Abstract
The valencies of the lanthanides vary more than was once thought. In addition to valencies associated with a half-full shell, there are valencies associated with a quarter- and three-quarter-full shell. This can be explained on the basis of Slater’s theory of many-electron atoms. The same theory explains the variation in complexing constants in the trivalent state (the “tetrad effect”). Valency in metallic and organometallic compounds is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fifty years ago, the lanthanidesFootnote 1 were little more than a chemical curiosity. They were thought of as being very similar, and placed together in the same box of the Periodic Table. Since then, their chemistry has been studied in more detail, and found to vary more than was once thought. Also, some now have important applications, e.g. neodymium in lasers and magnets.
Discussions of lanthanide compounds and ions are often conducted using a classification by oxidation state. However, certain distinctions that we make in this paper are not well served by this concept. For example, some lanthanide monosulphides, LnS, are metallic and others are semiconductors, but in both types of compound, the formal oxidation state of the lanthanide is +2. Here, therefore, we make the valency of the lanthanide element our classifying instrument and extend the classical definition of valency (Nelson 1997) to metals and metallic compounds by adding the number of electrons contributed to the metallic bonding. This gives sodium, for example, the same valency in the metal (Na+e−) as in its compounds (NaF, NaCl, etc.). An example more relevant to this paper is LaI2 in which the lanthanum atom has an oxidation number of +2. The compound, however, is bronze in colour, has a high electrical conductivity, and is diamagnetic [La2+(I−)2 would be paramagnetic]. It is accordingly formulated La3+(I−)2e−, in which the lanthanum has a valency of three. This definition of valency is essentially that adopted by Grimm and Sommerfeld (1926) and endorsed by Sidgwick (1927: 182): “it is numerically equal to the number of electrons of the atom ‘engaged’ (beansprucht) in attaching the other atoms”.
The variation in the valencies of the elements across the lanthanide series is of particular interest. Here we present a simple discussion of this problem based largely upon work which we have published elsewhere (Johnson 1969a, b, c, 1974, 1977, 1980, 1982a, b, 2006; Johnson and Corbett 1970).
Principal valency
The valencies of the lanthanides are shown in Table 1. Valencies that are stable enough to occur in aqueous solution are in bold type. A striking feature of the valencies is that they vary much less than they do for transition elements, and also for the actinides. Indeed, the most stable valency for all the elements is three.
In this valency, the lanthanides live up to their reputation of being very similar. Their most commonly observed reactions are complexing or precipitation processes in which the valency retains its value of three. In reactions of this kind, differences between elements are small. Such differences as there are are mainly associated with a gradual reduction in size across the series (“the lanthanide contraction”). This is illustrated in Fig. 1 for [Ln(H2O)9](C2H5OSO3)3 salts, all of which have the same structure (Gerkin and Reppart 1984; Persson et al. 2008). For some species, the reduction in size leads to a lowering of coordination number across the series. This is the case for Ln3+(aq) ions, in which there is an increasing shift from nine- to eight-coordination beyond dysprosium (D’Angelo et al. 2010).
Ln–O distances in [Ln(H2O)9](C2H5OSO3)3 salts (Gerkin and Reppart 1984) plotted against number of f electrons
For the reactions we have just described, in which the valency of three is retained, there are other factors that can lead to small differences in chemistry. As in the transition series, these are ligand-field splitting, shell expansion (“the nephelauxetic effect”), and spin-orbit coupling. The net effect of these, however, is very much smaller than in the transition series (Johnson 2006). The most important is the second, whose prominence sometimes gives rise to the small irregularities in complexing constants illustrated in Fig. 2 (Peppard et al. 1969; Nugent 1970). There are four humps separated by three minima, first at the f3/f4 pair, secondly at f7 and thirdly at the f10/f11 pair. This is called the “tetrad effect”.
The tetrad effect can be explained by using Slater’s theory of many-electron atoms (Jørgensen 1970; Nugent 1970).Footnote 2 This gives the following equation for the energy of an fn ion,
where E c is the energy of the core, E f the energy of each f electron in the field of the core, and E r is the repulsion energy between the f electrons. This energy is given in general by
where the E k’s are energy integralsFootnote 3 and c k ’s coefficients. From calculations using hydrogenic orbitals, E 3 is small, and E 2 very small, compared with E 0 and E 1. For the ground state, the terms in E 2 disappear and Eq. (2) can then be written:
where E p = E 0 + (9E 1/7) and E q = (9E 1/7); p is the number of pairs of electrons [½n(n − 1)] and q the number of pairs of electrons with parallel spins. The values of the coefficients are shown in Table 2.
The first term in Eq. (3) is approximately equal to the Coulomb energy between the electrons (J), and the second term to the exchange energy between electrons of the same spin (K).Footnote 4 This arises because electrons can keep apart better if their spins are parallel rather than antiparallel. The third term is associated with the ability of electrons to keep apart better if they are travelling round a nucleus in the same direction rather than in opposite directions. This lowers the repulsion between electrons whose m l values have the same sign compared with those whose m l values have the opposite sign.Footnote 5
From Eqs. (1) and (3), the equilibrium constant (K eq) for a complexing reaction is given byFootnote 6
where the first four terms arise from a change in the expansion of the f shell in the reaction, and the fifth term (ΔE′) from a change in the energies of other electrons. L is the Avogadro constant; the other symbols have their usual meanings. The only terms in this equation that should vary irregularly with n are those in q and c 3. Table 2 shows that the values of q have a cusp at f7. In the table, the values are compared with a base line drawn through the points for f0, f7, and f14, i.e. q b = 3n. The differences (δq = q − q b) are then zero at f0, f7 and f14. They rise from f4 to f7, and then fall, creating an upward cusp at f7. If, then, there is increased shell expansion (ΔE q < 0), this will lead to a downward cusp in ln K eq, which accounts for the minimum at f7 in Fig. 2.
Table 2 also shows that δq and c 3 bottom out at f3 and f4 and at f10 and f11. Since the terms in q and c 3 in Eq. (4) have opposite signs, the effect of this bottoming out will depend on the relative values of ΔE q and ΔE 3. Although ΔE 3/ΔE q is less than one, if it is sufficiently large, the variation in c 3 will predominate because of the large negative values at f3, f4, f10, and f11. In this case, when there is shell expansion (ΔE 3 < 0), the values of ln K eq will bottom out in the same way. This accords with Fig. 2, which can be reproduced with ΔE q = −36 cm−1 and ΔE 3 = −7 cm−1, coupled with a linear increase. A more precise analysis takes account of changes in spin-orbit coupling and ligand-field stabilization.
A secondary effect, which is not brought out by Eq. (3), is the higher repulsion exercised between electrons in the same orbital as compared with different orbitals (Blake 1981). We discuss this elsewhere (see footnote 4).
Other valencies
The valencies other than three are two, four, and, for praseodymium, five. Some of these can be obtained in aqueous solution. Others are obtained by reactions like
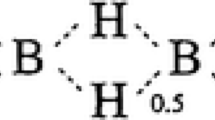
What is striking about these valencies is their distribution. As shown in Table 3, most correspond to ions whose f-electron configuration approaches or constitutes an empty (f0), half-full (f7), or full shell (f14). The nearer the configuration approaches one of these types, the more stable the valency. For example, Nd4+ (f2) can only form Cs3NdF7, whereas Ce4+ (f0) has an extensive chemistry. The remaining valencies (in blue in Table 3) correspond to a configuration approaching a three-quarter-full shell (f10.5), i.e. f10 for valency two and f11 for valency four.
This distribution reveals very marked chemical differences between the lanthanide elements. The similarities associated with processes in which trivalency is retained are no longer apparent: we are now concerned with reactions in which the valency changes. The distribution can be explained by considering the energy factors behind the oxidation and reduction of trivalent compounds (variations in entropy are relatively small). Consider, for example, the process
The enthalpy of this can be analysed in terms of the following cycle (all standard molar enthalpy changes at 298.15 K):

Figure 3 shows the variation in the hydration enthalpy, h 3, with the number of f electrons.Footnote 7 There is a detectable discontinuity between f0 and f1 (Johnson and Nelson 2017a, b) arising from the break between two subshells, 5p (4f0 being 5p6) and 4f. Nevertheless, despite this aberration, and the change in coordination number across the series, the variation is fairly smooth; the h 4 variation is expected to behave likewise. The ionization enthalpy i 4, however, varies irregularly, as shown in Fig. 4.Footnote 8 There is a general increase from f1 to f7, followed by a big drop to f8, and then a general increase again. This explains why oxidation becomes increasingly difficult across the first half of the series, becomes easier at the half-full shell, and then becomes increasingly difficult again.
A similar analysis can be made of the corresponding reduction:

Apart from the sub-shell break between f0 and f1, the variations in ΔH are mainly determined by those in −i 3, plotted in Fig. 5. In shape, this plot is the inverse of Fig. 4. There is a general decrease from f1 to f7, followed by a big rise to f8, and then a general decrease again. This explains why reduction becomes easier towards the half-full shell, then becomes more difficult, then becomes easier again. There is also a definite irregularity around the three-quarter-full shell, making the f10 ion Dy2+ more stable than it would otherwise be. There is a similar irregularity around the quarter-full shell, but this is smaller. The plot suggests that Pm2+ (f5) should be at least as stable as Nd2+ (f4). Analysis of the reduction of solid trivalent compounds, including by the metals, leads to similar conclusions (Johnson 1982b: Sect. 6.8). The heightened stability of Dy2+ explains why, in agreement with Table 1, it has been possible to prepare solid dihalides of dysprosium, but not those of erbium.
The marked irregularity around the three-quarter shell is also apparent in Fig. 4. It suggests that compounds of tetravalent thulium, such as Cs3TmF7, might be unexpectedly stable. Some workers claim to have prepared impure samples of this compound (Spitsin et al. 1982), but others have reported little or no oxidation [unpublished observations by the authors (1972), L. B. Asprey (1975) and R. Hoppe (1985)] and further confirmation is needed. This explains the question marks in Tables 1 and 3.
The reason for the big break in i 3 and i 4 is well known from the transition series. Repulsion energies between electrons are reduced by the exchange energy between electrons with the same spin. The latter builds up until a shell is half-full, then drops to zero as an electron is added with opposite spin. It then builds up again as more electrons are added with this spin.
This explanation is based on Eqs. (1) and (3) above. These give the following equation for the ionization enthalpy of an fn ion
If the energy integrals are approximated by the average values for the two ions involved (Lnz+ and Ln(z+1)+),
The values of Δi p, Δi q, and Δi c 3 are given in Table 4. The second of these determines the loss of exchange energy, and peaks at f7.
As with the tetrad effect, the irregularities in Δi c 3 centred on the quarter- and the three-quarter-full shell are associated with the ability of electrons to keep apart better if their m l values have the same sign rather than opposite. This eases the general increase in interelectronic repulsion in the first quarter of the series, where electrons have m l values of the same sign, relative to the second quarter, where they have both positive and negative values (Table 4); similarly in the third quarter over the fourth quarter.
Note that the pattern of variations exhibited by i 3 and i 4 holds for other processes involving the removal of an electron from an f shell (see below). Note also, with respect to the formation of 5-valent compounds by praseodymium, that this has the lowest fifth ionization energy of the lanthanides (Kramida et al. 2016).
Metallic compounds
In the reduction of trivalent compounds to metallic ones, there is no change in the number of f electrons. The ionization enthalpy −i 3 is accordingly no longer a factor. As a result, reductions are no longer restricted to bivalent elements. Thus, all of the elements form dihydrides (LnH2), monosulfides (LnS), monoselenides (LnSe), monotellurides (LnTe) and monoacetylides (LnC2), and most of them form diiodides (LnI2). What varies, however, is the distribution of metallic and salt-like compounds in these series, as shown in Table 5. Most compounds are metallic, but salt-like forms occur for some or all of the bivalent elements in Table 1.
This distribution can be explained by considering the following cycle (or the corresponding cycle for LnY):

This gives:
The first and third terms do not involve a change in the number of f electrons, and so they, along with the entropy term, are expected to vary fairly smoothly with n. Assuming that this variation is approximately linear, we can write:
The values of A and B for each series can be approximately determined by ensuring that ΔG < 0 for salt-like compounds and ΔG > 0 for metallic ones. The resulting values of ΔG are plotted against (Bn − i 3) in Fig. 6, where we have used a common value of B (13 kJ mol−1) and chosen the zero lines for each series to bring the graphs together. Comparison with Fig. 5 shows that the distribution of salt-like compounds is very largely determined by −i 3. Points lying below their specified zero line indicate salt-like character and an fn configuration, points lying above, metallic character and an fn−1 configuration. Where points lie close to a zero line, this is sometimes associated with states of mixed valency as for TmSe (Lebègue et al. 2005) and YbC2 (Link et al. 2011) or with pressure-induced salt-like → metallic transitions as in the case of SmS (Jayaraman et al. 1970; Johnson 1982a) and NdI2 (Beck and Schuster 1992).
Other systems in figure 6
The scope of Fig. 6 can be enlarged beyond salt-like/metallic distributions by using a classification that focuses on the 4fn configuration rather than on valency (Johnson 1977). Compounds or ions in which the lanthanide has the same 4f configuration as does the dipositive ion in an [Xe]4fn state are described as bi-f; those with the same 4fn configuration as the tripositive ion are called tri-f. Figure 6 then describes the distribution of systems between di-f and tri-f states. This allows us to include the free, gaseous dipositive ions which have either [Xe]4fn or [Xe]4fn−15d ground states, and the metals which may be (M2+2e−) or (M3+3e−). The bonding contribution of the outer 5d electron in the tri-f systems varies between zero in the gaseous ions to substantial in metallic compounds such as CeS where it populates a conduction band. Intermediate situations such as the dipositive ions in aqueous solution or fluoride host lattices also appear in Fig. 6. In the next section we encounter a case in which the d electron in tri-f compounds participates in π-bonding with ligands. This too has been included in Fig. 6.
Organometallic compounds
The lanthanides form a number of organometallic compounds (see, e.g. Cotton and Wilkinson 1988: 968–973). These are generally trivalent, e.g. the cyclopentadienyl compounds Ln(η-C5H5)3. Compounds with other valencies are formed by elements for which these valencies are most stable, e.g. Ce(η-C5Η5)4, Sm(η-C5Η5)2, Eu(η-C5H5)2, and Yb(η-C5H5)2.
Of interest are the ions [Ln(η-C5H4R)3]− [R = Si(CH3)3] (Hitchcock et al. 2008; Fieser et al. 2015; Evans 2016). These exhibit two valencies. Most have the same structure and size as Ln(η-C5H4R)3, but those for samarium, europium, thulium and ytterbium are significantly larger. These are elements forming bivalent compounds (Table 1). Fieser et al. (2015) and Evans (2016) suggest that the lanthanide atoms in the latter ions are bivalent and have the configuration fn (M3+ = fn−1) while those in the former have the configuration fn−1d1 and are trivalent, the d electron being involved in the bonding. This pattern of valencies can be explained in a similar way to the pattern in metallic compounds; we have accordingly included these ions in Fig. 6.
Also of interest are the arene complexes [Ln(η-C6H3Bu t3 )2]. Anderson et al. (1989) account for the relative stabilities and magnetic moments of these by proposing an fn−1d1s2 valence state for the lanthanide atoms. Promotion energies from fns2 into this state vary in a similar way to i 3 and i 4 (above).
Change history
03 April 2018
The authors regret that there are errors in equation (6) and subsequent discussion. The correct version is as follows:
16 September 2017
The authors regret that there are errors in equation (6) and subsequent discussion.
03 April 2018
The authors regret that there are errors in equation (6) and subsequent discussion. The correct version is as follows:
03 April 2018
The authors regret that there are errors in equation (6) and subsequent discussion. The correct version is as follows:
Notes
We take the lanthanides (Ln) to be the elements from lanthanum to lutetium inclusive. We take lutetium to be a transition element and lanthanum to ytterbium “inner” transition elements.
Although Slater’s theory fails for some systems, it holds for electrons outside cores (Johnson and Nelson 2014).
This is one of several sets of integrals in the literature. It was proposed by Racah (1949). The superscripts are labels.
Details and a more exact treatment will be given in Johnson and Nelson (paper in preparation).
For an approximate treatment of this term, see Johnson (1982b).
Taking ΔH θ ≈ ΔE.
Values calculated from the standard enthalpies of formation of Ln3+(g) and of Ln3+(aq) relative to H+(aq) (Cordfunke and Konings 2001; Konings and Benes 2010; Konings et al. 2014; Kramida et al. 2016; Johnson and Nelson 2017a, b), and from the standard intrinsic enthalpy of formation of H+(aq). For the latter, we took the value recommended by Hünenberger and Reif (2011: 460), made consistent with the standard states used by NBS/NIST (Wagman et al. 1982) and JANAF (Chase 1998). The uncertainty in this value is considerable (at least ± 30 kJ mol−1), but this does not affect the precision of the relative values of h 3, most of which have uncertainties less than ±15 kJ mol−1. We have used absolute values to simplify the equations.
References
Anderson, D.M., et al.: On the stability and bonding in bis(η-arene)lanthanide complexes. J. Chem. Soc., Chem. Commun. 53 (1989)
Beck, H.P., Schuster, M.: High-pressure transformations of NdI2. J. Solid State Chem. 100, 301 (1992)
Blake, A.B.: Exchange stabilization and the variation in ionization energy in the pn and dn series. J. Chem. Educ. 58, 393 (1981)
Chase, M.W.: JANAF Thermochemical Tables, 4th edn. American Institute of Physics, New York (1998)
Cordfunke, E.H.P., Konings, R.J.M.: The enthalpies of formation of lanthanide compounds. II. Ln3+(aq). Thermochim. Acta 375, 51 (2001)
Cotton, F.A., Wilkinson, G.: Advanced Inorganic Chemistry, 5th edn. Wiley, New York (1988)
D’Angelo, P., et al.: Analysis of the detailed configuration of hydrated lanthanoid(III) ions in aqueous solution and crystalline salts by using K- and L3-edge XANES spectroscopy. Chem. Eur. J. 16, 684 (2010)
Evans, W.J.: Tutorial on the role of cyclopentadienyl ligands in the discovery of molecular complexes of the rare-earth and actinide metals in new oxidation states. Organometallics 35, 3088 (2016)
Fieser, M.E., MacDonald, M.R., Krull, B.T., Bates, J.E., Ziller, J.W., Furche, F., Evans, W.J.: Structural, spectroscopic, and theoretical comparison of traditional vs recently discovered Ln2+ ions in the [K(2.2.2-cryptand)][(C5H4SiMe3)3Ln] complexes: the variable nature of Dy2+ and Nd2+. J. Am. Chem. Soc. 137, 369 (2015)
Gerkin, R.E., Reppart, W.J.: The structures of the lanthanide ethyl sulfate enneahydrates, M(C2H5SO4)3·9H2O [M = La-Lu (except Pm)], at 171 K. Acta Crystallogr. Sect. C. 40, 781 (1984)
Grimm, H.G., Sommerfeld, A.: Über den. zusammenhang des abschlusses der elektronengruppen im atom mit den chemischen valenzzahlen. Z. Physik 36, 36 (1926)
Hitchcock, P.B., Lappert, M.F., Maron, L., Protchenko, A.V.: Lanthanum does form stable molecular compounds in the +2 oxidation state. Angew. Chem. Int. Ed. 47, 1488 (2008)
Hünenberger, P., Reif, M.: Single Ion Solvation. Royal Society of Chemistry, London (2011)
Jayaraman, A., Narayanamurti, V., Bucher, E., Maines, R.G.: Continuous and discontinuous semiconductor-metal transition in samarium monochalcogenides under pressure. Phys. Rev. Lett. 25, 1430 (1970)
Johnson, D.A.: Third ionization potentials and sublimation energies of the lanthanides. J. Chem. Soc. A 1525 (1969a)
Johnson, D.A.: Variations in the relative stabilities of the di-, tri-, and tetra-positive oxidation states of the lanthanides and actinides. J. Chem. Soc. A 1529 (1969b)
Johnson, D.A.: Stabilities of the lanthanide dichlorides. J. Chem. Soc. A 2578 (1969c)
Johnson, D.A.: Relative stabilities of dipositive and tripositive lanthanoid ions in aqueous solution. J. Chem. Soc. Dalton Trans. 1671 (1974)
Johnson, D.A.: Recent advances in the chemistry of the less-common oxidation states of the lanthanide elements. Adv. Inorg. Chem. Radiochem. 20, 1 (1977)
Johnson, D.A.: Principles of lanthanide chemistry. J. Chem. Educ. 57, 475 (1980)
Johnson, D.A.: Thermodynamics of the semiconductor–metal transition in the lanthanide monosulphides. J. Chem. Soc. Dalton Trans. 2269 (1982a)
Johnson, D.A.: Some Thermodynamic Aspects of Inorganic Chemistry, 2nd edn. Cambridge University Press, Cambridge (1982b)
Johnson, D.A.: Inter-electron repulsion and irregularities in the chemistry of transition series. Inorg. Chem. Focus 3, 1 (2006)
Johnson, D.A., Corbett, J.D.: The relative stabilities of the rare earth metal diiodides: the metal-metal triiodide systems for terbium, dysprosium and holmium. Colloque. Inte. CNRS 180, 429 (1970)
Johnson, D.A., Nelson, P.G.: The status of Slater’s theory of many-electron atoms. Comments Inorg. Chem. 34, 178 (2014)
Johnson, D.A., Nelson, P.G.: Lanthanide ionization energies and the sub-shell break. Part 1. The second ionization energies. J. Phys. Chem. Ref. Data 46, 013108 (2017a)
Johnson, D.A., Nelson, P.G.: Lanthanide ionization energies and the sub-shell break. Part 2. The third and fourth ionization energies. J. Phys. Chem. Ref. Data 46, 013109 (2017b)
Jørgensen, C.K.: The “tetrad effect” of Peppard is a variation of the nephelauxetic ratio in the third decimal. J. Inorg. Nucl. Chem. 32, 3127 (1970)
Konings, R.J.M., Benes, O.: The thermodynamic properties of the f-elements and their compounds. 1. The lanthanide and actinide metals. J. Phys. Chem. Ref. Data 39, 043102 (2010)
Konings, R.J.M., et al.: The thermodynamic properties of the f-elements and their compounds. Part 2. The lanthanide and actinide oxides. J. Phys. Chem. Ref. Data 43, 013101 (2014)
Kramida, A., Ralchenko, Yu., Reader, J., and NIST ASD Team: NIST Atomic Spectra Database (version 5.4) [http://physics.nist.gov/asd]. National Institute of Standards and Technology, Gaithersburg, MD (2016)
Lebègue, S., et al.: Electronic structure and spectroscopic properties of thulium monochalcogenides. Phys. Rev. B 72, 245102 (2005)
Link, P., et al.: Yb valence states in YbC2: a HERFD-XANES spectroscopic investigation. Inorg. Chem. 50, 5587 (2011)
Meyer, G., Wickleder, M.S.: Handbook on the Physics and Chemistry of Rare Earths 28, 53 (2000)
Nelson, P.G.: Valency. J. Chem. Educ. 74, 465 (1997)
Nugent, L.J.: Theory of the tetrad effect in the lanthanide(III) and actinide(III) series. J. Inorg. Nucl. Chem. 32, 3485 (1970)
Peppard, D.F., Mason, G.W., Lewey, S.: A tetrad effect in the liquid-liquid extraction ordering of lanthanides(III). J. Inorg. Nucl. Chem. 31, 2271 (1969)
Persson, I., D’Angelo, P., De Panfilis, S., Sandström, M.: Hydration of lanthanoid(III) ions in aqueous solution and hydrates studied by EXAFS spectroscopy and crystallography: the myth of the “gadolinium break”. Chem. Eur. J. 14, 3056 (2008)
Racah, G.: Theory of complex spectra. IV. Phys. Rev. 76, 1352 (1949)
Sidgwick, N.V.: The Electronic Theory of Valency. Oxford University Press, Oxford (1927)
Spitsin, V.I., Martynenko, L.I., Kiselew, J.U.M.: Anwendung der edelgasfluoride zur darstellung von verbindungen vierwertiger lanthanoide. Z. Anorg. Allgem. Chem. 495, 39 (1982)
Wagman, D.D., et al.: The NBS Tables of Chemical Thermodynamic Properties. American Institute of Physics, New York (1982)
Zhang, Q., Hu, S.-X., Qu, H., Su, J., Wang, G., Lu, J.-B., Chen, M., Zhou, M., Li, J.: Pentavalent lanthanide compounds: formation and characterization of praseodymium(V) oxides. Angew. Chem. Int. Ed. 55, 6896 (2016)
Acknowledgements
We are grateful to a reviewer for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Johnson, D.A., Nelson, P.G. Valencies of the lanthanides. Found Chem 20, 15–27 (2018). https://doi.org/10.1007/s10698-017-9291-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10698-017-9291-6