Abstract
In this short essay I address the central topic of the Centenary Workshop on Acidity, that is the relations of the classical protonist acid–base theory by Brønsted and the electronist approach by Lewis. Emphasis is laid on the empirical background of both approaches and the over-theoretization of chemical phenomena (essentialism) is criticized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Summing up his critique of Hilary Putnam’s theory of meaning, Ian Hacking states in Representing and Intervening: “We should worry about kinds of acids, not kinds of meaning” (Hacking 1983, 85). In his critical examination of Norman Malcolm’s theoretical assumptions in the work Dreaming, Putnam had reflected on the historicity of scientific concepts and invented an example regarding the origins of multiple sclerosis. Putnam adds to this a real example:
A similar case is afforded by the history of the term ‘acid’ in chemistry. Two hundred years ago a chemist might have had only two or three criteria for a substance’s being an acid: being soluble in water; sour taste (in water solution; turning litmus paper red. Today we have a theoretical definition in terms of the notion ‘proton-donor.’ Yet I feel sure that any chemist would want to say he is talking about the same chemical substances that the eighteenth century chemist called ‘acids’. Is there any decisive reason for rejecting this ‘naïve’ view? (Putnam 1975, 269)
Putnam admits that there may have been cases in which one criterion or the other could not be observed yet one had nonetheless kept the designation “acid”.Footnote 1 He then continues:
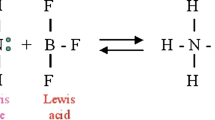
Main sections of the use of the notion (or concept of) acid (This is (on purpose) not a Venn diagram as it is occasionally found in the literature, for example in Hall (1940). Cf. also the contributions of Gerd-Uwe Flechsig and Pieter Thyssen)
But why not say that ‘in the eighteenth century sense’ only substances satisfying the eighteenth century criteria were acids?—Simply because this does not do justice to the probable intelligence of eighteenth century chemists. In all likelihood, they knew perfectly well that their criteria were crude ways of detecting a ‘natural kind’ of chemical; they would have thought it unlikely that their criteria exactly ‘caught’ the boundaries of that kind. Of course, even in the light of later theory, the ‘boundaries’ of the kind in question may require more or less arbitrary legislation: in this sense some stipulation may have entered into the present technical definition. (Putnam 1975, 312, emphases original)
Putnam hence indicates that the image of the acids (and bases) in the respective context can change, especially as scientific developments progress. As is the case with his more well known example (water) from The Meaning of Meaning the “vector” of the conceptual context for the acids as well can be subdivided into “syntactic markers,” “semantic markers”, “stereotypical properties”, and an “extension”. If according to Putnam the extension (the “rigid designator”) for water is the compositional formula H2O (Fig. 1), then for acids it is the theoretical statement “proton-donor”.
What exactly do we mean when we use the expression “acid”? To answer this question it is first necessary to specify the “we” being used. As the concept is customarily used in everyday practice in kitchens, hospitals, workshops, and laboratories, we find ourselves very close to Putnam's description: We think of something liquid that has a specific taste, can change certain plant-based colorings, dissolves certain materials, and maybe even attacks metals. If we ask experts from the environmental sciences or clinical medicine, then we soon hear talk of pH values, and most people we talk to will—in keeping with their scientific training—agree on characterizing those liquids as “acidic” that exhibit pH values below 7 under standard conditions. The experts in the field of organic chemistry often refer to Lewis acids and bases, particularly in discussions about reaction mechanisms. Finally, if we ask current chemical theorists, that is quantum chemists, what acids are, we might receive an answer like this: a substrate with the highest occupied molecular orbital (HOMO).Footnote 2 Hence, when it comes to acids (and bases) one must expect to encounter a range of different views. In any case one can hardly speak of unanimity.Footnote 3
Putnam’s mention of the historicity of the professional characterization of acidity is actually as correct as it is essentially trivial. As regards the example from the history of chemistry discussed here, the situation is nonetheless particularly interesting. In his 1975 writings he refers exclusively to Brønsted’s acid–base theory, which the latter presented in a comprehensive article in Brønsted (1923). Hacking is apparently accusing Putnam of having at least not recounted the actual episode in its entirety, for Putnam makes no mention of Gilbert Lewis’ theoretical proposition of the same year. This is astounding because in 1975 the acid–base theory of the American chemist Lewis, that is his “Lewis acids” and “Lewis bases”, had long since become established in mainstream chemistry. Additionally, Lewis’ approach fits in nicely with Putna’s argumentation. The meaning of the term “acid” as Lewis understands it is in fact different from Brønsted’s “classical” version from the protonistic tradition.
Does Hacking in his critique actually think that there are many kinds of acids? What would then be the common element that would justify using the same expression? I will examine the latter question in particular in the following section.
The relationship between acids and bases has been asymmetrical from the beginning: Due to their striking sensory characteristics some examples achieved the status of chemical individuals very early whereas the bases have remained in the shadows so to speak.Footnote 4 Even in Lewis’ new definition bases are initially defined negatively, that is as non-acids:
When we discuss aqueous solutions of substances which do not contain hydroxyl, it is simplest to define a base as a substance which adds hydrogen ion. (Lewis 1923, 141)Footnote 5
Lewis introduces the point at which he effects the bifurcation discussed here by stating that in prototropic solutions acids give off hydrogen ion and bases accept it. Up to this point his description remains compatible with Brønsted’s definition, and in particular it is more general than the classic water-based dichotomy of H+ ions and OH− ions.
Right at the beginning of his trail-blazing article of Brønsted (1923), Brønsted had expressed the relationship between acids and bases schematically as: A → B + H+. He thus emphasizes the asymmetry mentioned above although in the text he expressly mentions (reading the equation from right to left) that the placeholder “B” does not necessarily mean “OH−”. Brønsted states that the acid definition expressed by this “has probably never been seriously disputed”.
That same year, without any direct reference to Brønsted, Lewis stands at the parting of the ways and does precisely what the Dane felt was undisputed:
Another definition of acid and base in any given solvent would be the following: An acid is a substance which gives off the cation or combines with the anion of the solvent; a base is a substance which gives off the anion or combines with the cation of the solvent”. (Lewis 1923, 142)
This version of a definition underscores the conceptional rupture that the emergence of electronistic thinking introduces into the characterization of acidity.Footnote 6 In any case the introduction of the concepts of cation and anion is associated with an expansion of the range of reference in which the “classic” acid properties no longer play any role. Lewis himself notes that in this context potassium amide (KNH2) in liquid ammonia (NH3) and potassium chloride (KCl) in liquid hydrogen chloride (HCl) would be bases. Yet associating the adjective “acidic” with all solvated cations and the adjective “alkaline” with all solvated anions would hardly be comprehensible: One could easily leave it at the ionotropic descriptions without sacrificing meaning. Lewis nonetheless pursues this ionotropic train of thought in Valence. He states: “We are inclined to think of substances as possessing acid or basic properties, without having a particular solvent in mind”.
Upon closer examination this proves to be a questionable proposition and is certainly the point of contention in the debate about the bifurcation under discussion here. What defines such an inclination to generalize? Is it really helpful to withdraw in such an extreme way from conventional acid–base concepts that undoubtedly stem from sensory experience and have their most common applications in the substantial environment of humans? Would it not suffice to describe the relationships with the concept pair “electrophilic” and “nucleophilic”?Footnote 7 Acids in this sense would be chemical entities (substances) that act as specific electrophiles and bases as specific nucleophiles. However, it would not be absolutely necessary to describe all electrophiles as acids and all nucleophiles as bases (see below). In his 1923 proposition Lewis famously chooses a borderline case of this description:Footnote 8
It seems to me that with complete generality we may say that a basic substance is one which has a lone pair of electrons which may be used to complete the stable group of another atom, and that an acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. In other words, the basic substance furnishes a pair of electrons for a chemical bond, the acid substance accepts such a pair”. (Lewis 1923, 142, original emphases).
In this way borderline electrophilic cases (compounds lacking electrons) are indeed declared acids and borderline nucleophilic cases of (compounds with superfluous electrons) are declared bases. This becomes particularly clear in an essay that Gilbert Lewis wrote 15 years after the initial proposition. In the interim he had essentially made no further mention of acidity. In reality he was not even interested in the clarification of the phenomenon of acidity but in the theory of chemical bonds, specifically in defending and propagating his electron pair theory. In a 1938 essay he lists the particularly important dispositions of “his” acids and bases (Lewis 1938, 302):
-
I.
When an acid and a base can combine, the process of combination, or neutralization, is a rapid one.
-
II.
An acid or base will replace a weaker acid or base from its compounds.
-
III.
Acids and bases may be titrated against one another by the use of substances, usually colored, known as indicators.
-
IV.
Both acids and bases play an extremely important part in promoting chemical processes through their action as catalysts.
In this way, which looks similar to the customary view of acidity in the first place, Lewis reverses the relationship between explanatory model and explanandum and abruptly declares electrophilic and nucleophilic chemical entities to be acids and bases (see my critical discussion below).
Let us accordingly assume that the electronistic approach has a fundamentally different characteristic than the protonistic approach. On what grounds does Lewis regard his approach as an improvement of the conventional concept (which in 1938 he explicitly links to the name Brønsted)? First and foremost he is interested in generalization: His criticism centers on what he regards as the inappropriately limited scope of the “prototropic” acids and bases in aqueous solutions. Whereas he mentions Brønsted’s description of bases other than OH− favorably, he then goes on to state:
On the other hand, any similar valuable and instructive extension of the idea of acids has been prevented by what I am tempted to call the modern cult of the proton. To restrict the group of acids to those substances which contain hydrogen interferes as seriously with the systematic understanding of chemistry as would the restriction of the term oxidizing agent to substances containing oxygen. (Lewis 1938, 297)
But why is an expansion of the acidity concept in the context of a “systematic understanding of chemistry” desirable at all? Let us briefly compare this with another example already mentioned, oxidation. This phenomenon still bears the same name today that stems from a number of compounds of the element oxygen, the oxides (such as calcium oxide, carbon monoxide, etc.). The expansion of the original concept of oxidation (as a reaction with oxygen) occurred during the course of theorization: The release of electrons (or the increase in the oxidation number) is deemed to be oxidation. Current doctrine holds that there are a large number of oxidants and that oxygen, which gave its name to the process, is now only one of them. The essential difference to acidity is this: Empirically identified examples of such substances have been around for many centuries, and accumulated experience pertains primarily to aqueous matrices. As for oxidation, this is (merely) a theoretical reclassification. Lewis’ electronistic approach to explaining the phenomenon is undoubtedly noteworthy, yet his suggestion to subordinate centuries of accumulated experience together with its primarily sensory characterization to a supposedly better “systematic understanding” seems to me to go too far, apart from the impossibility of any operational access to the chemical kinds of Lewis acidity. It is a trivial fact that the concentration of many chemical substances can be determined in all possible media. Yet only stipulating that one or other of these substances be called “acid” is hardly a genuine examination of acid: Lewis acids and bases as such cannot be analyzed.Footnote 9 It is quite different with the protonistic image: Measuring pH may not detect all significant characteristics of a (classic) acid, but it is specific for detecting hydrogen ions in aqueous media.
Apart from that, compositionistic examination methods are not absolutely necessary in order to fully characterize substances such as hydrogen chloride, nitric acid, and sulfuric acid. Chemical analytics not only studies the composition of a substance sample,Footnote 10 but also its chemical behavior (affinity, reactivity). In his operational approach to defining affinity (or better, acid strength) of certain chemical entities, Wilhelm Ostwald, for example, had no need of a microstructural theory in his doctoral thesis (the results of which were, by the way, an important part of the basis for his Nobel prize in 1909).
In an ingenious glass apparatus he designed himself (Fig. 2), he combined defined quantities of potassium hydroxide solution or sodium hydroxide solution (storage vessel, top) with the acid solutions and read the increase in volume off the graduated scales of a slightly modified pipette. The affinity scale obtained in this manner largely corresponds to the current pKa values (except in the case of HNO3). Ostwald never provided an explanation for this behavior he used in measuring. As described above, it would be difficult to imagine a similar scale for Lewis acids. Given the far more complex relational structure (Brønsted’s concept involves only one relevant acid particle, the “proton”) scaling in the context of Lewis' concept is also fundamentally more difficult.
The apparatus Ostwald used for his affinity measurements (Ostwald 1878, 4, 37)
It is instructive to examine the bifurcation discussed here against the backdrop of Thomas Kuhn’s ideas. At first glance the invention of the electronistic acid–base theory would not appear to fit into a normal-science phase of the chemical sciences because in a certain sense it breaks out of the prevailing paradigm. Normal scientific research in Kuhn’s sense would perhaps have searched for further examples within the protonistic framework. If one reflects on how observers can know what kind of acid or base the respective entity in question belongs to, then the protonists will be able to identify “their” acids and bases relatively easily as their concept allows operational access to “reality”, that is to their “natural kinds”.Footnote 11 Whereas the electronists can follow Lewis’ list of dispositions, they essentially have to know in advance what for them is to be an acid and what is to be a base. Here at least the following aspects need to be considered: Rapid reactions (item I) need not be neutralizations (unless we are willing to transform the meaning of this term as well). Strength or weakness in displacement reactions (item II) are not describable by an exchange of electrons alone. Titrations (item III) are possible when a defined reaction sequence and an indicator are present, whereby neither protonistic nor electronistic acids and bases need be involved. Catalytic effects (item IV) are also not limited to acids or bases regardless of which concept one uses to interpret them. With all the items Lewis mentions one can find examples that do not fit into the system, examples which are absolutely atypical of an electronistic classification as acid or base. Consequently, Lewis’ list of dispositions is useless in an operational setting.Footnote 12
As we know today—due in no small measure to the great work of Gilbert Lewis—it is scarcely possible to explain relationships within a theory of chemical bonds without involving electrons. Lewis goes very far with his theory. As was suggested, one can even take the approach so far that practically all chemical reactions appear to be electronistic. Yet doing so would ignore the operational or empirical aspect of every examination in chemistry and would oversimplify our theoretical grasp of the phenomenon. Direct experimentation with electron pairs is obviously not possible. Rather they are theoretical concepts that have been derived from certain empirical findings. The following comment by Thomas Kuhn addresses the general character of the incommensurability inherent in this situation as well:
[…] the parties to such debates inevitably see differently certain of the experimental or observational situations to which both have recourse. Since the vocabularies in which they discuss such situations consist, however, predominantly of the same terms, they must be attaching some of those terms to nature differently, and their communication is inevitably only partial. As a result, the superiority of one theory to another is something that cannot be proved in the debate (Kuhn 1996, 198).
The disrupted communication between the protonists and the electronists essentially stems from differences in the meaning assigned to the same words. A classic example for this situation from physics is the different meanings assigned to the word “mass”: In the classic sense mass is an invariant quantity, whereas in the modern relativistic sense it is not. Compared with classic oxidation as a reaction with oxygen and the modern notion of the release of electrons (increasing the oxidation number) this shift in meaning is undoubtedly a more fundamental one. The introduction of the oxidation number and the modern understanding of oxidations and reductions does not require any fundamental change in world view (ontology) but can be understood as a more precise rendering of the description of chemical processes. The electronists understand the introduction of Lewis’ doctrine in this same sense, yet they have failed to argue convincingly why the same words, “acids” and “bases”, should be used. It is true that hydrogen ions, the relevant particles for acids, can be described as electrophiles, but not all electrophiles are acids. Aside from this, from a practical empirical perspective it is no less important that the theory fails to provide operational indications for a clear classification of the entities observed.
It is trivial that not every theoretical invention leads to the establishment of a new paradigm. Lewis’ electronistic proposition supplements the arsenal of chemical knowledge while in no way replacing the protonistic acid–base theory. Hasok Chang provides a very useful description of the general historical context in which he introduces the plausible framework concept “epistemic iteration”. He observes a series of decisive steps for the example of acidity, which he ends with Lewis. He summarizes:
The case of acidity is instructive as it shows the whole arc of development: starting with bare sensations and then simple operations and crude theoretical presumptions, we witness a gradual building of precision, coherence, sophistication and range. It also illustrates how a natural kind can become fractured. Up to stage 5 [the protonist concept of 1923] was a coherent process of iterative development, but Lewis made a significant departure […] The Lewis and Brønsted-Lowry concepts each constitute natural kinds, but they point at different things. They are lumped together under the 'acid' rubric only through historical connections. Had atomic structure been elucidated first before scientists learned about acids, relabelling all electron-pair acceptors as ‘acids’ would have seemed a very peculiar proposal (Chang 2016, 42).
Chang then concludes this paragraph with another elucidating explanation:
Lewis’ concept of electron-pair receptivity designates a coherent and interesting property, but calling it ‘acidity’ is a misnomer, just as 'oxidation' is a misnomer to the extent that it doesn’t have anything inherently to do with oxygen.
Even the identity of the “classic” acids is admittedly relational. Sulfuric acid for example is not sufficiently characterized solely by its empirical formula and a few thermodynamic data. An intended classification can only be verified or excluded upon contact with other substances, including the solvent. Wilhelm Ostwald’s “volume-chemical” studies represent a very creative example of the utilization of the relational behavior of chemical substances, even along a quantitative scale.
Not every conventional classification criterion need apply in the positive case as long as the members of the scientific community can logically follow such a classification decision.Footnote 13 In any case it is useful to stick with conventional designations, which admittedly requires one to speak the protonistic language. Acetic acid remains an acid even when it is "displaced" by the stronger hydrochloric acid. Lewis acids and Lewis bases bear their names only in a specific relational context which invariably is discussed a posteriori.
Lewis is not the only researcher demanding an expansion of the acid–base concept in 1923.Footnote 14 Brønsted, too, is interested in a generalization, although his is far less overburdened with theory. His approach expands the base part of the theory by introducing the relationality that we refer to today as the principle of corresponding acids and bases. As an example, Brønsted explains the relationship between ammonium and ammonia in his scheme as NH4+ = NH3 + H+, whereby ammonium is described as an acid and ammonia as its corresponding base. He emphasizes the advantage of this general representation over the water-based variant NH4+ + OHˉ = NH4OH. Seen in this manner, it becomes clear that this approach gives the acid priority or, in other words, the relationship between acids and bases is asymmetrical. Brønsted then goes on to say:
Yet the acidic and alkaline characteristics are fundamentally independent of the nature of the solvent, and the concepts of the acids and bases are indeed of such general character that we must regard it as a necessary requirement to formulate these concepts in a general form independent of the nature of the actual solvent (Brønsted 1923, 719).
One can take issue with the statement at the beginning of this sentence. The exact opposite is namely the case. What would be the characteristic features of acids and bases that one could determine independently of the solvent? Can the hydrogen ion form the acid principle in all possible matrices as Brønsted postulates? The author provides an interesting explanation of this relationship in a lengthy essay published later in German under the title Zur Theorie der Säure-Basen-Funktion. In that discussion of acid strength in neutral solvents such as benzene, he concludes that in this solvent there is no “hydrogen ion concentration”; prototropic acids are known not to dissolve in neutral organic liquids. Yet then he continues:
Nonetheless in such a solvent the hydrogen ion activity or, as we may better express it, the proton activity, possesses the same significance as in an aqueous solution, and it is possible and expedient to generally define the acidity of a solution by it proton activity. The basicity of a solution is then equal to the reciprocal value of the acidity (Brønsted 1928, 2056, emphases original).
This “proton activity” must not be read as a specification of the effective concentration as it was introduced by Gilbert Lewis but as effective potential (through the phase boundary between the unmixable liquids). This effect is demonstrated by many practical applications, especially in synthetic organic chemistry. Here one refers to “protonation” when describing the following intermediate step that (in abbreviated notation) represents the catalytic effect of the acid independent of the solvent: CH3OH + H+ → CH3OH2+. The protonated methanol molecule now receives the preformed good cleavage group H2O so that in the presence of a nucleophilic reaction partner such as bromide (Br−) a substitution now takes place which cannot be realized on the formally simpler path CH3OH + Br− → CH3Br + OH− because the hydroxide ion is a poor cleavage group. Using such examples it can be shown that Brønsted with his protonism further developed notions that were anything but a weakly established “cult”. On the contrary, he presented a theoretical foundation for the central and indeed paradigmatic case of the effect of acids.
In a very early attempt to clear up the different approaches, Norris Hall names A.F.O. Germann, M. Usanovich, and G.N. Lewis as explicit opponents of the “hydrogen theory”. Hall discusses in detail the idea of expanding the classic matrix water to other solvents (the “general solvo-system”), which also seems to appeal to Brønsted as we have seen. However, Brønsted sticks to his fundamental protonistic idea when it comes to acidity. Hall on the whole takes a very balanced position. For example he also discusses arguments against theories of a generalized solvo-system (Hall 1940, 125): (1) Despite the formal advantage, the practical significance of non-aqueous systems is negligible. (2) Giving up the hydrogen theory leads to exaggerated generalization; “there is something intrinsically and exclusively acidic about hydrogen compounds”. (3) Some might say that such a theory is not general enough. “Such critics would feel that the solvo-system is too much an empty formalism which fails to emphasize the essential underlying mechanism of acid–base action”. Hall then states: “Whether for these or for other reasons, the generalized solvo-system has had more lip service than actual use”.
The excessive formalization and exaggerated generalization are also aspects that may be found among the electronists.Footnote 15 Eric Scerri goes as far as to interpret the Venn diagram published by Hall as evidence of his support for the reducibility of Brønsted’s theses to Lewis’ electronism.Footnote 16 Yet this is a rather tenuous proposition. Hall himself states with reference to this diagram: “In summary, a diagram […] may serve to emphasize certain of the relationships discussed above” (Hall, 128). Here he refers to certain relationships, not to the total subsumption of the one theory by the other. Successful reduction of a theory only occurs when the reducing theory takes on all the characteristics of the reduced theory, and that is questionable. Does Lewis explain what an acid is? No, he doesn’t; he takes over this term, classifies the existing examples in a purely theoretical manner, and expands the content of the existing inventory, but in this expansion goes as far as to give relevance only to specific microphysical terms—electron pairs. Acids (and bases) in this system can no longer be operationally classified because empirical access to electron pairs can only be obtained indirectly. Finally, Lewis (like Usanovich and Pearson later) redefines acidity whereby reference to electrons supersedes that of substance characteristics. This is the bifurcation that Hacking refers to in Representing and Intervening, and it is undoubtedly a specific example of the larger paradigm shift that has been part of chemistry for over a hundred years now, that is after the Long 19th Century.Footnote 17
The physicalistic ideal of earlier philosophy of science was the concept that phenomena can be reduced to fundamental mathematical laws. This ideal plays only a subordinate role in chemistry’s central task, which is the study of the nature of substances. The idea, however, that the essence of a substance class such as the acids could be reduced to its composition is undoubtedly legitimate and plausible. It is a variant of the old philosophical notion of transferring our empirical experiences into “actual” ontological reality. The “essentialists”, who strive for ontological certainty and in so doing develop their own “ontography”, often forget that their thoughts also arise from active interaction with empirical objects.Footnote 18 The “descriptivists” on the other hand advocate their own “ontography” which usually comes off as less ambitious but can (and does) at least claim the old positivistic principle that all knowledge begins with empirical experience. Differing at least partially, the two ontographies lead to communication difficulties such as those discussed here. I feel it is not helpful to seek to definitively resolve the question once and for all as to which approach in the acid–base descriptions is the “correct” one. Even posing this question should be regarded critically. There is probably no definitive answer to the question of what a chemical substance is and consequently the question of what an acid is becomes no easier to answer. Therefore I believe it is better to advocate a pluralistic standpoint with respect to this and similar questions.Footnote 19
Conclusion
There are good reasons for retaining the conventional meaning of the expression “acid” and, when it appears desirable and expedient in specific cases, for using the terms “Lewis acid” and “Lewis base”, respectively. From my perspective, Lewis’ theory describes special or borderline cases of acidity, and not vice versa. Hence, I cannot see a “cult of the proton”, and I can also not see a “fork in the road”, that is the necessity to colonialize the descriptivist picture. Hacking was right: We must worry about kinds of acids. However, this does not work without worrying about meaning…
Notes
Even now many substance entities that do not exhibit the full range of characteristic “classic” features of acids are referred to as such. Such substances include the so-called amino acids and nucleic acids. In these cases, one deduces the substance class from microstructural units such as the carboxyl group. See Fig. 1.
Cf. Tantillo and Seeman (2023). I am not referring here to the negative role the authors reserve for philosophers of chemistry…
At the Centenary Workshop, Hasok Chang (Cambridge) pointed at the fact that all known acids show partly extremely different behaviour: Some of them, for example, dissolve metals and produce hydrogen, others do not, or they dissolve metals and yield nitrogen oxide. Hence, there are limits to a unification of a theory of acidity. Chang as well presented his view of a conceptual engineering perspective, see (Chang 2022).
Cf. Ruthenberg and Chang (2017).
Note, by the way, that Lewis, like Ostwald (in German), for example, uses expressions like “hydrogen ion” rather than “hydrogen ions”. Lewis proceeds: “Thus ammonia adds hydrogen ion to form ammonium ion, and the degree to which this occurs will vary as we substitute other radicals for hydrogen. Indeed if we wish, we may consider ammonium ion as an acid and say that its strength as an acid is increased when hydrogen is replaced by Cl or OH or NH2”.
It is noteworthy that Brønsted in his (Brønsted 1928) essay does not mention Lewis’ approach although he is quite open to expanding the theory of acidity to include non-aqueous solvents.
Christopher Kelk Ingold introduces the modern meaning of the terms electrophilic and nucleophilic in his extensive article of Ingold (1934).
At this point it should be noted that Thomas Martin Lowry sympathetically mentions Lewis’ definition from the proofs of Valence, which (along with other aspects) indicates that his role as a co-advocate of the modern protonistic acid–base theory (“Brønsted–Lowry”) is a legend. Cf. (Lowry 1923, 1050–1051). This other centenary story will be investigated in detail elsewhere.
Scerri (2022) errs in claiming that “Lewis acidity can be quantified through the well-known use of a stability constant for any reaction” in his polemic paper against Chang. Equilibrium constants are not specific for Lewis acids or bases, whereas the pH is specific for protonic acidity. At the Centenary Workshop, Camilo Martinez Gonzalez (Buenos Aires) pointed at the differences between measurement and quantification and connected the former to pH and the latter to the determination of equilibrium constants. Cf. Ruthenberg and Martinez Gonzalez (2017).
The study of composition is just one part of the search for the nature of a chemical substance, not its core.
Regarding natural kinds consider the contribution of Pieter Thyssen. At the Centenary Workshop, Eric Scerri (Los Angeles) surprisingly answered in the negative to the question whether acids are natural kinds. According to him, they are not natural kinds because of their relational character. In contrast to elements, which would be determined by their atomic number, acids are strongly context dependent.
It should be mentioned that Jannik Bjerrum (1951) discusses Lewis´ criteria (here item I–IV) critically but tries to harmonize the tension between the two theories by suggesting the notion of “antibase”.
Another example from the substance realm is the expression “metal”: A certain series of characteristics (solid state, electrical conductivity, thermal conductivity, malleability, luster) applies to typical cases. Yet one also refers liquid metals, and not every metal is shiny. At the Centenary Workshop, Nikos Psarros (Leipzig) pointed out that it would be more appropriate to speak of “genera” than of “kinds”.
Although he belonged to the “protonist fraction”, William Mansfield Clark wrote in his monumental work The Determination of Hydrogen Ions a few years after the bifurcation: “When a more comprehensive formulation capable of extension to all sorts of non-aqueous solutions is desired, those presented by Brønsted (1923) and by Lewis in Valence will be found useful” (Clark 1928, 49).
See Scerri (2022).
I mean the shift from descriptive “substantialism” to essentialist “electronism”. A brief look at the historical development of chemistry reveals that atomism and microstructuralism eventually took over after the First World War. Note that chemistry was practically active and widely successful long before (see Ostwald).
Quantum mechanics, for example, is often presented as an ahistorical truth that is to be accepted without question.
Cf. Ruthenberg & Mets (2020).
References
Bjerrum, J.: Über die Notwendigkeit eines besonderen Antibasenbegriffes. Angew. Chem. 63, 527–530 (1951)
Brønsted, J.N.: Einige Bemerkungen über den Begriff der Säuren und Basen. Recueil Des Travaux Chimique Des Pays-Bas 42, 718–728 (1923)
Brønsted, J.N.: Zur Theorie der Säure-Basen-Funktion. Ber. Dtsch. Chem. Ges. 61, 2049–2063 (1928)
Chang, H.: Acidity: the persistence of the everyday in the scientific. Philos Sci 79, 690–700 (2012)
Chang, H.: The rising of chemical natural kinds through epistemic iteration. In: Kendig, C. (ed.) Natural Kinds and Classification in Scientific Practice, pp. 33–46. Routledge, London (2016)
Chang, H.: Realism for Realistic People. Cambridge University Press, Cambridge (2022)
Clark, W.M.: The Determination of Hydrogen Ions, 3rd edn. Williams & Wilkins Co, Baltimore (1928)
Hacking, I.: Representing and Intervening. Cambridge University Press, Cambridge (1983)
Hall, N.F.: Systems of acids and bases. J. Chem. Educ. 17, 124–128 (1940)
Ingold, C.K.: Principles of an electronic theory of organic reactions. Chem. Rev. 15, 225–274 (1934)
Kuhn, T.: The Structure of Scientific Revolutions, 3rd edn. Chicago University Press, Chicago (1996)
Lewis, G.N.: Valence and the Structure of Atoms and Molecules. The Chemical Catalog Company, New York (1923)
Lewis, G.N.: Acids and bases. J. Franklin Inst. 226, 293–313 (1938)
Lowry, T.M.: Co-ordination and acidity. Chem. Ind. 42, 1048–1052 (1923)
Ostwald, W.: Volumchemische und Optisch-Chemische Studien. Druck von H. Laakmann, Dorpat (1878).
Putnam, H.: Mind, language and reality. Philosophical Papers, vol. 2. Cambridge University Press, Cambridge (1975).
Ruthenberg, K., Chang, H.: Acidity: Modes of characterization and quantification. Stud. Hist. Philos. Sci. 65–66, 121–131 (2017)
Ruthenberg, K., Martínez Gonzalez, J.C.: Electronegativity and its multiple faces: persistence and measurement. Found. Chem. 19, 61–75 (2017)
Ruthenberg, K., Mets, A.: Chemistry is pluralistic. Found. Chem. 22, 403–419 (2020)
Scerri, E.: Hasok Chang on the nature of acids. Found. Chem. 24, 389–404 (2022)
Tantillo, D.J., Seeman, J.I.: On a unified theory of acids and bases: Hasok Chang, Eric R. Scerri, modern theoretical chemistry, and the philosophy of chemistry. Found Chem 25, 1–22 (2023)
Acknowledgements
I thank John Grossman for his help with the translation of large parts of the text. I greatly appreciate recent discussions with and comments of Pieter Thyssen, Gerd Flechsig and Paul Needham. Extra special thanks go to Hasok Chang and Eric Scerri.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruthenberg, K. Bifurcations. Found Chem (2023). https://doi.org/10.1007/s10698-023-09484-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10698-023-09484-9