Abstract
High temperature hydrogen attack (HTHA) is degradation of steels exposed to hydrogen gas at high temperatures and pressures. Hydrogen in steels reacts with carbon from carbides to produce methane gas bubbles typically on grain boundaries which grow and coalesce, leading to loss of strength and fracture toughness. Current design practice against HTHA is based on the Nelson curves which define the conditions for safe operation in a temperature/hydrogen-partial-pressure diagram. Nelson curves are phenomenological in nature and do not account for the underlying failure mechanism(s), material microstructure, carbide stability, and applied stresses. In light of experimental evidence of predominant cavitation ahead of cracks reported by Martin et al. (Acta Mater 140:300–304, 2017), it is expected that void growth is accelerated by the triaxial stresses associated with microstructural flaws. To this end, we propose a three-dimensional, axisymmetric, constraint-based void-growth model extending the “one-dimensional” model of Dadfarnia et al. (Int J Fract 219:1–17, 2019). The present model is shown to yield satisfactory agreement with the available experimental data from hydrogen attack of 2¼Cr–1Mo steel at temperatures ranging from 500 to 600 °C. In addition, the model is used to construct Nelson type curves in the temperature/hydrogen-partial-pressure diagram. These curves represent failure times for given applied stresses and triaxiality. The proposed methodology can be viewed as providing a step toward improving the current design practice against HTHA while maintaining the simplicity of the original Nelson curve approach.




















Similar content being viewed by others
Notes
In this study we adopt experimental data of Viswanathan since their creep experiments were conducted at temperatures which are of interest to us from simulations point of view. Klueh conducted creep test at temperatures only as high as 566 °C which is lower than 600 °C at which we are interested in simulating HTHA.
References
API TR 941 (2008) The technical basis document for API RP 941. American Petroleum Institute Technical Report 941, API Publishing Services
API RP 941 (2016) Steels for hydrogen service at elevated temperatures and pressures in petroleum refineries and petrochemical plants, 8th edn. American Petroleum Institute Recommended Practice 941, API Publishing Services
Bao G, Hutchinson JW, McMeeking RM (1991) Particle reinforcement of ductile matrices against plastic flow and creep. Acta Metall Mater 39:1871–1882. https://doi.org/10.1016/0956-7151(91)90156-U
Chao B (1987) Kinetics and mechanisms of hydrogen attack in 2.25 Cr-1 Mo steel. University of California, Santa Barbara
Chuang T-J, Kagawa KI, Rice JR, Sills LB (1979) Overview no. 2: non-equilibrium models for diffusive cavitation of grain interfaces. Acta Metall 27:265–284. https://doi.org/10.1016/0001-6160(79)90021-X
Cocks ACF, Ashby MF (1982) On creep fracture by void growth. Prog Mater Sci 27:189–244. https://doi.org/10.1016/0079-6425(82)90001-9
Dadfarnia M, Martin ML, Moore DE et al (2019) A model for high temperature hydrogen attack in carbon steels under constrained void growth. Int J Fract 219:1–17. https://doi.org/10.1007/s10704-019-00376-8
Dyson BF (1976) Constraints on diffusional cavity growth rates. Metal Sci 10:349–353. https://doi.org/10.1179/030634576790431417
Erwin WE (1982) Mechanisms of hydrogen attack in 2 1/4Cr-1Mo reactor steel. Proc Am Pet Inst, Refin Dep; (United States) 61
Frost HJ, Ashby MF (1982) Deformation mechanism maps: the plasticity and creep of metals and ceramics. Pergamon Press, Oxford
Grabke H, Martin E (1973) Kinetics and thermodynamics of the carburization and decarburization of α-iron in CH4-H2 mixtures. Arch Fuer Das Eisenhuettenwes 44:837–842
Hull D, Rimmer DE (1959) The growth of grain-boundary voids under stress. The philosophical magazine. J Theor Exp Appl Phys 4:673–687. https://doi.org/10.1080/14786435908243264
Klueh RL (1980) Creep and creep-rupture behaviour of a bainitic 2 1/4 Cr-1 Mo steel. Int J Press Vessels Pip 8:165–185. https://doi.org/10.1016/0308-0161(80)90023-X
Lubliner J (2008) Plasticity theory. Courier Corporation
Martin ML, Dadfarnia M, Orwig S et al (2017) A microstructure-based mechanism of cracking in high temperature hydrogen attack. Acta Mater 140:300–304. https://doi.org/10.1016/j.actamat.2017.08.051
Needleman A, Rice JR (1980) Plastic creep flow effects in the diffusive cavitation of grain boundaries. Acta Metall 28:1315–1332. https://doi.org/10.1016/0001-6160(80)90001-2
Odette GR, Vagarali SS (1982) An equation-of-state for methane for modeling hydrogen attack in ferritic steels. Metall Trans A 13:299–303. https://doi.org/10.1007/BF02643320
Pillot S, Coudreuse L (2012) 2-Hydrogen-induced disbonding and embrittlement of steels used in petrochemical refining. In: Gangloff RP, Somerday BP (eds) Gaseous hydrogen embrittlement of materials in energy technologies. Woodhead Publishing, Sawston, pp 51–93
Sagüés AA, Okray Hall B, Wiedersich H (1978) On the mechanisms of hydrogen attack. Scr Metall 12:319–326. https://doi.org/10.1016/0036-9748(78)90121-7
Sham T-L, Needleman A (1983) Effects of triaxial stressing on creep cavitation of grain boundaries. Acta Metall 31:919–926. https://doi.org/10.1016/0001-6160(83)90120-7
Shewmon PG (1976) Hydrogen attack of carbon steel. Metall Trans A 7:279–286. https://doi.org/10.1007/BF02644468
Shih H-M, Johnson HH (1986) A model calculation of the Nelson curves for hydrogen attack. In: Ashby MF, Hirth JP (eds) Perspectives in hydrogen in metals. Pergamon, Bergama, pp 163–171
Stone D (1984) Hydrogen attack in 2.25 Cr–1.0Mo steels. Cornell University
Sundararajan G, Shewmon PG (1981) The kinetics of hydrogen attack of steels. Metall Trans A 12:1761–1775. https://doi.org/10.1007/BF02643758
Takazaki D, Tsuchiyama T, Komoda R et al (2020) Effect of hydrogen on creep properties of SUS304 austenitic stainless steel. Corrosion 77:256–265. https://doi.org/10.5006/3678
U.S. Chemical Safety and Hazard Investigation Board (2014) Investigation report: catastrophic rupture of heat exchanger
Van der Burg MWD, van der Giessen E, Brouwer RC (1996) Investigation of hydrogen attack in 2.25Cr-1Mo steels with a high-triaxiality void growth model. Acta Mater 44:505–518. https://doi.org/10.1016/1359-6454(95)00203-0
Van Der Giessen E, Van Der Burg MWD, Needleman A, Tvergaard V (1995) Void growth due to creep and grain boundary diffusion at high triaxialities. J Mech Phys Solids 43:123–165. https://doi.org/10.1016/0022-5096(94)00059-E
Viswanathan R (1974) Strength and ductility of 21/4Cr–1Mo steels in creep at elevated temperatures. Metals Technol 1:284–294. https://doi.org/10.1179/030716974803287546
Yokogawa K, Fukuyama S, Kudo K (1982) Apparatus for creep rupture testing in high-pressure hydrogen at elevated temperatures. Rev Sci Instrum 53:86–89. https://doi.org/10.1063/1.1136822
Yokogawa K, Fukuyama S, Kudo K, Shewmon PG (1989) Effect of hydrogen attack on tensile and creep properties of low carbon steel. Int J Press Vessels Pip 37:365–385. https://doi.org/10.1016/0308-0161(89)90030-6
Acknowledgements
The authors would like to acknowledge the funding and technical support from bp through the bp International Centre for Advanced Materials (bp-ICAM) which made this research possible.
Author information
Authors and Affiliations
Contributions
K.V., M.D., and P.S. designed the model and the computational framework. M.K., A.S., K.W., J.A.P., and T.J.E., provided critical feedback for the model. K.V. and M.D. carried out the numerical simulations. K.V., M.D., and P.S. drafted the manuscript. M.K., A.S., K.W., J.A.P., and T.J.E. provided critical revision of the paper. All authors reviewed the manuscript and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Constitutive model for the of 2¼Cr–1Mo steel
Dadfarnia et al. (2019) studied the creep response data of 2¼Cr–1Mo steel from Klueh (1980) and Viswanathan (1974) and observed that the creep exponent, \(n\), and the activation energy, \(Q_{v}\), vary significantly with temperature. Accordingly, the experimental data of Viswanathan (1974) can be fit at different temperaturesFootnote 1 by the power-law equation
in which the reference stress \(\sigma_{0}\) is taken equal to 1 MPa the reference strain rate \(\dot{\varepsilon }_{0}\) equal to \(5.69 \times 10^{ - 4} /{\text{s}}\), and R is the universal gas constant equal to 8.31 J/mol.K. The creep exponent \(n\) and the activation energy \(Q_{v}\) are estimated from the experimental data as shown in Fig.
Experimental data for creep of 2¼Cr–1Mo steel at four different temperatures by Viswanathan (1974) shown as log of normalized strain rate vs log of normalized stress along with the corresponding power law function fit (dashed lines)
21 The values of the fitted parameters are listed in Table
2, and linear interpolation, as shown in Fig.
Linear interpolation of the Table 2 data for a creep exponent and b creep activation energy for 2¼Cr–1M steel as function of temperature
22, yields
where the temperature \(\Theta\) is measured in Kelvin and the activation energy \(Q_{v}\) in kJ/mole. In the computations, the creep response of the material, in both cavitated and uncavitated regions, at any given temperature in between range 500–600 °C, is described by the power law creep equation (6) in which \(n\left( \Theta \right)\) and \(\dot{\varepsilon }\left( \Theta \right) = \dot{\varepsilon }_{0} \exp \left( { - Q_{v} \left( \Theta \right)/R\Theta } \right)\) are calculated through Eqs. (A2).
Appendix B: Void growth model of Van Der Giessen et al. (1995)
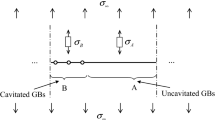
The deformation of the imbedded \(C\) cylinder given in Fig. 4b is studied in this section. The mean and effective macroscopic stress associated with this state of stress in cylinder \(C\) are
Void growth model of Van Der Giessen et al. (which is based on the previous studies by Sham and Needleman 1983 and Needleman and Rice 1980), provides the volumetric growth rate as the summation of void growth due to creep and grain boundary diffusion:
Here, L and H denote low and high triaxiality ratios, respectively.
Void growth rate due to creep is given by
where \(\dot{\varepsilon }_{m}^{C} = \dot{\varepsilon }(\Theta )\left| {\frac{{\sigma_{m}^{C} }}{{\sigma_{0} }}} \right|^{n} ,\alpha_{n} = \frac{3}{2n},\beta_{n} = \frac{(n - 1)(n + 0.4319)}{{n^{2} }},m = \frac{{sign(S_{app}^{B} - T)}}{{sign(\sigma_{m}^{C} )}}\).
For the contribution of diffusion
Here,
where \(p\) is the pressure due to gas in cavity, \(\sigma_{s} = \left( {1 - f} \right)2\gamma_{s} \sin \psi /a\) is the sintering stress, \(\gamma_{s}\) is the surface free energy, \(D\left( \Theta \right) = D_{B} \delta_{B} \Omega /k\Theta\) is the grain boundary diffusion parameter, \(D_{B} \delta_{B}\) is the boundary diffusion coefficient, \(\Omega\) is the atomic volume, \(k\) is the Boltzmann’s constant and \(L_{NR} = \left( {D(\Theta )\sigma_{e}^{C} /\dot{\varepsilon }_{e}^{C} } \right)^{1/3}\) is the diffusion length parameter introduced by Needleman and Rice (1980), \(\sigma_{e}^{C}\) is the effective stress in the imbedded cylinder C given by (B1), and \(\dot{\varepsilon }_{e}^{C}\) is the effective strain rate in the imbedded cylinder C given by
Appendix C: Cavity pressure
Grabke and Martin (1973) proposed the kinetics of methane formation on iron surface as
where \(N_{{CH_{4} }}\) is the number of methane molecules, \(A\) is the reaction area, \(C_{C}\) is the concentration of carbon atoms at the reaction surface area, \(f_{{H_{2} }}\) and \(f_{{CH_{4} }}\) are the fugacities of hydrogen and methane gas, respectively, \(K_{1}\) and \(K_{2}\) are the reaction rate constants for formation and dissociation reactions of methane, respectively, which are given by
where \(R = 8.314\) J/mol K is the universal gas constant and \(\Theta\) is the temperature in Kelvins. Dadfarnia et al. (2019) showed that under typical HTHA conditions, the kinetics of methane formation, based on the Grabke and Martin (1973) model, are such that carbon and hydrogen gas can very well be assumed to be in chemical equilibrium. Thus, Eq. (C1) provides
The fugacity of hydrogen gas is assumed to be the same as the hydrogen partial pressure. The concentration of carbon, in mol/m3, is calculated by
where, \(a_{C}\) is the activity of carbon in steel and \(C_{gr} = 66.79\exp \left( { - 68,700/R\Theta } \right)\) is the solubility of graphite in ferrite in percent weight. Based on the carbon concentration and hydrogen fugacity, equilibrium methane fugacity at a given temperature can be calculated using Eq. (C3). Odette and Vagarali (1982) proposed the relationship between methane fugacity, \(f_{{CH_{4} }}\), and methane partial pressure, \(p_{{CH_{4} }}\), as
where \(C(\Theta )\) is given by
Finally, the total pressure is assumed to be the sum of partial pressures of hydrogen gas and methane gas,
It should be noted that Eq. (C7) is a simplified assumption which has been conventionally adopted by researchers for modeling the void-growth during HTHA (Van der Burg et al. 1996; Dadfarnia et al. 2019).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vijayvargia, K., Dadfarnia, M., Sofronis, P. et al. Three-dimensional constraint-based void-growth model for high temperature hydrogen attack. Int J Fract 243, 203–228 (2023). https://doi.org/10.1007/s10704-023-00739-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10704-023-00739-2