Abstract
Interferon regulatory factors (IRFs) are transcription factors of the interferon (IFN)-inducible signaling pathway essential for host immunity against antimicrobial infection by virus and bacteria. Interferon regulatory factor 3 (IRF3) regulates the expression of IFNs and IFN-stimulated genes by binding to the IFN stimulatory response element (ISRE). In this study, we analyze the thymus transcriptome of the mandarin fish Siniperca chuatsi and report the functional analysis of Irf3 from the thymus as an emerging model of antiviral approaches. The predicted S. chuatsi IRF3 (Sc-Irf3) protein has 465 amino acid residues and evolutionarily conserved domains and is clustered in the IRF3 subfamily on a phylogenetic tree. Sc-Irf3 upon transgenic expression was mainly found in the cytoplasm through Western blot analysis and microscopy, but it translocated to the nucleus after polyinosinic:polycytidylic acid (ploly I:C) treatment. Endogenous Sc-irf3 RNA expression was detected in all eight adult organs examined. Importantly, Sc-irf3 RNA expression was significantly upregulated by ploly(I:C) treatment in the adult organs. Concurrently, reporter assays revealed that Sc-Irf3 increased the transcriptional activity of the ifnβ promoter, a minimal ISRE-containing promoter, and ifn promoter of mandarin fish. Therefore, Sc-Irf3 plays a major role in the IFN immune defense system against virus infection.





Similar content being viewed by others
References
Aggad D, Stein C, Sieger D, Mazel M, Boudinot P, Herbomel P, Levraud J-P, Lutfalla G, Leptin M (2010) In vivo analysis of Ifn-γ1 and Ifn-γ2 signaling in zebrafish. J Immunol 185(11):6774–6782
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet 25(1):25–29. https://doi.org/10.1038/75556
Barnes B, Lubyova B, Pitha PM (2002) Review: on the role of IRF in host defense. J Interf Cytokine Res 22(1):59–71
Bergstroem B, Johnsen IB, Nguyen TT, Hagen L, Slupphaug G, Thommesen L, Anthonsen MW (2010) Identification of a novel in vivo virus-targeted phosphorylation site in interferon regulatory factor-3 (IRF3). J Biol Chem 285(32):24904–24914
Bowden TJ, Cook P, Rombout JH (2005) Development and function of the thymus in teleosts. Fish Shellfish Immunol 19(5):413–427. https://doi.org/10.1016/j.fsi.2005.02.003
Briolat V, Jouneau L, Carvalho R, Palha N, Langevin C, Herbomel P, Schwartz O, Spaink HP, Levraud J-P, Boudinot P (2014) Contrasted innate responses to two viruses in zebrafish: insights into the ancestral repertoire of vertebrate IFN-stimulated genes. J Immunol 192(9):4328–4341
Chen XW, Jiang S, Gu YF, Shi ZY (2014) Molecular characterization and expression of cyp19a gene in Carassius auratus. J Fish Biol 85(2):516–522. https://doi.org/10.1111/jfb.12418
Fänge R (1986) Lymphoid organs in sturgeons (Acipenseridae). Vet Immunol Immunopathol 12(1–4):153–161
Feng H, Liu H, Kong R, Wang L, Wang Y, Hu W, Guo Q (2011) Expression profiles of carp IRF-3/-7 correlate with the up-regulation of RIG-I/MAVS/TRAF3/TBK1, four pivotal molecules in RIG-I signaling pathway. Fish & shellfish immunology 30(4):1159–1169
Feng H, Zhang YB, Zhang QM, Li Z, Zhang QY, Gui JF (2015) Zebrafish IRF1 regulates IFN antiviral response through binding to IFNvarphi1 and IFNvarphi3 promoters downstream of MyD88 signaling. J Immunol 194(3):1225–1238. https://doi.org/10.4049/jimmunol.1402415
Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao S-M, Maniatis T (2003a) IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4(5):491–496
Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT (2003b) LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J Exp Med 198(7):1043–1055
Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN (2004) The viral mimic, polyinosinic: polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Phys Regul Integr Comp Phys 287(4):R759–R766
Furukawa S, Wingenfeld L, Morita S, Takaya A, Nakagawa T, Sakaguchi I, Matsuda W, Nishi K (2012) Histochemical and morphological characteristics of the Hassall’s corpuscles. The stress affects involution of the ectopic intra-thyroidal and the normal position thymus and morphological changes of the Hassall’s corpuscles. J Forensic Res 3:9. https://doi.org/10.4172/2157-7145.1000165
Gu YF, Fang Y, Jin Y, Dong WR, Xiang LX, Shao JZ (2011) Discovery of the DIGIRR gene from teleost fish: a novel Toll-IL-1 receptor family member serving as a negative regulator of IL-1 signaling. J Immunol 187(5):2514–2530. https://doi.org/10.4049/jimmunol.1003457
Gu YF, Wei Q, Tang SJ, Chen XW, Zhao JL (2016) Molecular characterization and functional analysis of IRF3 in tilapia (Oreochromis niloticus). Dev Comp Immunol 55:130–137
He JG, Wang SP, Zeng K, Huang ZJ, Chan SM (2000) Systemic disease caused by an iridovirus-like agent in cultured mandarinfish, Siniperca chuatsi (Basilewsky), in China. J Fish Dis 23(3):219–222
He JG, Deng M, Weng SP, Li Z, Zhou SY, Long QX, Wang XZ, Chan SM (2001) Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology 291(1):126–139. https://doi.org/10.1006/viro.2001.1208
Holland JW, Bird S, Williamson B, Woudstra C, Mustafa A, Wang T, Zou J, Blaney SC, Collet B, Secombes CJ (2008) Molecular characterization of IRF3 and IRF7 in rainbow trout, Oncorhynchus mykiss: functional analysis and transcriptional modulation. Mol Immunol 46(2):269–285. https://doi.org/10.1016/j.molimm.2008.08.265
Honda K, Taniguchi T (2006) IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6(9):644–658
Hu GB, Xia J, Lou HM, Chen XL, Li J, Liu QM (2011) An IRF-3 homolog that is up-regulated by DNA virus and poly I: C in turbot, Scophthalmus maximus. Fish & Shellfish Immunology 31(6):1224–1231
Huang B, Qi ZT, Xu Z, Nie P (2010) Global characterization of interferon regulatory factor (IRF) genes in vertebrates: glimpse of the diversification in evolution. BMC Immunol 11(1):22
Huang HY, Houwing S, Kaaij LJ, Meppelink A, Redl S, Gauci S, Vos H, Draper BW, Moens CB, Burgering BM (2011) Tdrd1 acts as a molecular scaffold for Piwi proteins and piRNA targets in zebrafish. EMBO J 30(16):3298–3308
Huang Y, Huang X, Cai J, OuYang Z, Wei S, Wei J, Qin Q (2015) Identification of orange-spotted grouper (Epinephelus coioides) interferon regulatory factor 3 involved in antiviral immune response against fish RNA virus. Fish & shellfish immunology 42(2):345–352
Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M (2010) KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res 38(Database issue):D355–D360. https://doi.org/10.1093/nar/gkp896
Kawai T, Akira S (2006) Innate immune recognition of viral infection. Nat Immunol 7(2):131–137
Langenau DM, Zon LI (2005) The zebrafish: a new model of T-cell and thymic development. Nat Rev Immunol 5(4):307–317. https://doi.org/10.1038/nri1590
Lazear HM, Lancaster A, Wilkins C, Suthar MS, Huang A, Vick SC, Clepper L, Thackray L, Brassil MM, Virgin HW, Nikolich-Zugich J, Moses AV, Gale M Jr, Fruh K, Diamond MS (2013) IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog 9(1):e1003118. https://doi.org/10.1371/journal.ppat.1003118
Levraud J-P, Boudinot P, Colin I, Benmansour A, Peyrieras N, Herbomel P, Lutfalla G (2007) Identification of the zebrafish IFN receptor: implications for the origin of the vertebrate IFN system. J Immunol 178(7):4385–4394
Li S, Lu LF, Feng H, Wu N, Chen DD, Zhang YB, Gui JF, Nie P, Zhang YA (2014) IFN regulatory factor 10 is a negative regulator of the IFN responses in fish. J Immunol 193(3):1100–1109
López-Muñoz A, Roca FJ, Meseguer J, Mulero V (2009) New insights into the evolution of IFNs: zebrafish group II IFNs induce a rapid and transient expression of IFN-dependent genes and display powerful antiviral activities. J Immunol 182(6):3440–3449
Lustig A, Weeraratna AT, Wood WW 3rd, Teichberg D, Bertak D, Carter A, Poosala S, Firman J, Becker KG, Zonderman AB, Longo DL, Taub DD (2007) Transcriptome analysis of age-, gender- and diet-associated changes in murine thymus. Cell Immunol 245(1):42–61. https://doi.org/10.1016/j.cellimm.2007.03.008
Meylan E, Tschopp J, Karin M (2006) Intracellular pattern recognition receptors in the host response. Nature 442(7098):39–44
Mohammad MG, Chilmonczyk S, Birch D, Aladaileh S, Raftos D, Joss J (2007) Anatomy and cytology of the thymus in juvenile Australian lungfish, Neoceratodus forsteri. J Anat 211(6):784–797. https://doi.org/10.1111/j.1469-7580.2007.00814.x
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628. https://doi.org/10.1038/nmeth.1226
Paun A, Pitha P (2007) The IRF family, revisited. Biochimie 89(6):744–753
Petrie-Hanson L, Peterman A (2005) American paddlefish leukocytes demonstrate mammalian-like cytochemical staining characteristics in lymphoid tissues. J Fish Biol 66(4):1101–1115
Pulsford A, Tomlinson M, Lemaire-Gony S, Glynn P (1994) Development and immunocompetence of juvenile flounder Platichthys flesus, L. Fish & shellfish immunology 4(1):63–78
Rasmussen A-S, Arnason U (1999) Molecular studies suggest that cartilaginous fishes have a terminal position in the piscine tree. Proc Natl Acad Sci 96(5):2177–2182
Severa M, Remoli ME, Giacomini E, Annibali V, Gafa V, Lande R, Tomai M, Salvetti M, Coccia EM (2007) Sensitization to TLR7 agonist in IFN-beta-preactivated dendritic cells. J Immunol 178(10):6208–6216
Sharma S, Grandvaux N, Zhou G-P, Lin R, Hiscott J (2003) Triggering the interferon antiviral response through an IKK-related pathway. Science 300(5622):1148–1151
Shi J, Zhang YB, Zhang JS, Gui JF (2013) Expression regulation of zebrafish interferon regulatory factor 9 by promoter analysis. Dev Comp Immunol 41(4):534–543
Sun BJ, Nie P (2004) Molecular cloning of the viperin gene and its promoter region from the mandarin fish Siniperca chuatsi. Vet Immunol Immunopathol 101(3–4):161–170. https://doi.org/10.1016/j.vetimm.2004.04.013
Sun F, Zhang YB, Liu TK, Gan L, Yu FF, Liu Y, Gui JF (2010) Characterization of fish IRF3 as an IFN-inducible protein reveals evolving regulation of IFN response in vertebrates. J Immunol 185(12):7573–7582. https://doi.org/10.4049/jimmunol.1002401
Sun H, Liu P, Nolan LK, Lamont SJ (2016) Thymus transcriptome reveals novel pathways in response to avian pathogenic Escherichia coli infection. Poult Sci 95(12):2083–2814. https://doi.org/10.3382/ps/pew202
Tamura T, Yanai H, Savitsky D, Taniguchi T (2008) The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 26(1):535–584. https://doi.org/10.1146/annurev.immunol.26.021607.090400
Tanaka Y, Chen ZJ (2012) STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal 5(214):ra20
Tanaka N, Izawa T, Kuwamura M, Higashiguchi N, Kezuka C, Kurata O, Wada S, Yamate J (2014) The first case of infectious spleen and kidney necrosis virus (ISKNV) infection in aquarium-maintained mandarin fish, Siniperca chuatsi (Basilewsky), in Japan. J Fish Dis 37(4):401–405. https://doi.org/10.1111/jfd.12134
Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19(1):623–655
Wang L, Feng Z, Wang X, Wang X, Zhang X (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26(1):136–138. https://doi.org/10.1093/bioinformatics/btp612
Xiang LX, He D, Dong WR, Zhang YW, Shao JZ (2010) Deep sequencing-based transcriptome profiling analysis of bacteria-challenged Lateolabrax japonicus reveals insight into the immune-relevant genes in marine fish. BMC Genomics 11(1):472
Yao CL, Huang XN, Fan Z, Kong P, Wang ZY (2012) Cloning and expression analysis of interferon regulatory factor (IRF) 3 and 7 in large yellow croaker, Larimichthys crocea. Fish & shellfish immunology 32(5):869–878
Zehn D, Lee SY, Bevan MJ (2009) Complete but curtailed T-cell response to very low-affinity antigen. Nature 458(7235):211–214. https://doi.org/10.1038/nature07657
Zhang Q, Li Z (1999) Three different viruses observed from the tissues of diseased mandarin fish Siniperca chuatsi. Chin Sci Bull 44(5):437–441
Zhu LY, Nie L, Zhu G, Xiang LX, Shao JZ (2013) Advances in research of fish immune-relevant genes: a comparative overview of innate and adaptive immunity in teleosts. Dev Comp Immunol 39(1):39–62
Funding
This work was financially supported by the China Agriculture Research System (CARS-46), the Shanghai Collaborate Innovation Center for Aquatic Animal Genetics and Breeding (ZF1206), and the Talent Development Special Fund of Anhui Academy of Agricultural Sciences (16F0505).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved and authorized by the State Council of the People’s Republic of China.
Electronic supplementary material
Figure S1
cDNA sequence of SC-irf3 and the 5′-flanking sequence of SC-ifn. (A) cDNA (black letters) and deduced protein (blue letters) sequence of SC-irf3; (B) Sequence of the 5′-flanking region of Sc-ifn. Boxed ATG indicates the start codon site. The predicted TATA box is boxed. Predicted NF-kappaB binding sites are wave lined, and the interferon-stimulated response element (ISRE) is underlined. (PNG 6647 kb)
Figure S2
Multiple alignment of Sc-Irf3 with its homologues. Residues shaded in black are completely conserved across all species, and residues shaded in gray are similar in terms of side chains. The dashes in the amino acid sequences indicate the gaps introduced to maximize alignment. Putative DNA-binding domain (DBD) in the N-terminal and IRF association domain (IAD) in the C-terminal are underlined. Serine-rich C-terminal domain is boxed. Black triangle represents the tryptophan residue cluster. (PNG 7481 kb)
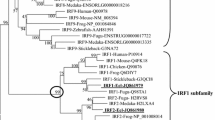
Figure S3
Phylogenetic analysis of the IRF3 family phylogenetic tree was constructed with the neighbor-joining method by Mega 6.0. Node values represent percent bootstrap confidence derived from 100 replicates. (PNG 912 kb)
Table S1
List of primers used in this study. (DOC 37 kb)
Supplementary data 1
All unigenes from the thymus of S. chuatsi. (FA 33487 kb)
Supplementary data 2
Expression analysis and annotation of all unigenes from the thymus of S. chuatsi. (XLSX 3948 kb)
Rights and permissions
About this article
Cite this article
Chen, X., Shen, Y., Wu, M. et al. Irf3 from mandarin fish thymus initiates interferon transcription. Fish Physiol Biochem 45, 133–144 (2019). https://doi.org/10.1007/s10695-018-0543-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0543-8