://doi.org/10.1007/s10705-020-10070-0
Adhikari KP, Bishop P, Saggar S (2021a) Methods for extracting and analysing DMPP and Nitrapyrin in soil and plant samples from grazed pasture. Plant Soil 469:149–160. https://doi.org/10.1007/s11104-021-05151-0
Adhikari KP, Chibuike G, Saggar S, Simon PL, Luo J, de Klein CAM (2021b) Management and implications of using nitrification inhibitors to reduce nitrous oxide emissions from urine patches on grazed pasture soils – a review. Sci Total Environ 791:148099. https://doi.org/10.1016/j.scitotenv.2021.148099
Chibuike G, Palmada T, Saggar S, Giltrap D, Luo J (2022) A comparison of the threshold concentrations of DCD, DMPP and nitrapyrin to reduce urinary nitrogen nitrification rates on pasture soils–a laboratory study. Soil Res 61:187–197. https://doi.org/10.1071/SR22133
Clough TJ, Di HJ, Cameron KC, Sherlock RR, Metherell AK, Clarke H, Rys G (2007) Accounting for the utilization of a N2O mitigation tool in the IPCC inventory methodology for agricultural soils. Nutr Cycl Agroecosyst 78:1–14. https://doi.org/10.1007/s10705-006-9069-z
de Klein CA, Barton L, Sherlock RR, Li Z, Littlejohn RP (2003) Estimating a nitrous oxide emission factor for animal urine from some New Zealand pastoral soils. Soil Res 41:381–399. https://doi.org/10.1071/SR02128
Di HJ, Cameron KC (2006) Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide. Biol Fertil Soils 42:472–480. https://doi.org/10.1007/s00374-005-0038-5
Di HJ, Cameron KC (2016) Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. J Soils Sediments 16:1401–1420. https://doi.org/10.1007/s11368-016-1403-8
Di HJ, Cameron KC, Sherlock RR, Shen JP, He J, Winefield C (2010) Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J Soil Sediments 10:943–954. https://doi.org/10.1007/s11368-009-0174-x
FAO-UNESCO (1998) Soil map of the world. World soil resources report No 84. FAO, Rome
Giltrap D, Singh J, Saggar S, Zaman M (2010) A preliminary study to model the effects of a nitrification inhibitor on nitrous oxide emissions from urine-amended pasture. Agric Ecosyst Environ 136:310–317. https://doi.org/10.1016/j.agee.2009.08.007
Giltrap D, Portegys N, Saggar S, Hanly JA (2022) The proportion of deposited urine patch intercepted by a delayed inhibitor application. Environ Technol 43:3755–3764. https://doi.org/10.1080/09593330.2021.1934561
Hewitt AE (2010) New Zealand soil classification. Manaaki Whenua-Landcare Research New Zealand Ltd Lincoln, N.Z.: Manaaki Whenua Press, 2010. ISBN 9780478347104 (pbk.)
Jolly B, Luo J, Mehra P, Forrestal P, O’Neill M, Richards K, Singh BP, Bates G, Saggar S (2021) Evaluation of proximal sensing technologies for mapping bovine urine patches in grazing pastures. Comput Electron Agric 188:106309. https://doi.org/10.1016/j.compag.2021.106309
Kim D-G, Giltrap D, Saggar S, Palmada T, Berben P, Drysdale D (2012) Fate of the nitrification inhibitor dicyandiamide (DCD) sprayed on a grazed pasture: effect of rate and time of application. Soil Res 50:337–347. https://doi.org/10.1071/SR12069
Maire J, Gibson-Poole S, Cowan N, Reay DS, Richards KG, Skiba U, Rees RM, Laniga GJ (2018) Identifying urine patches on intensively managed grassland using aerial imagery captured from remotely piloted aircraft systems. Front Sustain Food Syst 2:10. https://doi.org/10.3389/fsufs.2018.00010
MfE (Ministry for the Environment) (2022) New Zealand's greenhouse gas inventory 1990–2020 Available at https://environment.govt.nz/publications/new-zealands-greenhouse-gas-inventory-1990-2020/. Accessed 13 Jan 2023
Moir JL, Cameron KC, Di HJ, Fertsak U (2011) The spatial coverage of dairy cattle urine patches in an intensively grazed pasture system. J Agric Sci 149:473–485. https://doi.org/10.1017/S0021859610001012
MPI (Ministry for Primary Industries) (2013) New Zealand Government assures safety of country's dairy products. Available at https://www.mpi.govt.nz/news/media-releases/new-zealand-government-assures-safety-of-countrys-dairy-products/. Accessed 25 Aug 2021
Mulvaney RL (1996) Extraction of exchangeable ammonium, nitrate and nitrite. In: Sparks DL, Page AL, Helmke PA, Loeppert RH (eds) Methods of Soil Analysis Part 3: Chemical Methods. Soil Science Society of America, American Society of Agronomy, Madison, pp 1129–1131
Qiao CL, Liu LL, Hu SJ, Compton JE, Greaver TL, Li QL (2015) How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob Chang Biol 21:1249–1257. https://doi.org/10.1111/gcb.12802
Rodriguez MJ, Saggar S, Berben P, Palmada T, Lopez-Villalobos N, Pal P (2021) Use of a urease inhibitor to mitigate ammonia emissions from urine patches. Environ Technol 42:20–31. https://doi.org/10.1080/09593330.2019.1620345
Saggar S, Singh J, Giltrap DL, Zaman M, Luo J, Rollo M, Kim DG, Rys G, van der Weerden TJ (2013) Quantification of reductions in ammonia emissions from fertiliser urea and animal urine in grazed pastures with urease inhibitors for agriculture inventory: New Zealand as a case study. Sci Total Environ 465:136–146. https://doi.org/10.1016/j.scitotenv.2012.07.088
Schwarzer C, Haselwandter K (1996) Rapid quantification of the nitrification inhibitor dicyandiamide in soil samples, nutrient media and bacterial cell-free extracts. J Chromatogr A 732:390–393. https://doi.org/10.1016/0021-9673(95)01283-4
Selbie DR, Buckthought LE, Shepherd MA (2015) The challenge of the urine patch for managing nitrogen in grazed pasture systems. Adv Agron 129:229–292. https://doi.org/10.1016/bs.agron.2014.09.004
Zaman M, Nguyen ML (2012) How application timings of urease and nitrification inhibitors affect N losses from urine patches in pastoral system. Agric Ecosyst Environ 156:37–48. https://doi.org/10.1016/j.agee.2012.04.025
Acknowledgements
We are grateful to Massey University and AgResearch for providing access to dairy grazed pasture sites in Palmerston North and Hamilton for conducting field experiments, and to Massey University for HPLC and GCMS facilities to analyse inhibitor residues. Our thanks to field and laboratory staff from the Manaaki Whenua – Landcare Research (MWLR) Palmerston North (Peter Berben, Thilak Palmada), and AgResearch Hamilton (Stuart Lindsey, Moira Dexter) for their contributions in the maintenance of the sites, application of treatments, soil and plant samples processing and laboratory analysis. The efforts of Dr Liyin Liang and Helen O'Leary from MWLR NZ Ltd for reviewing and editing this research paper, respectively are also appreciated.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Kamal P. Adhikari: Conceptualization, Methodology, Supervision, Formal analysis, Data curation, Writing – original draft, Writing – review & editing.
Jiafa Luo: Conceptualization, Methodology, Resources, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Surinder Saggar: Conceptualization, Methodology, Resources, Project administration, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition.
Donna Giltrap: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Financial support
This research was funded by the New Zealand Agricultural Greenhouse Gas Research Centre, and in-kind and cash contributions from Pastoral Robotics Limited, and supported by Strategic Science Investment Fund (SSIF) for Crown Research Institutes from the Ministry of Business, Innovation and Employment’s Science Innovation Group.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This special issue, titled ‘Towards Net Zero Emissions Without Compromising Agricultural Sustainability: What Is Achievable?’, is guest-edited by M.I. Khalil, B. Osborne, and A. Wingler. It originates from theISCRAES 2022 (https://www.iscraes.org/) Panel Discussion, which was sponsored by the OECD Co-operative Research Programme: Sustainable Agricultural and Food Systems.
The OECD disclaimer is: The opinions expressed and arguments employed in this publication are the sole responsibility of the authors and do not necessarily reflect those of the OECD or of the governments of its Membercountries.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adhikari, K.P., Luo, J., Saggar, S. et al. Inhibitors application time and pasture canopy capture regulate gaseous losses of urine-N. Nutr Cycl Agroecosyst (2024). https://doi.org/10.1007/s10705-024-10351-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10705-024-10351-y