Abstract
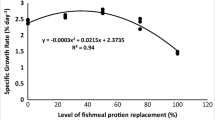
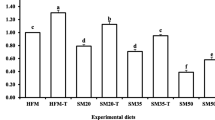
We determined the effects of complete fishmeal (FM) replacement by alternative protein (soy protein concentrate, SPC) with guanosine monophosphate (GMP) supplementation on growth, digestibility, immunity, blood chemistry profile, and stress resistance of juvenile red sea bream, Pagrus major. FM protein of a FM-based control diet (FM0) was replaced with 33.3 (FM33.3), 66.6 (FM66.7), and 100% (FM100) by SPC protein, and each replacement group was supplemented with 0.4% GMP to formulate four experimental diets. Each diet was randomly allocated to triplicate groups of fish (4.8 g) for 56 days. Results demonstrated that fish fed diet group FM33.3 had the significantly highest final weight, weight gain-specific growth rate, and feed intake. Meanwhile, in comparison to control, growth performance and feed utilization did not significantly differ with 66.7% FM replacement by SPC with GMP supplementation. Apparent digestibility coefficient of protein and lipid also followed a similar trend. All growth, feed utilization, and digestibility parameters were significantly lower in FM100 diet group. Blood urea nitrogen (BUN) and triglycerides (TG) increased (P < 0.05) with increasing FM replacement level by SPC. Interestingly, total cholesterol level reduces with the increasing level of FM replacement by SPC with GMP supplementation. Fish fed FM0 diet group showed the best condition of both oxidative and freshwater stress resistance. Meanwhile, FM33.3 and FM66.7 diet groups showed acceptable conditions. Innate immune responses enhanced with the increasing FM replacement level by SPC with GMP supplementation. In conclusion, FM could be replaced ≤66.7% by SPC with GMP supplementation in diets for red sea bream without any adverse effects on fish performances.


Similar content being viewed by others
References
Afuang W, Siddhuraju P, Becker K (2003) Comparative nutritional evaluation of raw, methanol extracted residues and methanol extracts of moringa (Moringa oleifera Lam.) leaves on growth performance and feed utilization in Nile tilapia (Oreochromis niloticus L.) Aquac Res 34:1147–1159
Anderson DP, Siwicki AK (1995) Basic hematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian aquaculture II. Philippines, Fish Health Section, Asian Fisheries Society, Manila, pp 185–202
AOAC (1990) Official methods of analysis of the Association of Official Analytical Chemists, 15th edn. Association of Official Analytical Chemists, Arlington
Aragão C, Conceicção LEC, Dias J, Marques AC, Gomes E, Dinis MT (2003) Soy protein concentrate as a protein source for Senegalese sole (Solea senegalensis Kaup 1858) diets: effects on growth and amino acid metabolism of postlarvae. Aquac Res 34:1443–1452
Berge GM, Grisdale-Helland B, Helland SJ (1999) Soy protein concentrate in diets for Atlantic halibut (Hippoglossus hippoglossus). Aquaculture 178:139–148
Bjerkeng B, Refstie S, Fjalestad KT, Storebakken T, Rodbotten M, Roem AJ (1997) Quality parameters of the flesh of Atlantic salmon (Salmo salar) as affected by dietary fat content and full-fat soy bean meal as a partial substitute for fish meal in the diet. Aquaculture 157:297–309
Burrells C, Williams PD, Southgate PJ, Crampton VO (1999) Immunological, physiological and pathological responses of rainbow trout (Oncorhynchus mykiss) to increasing dietary concentrations of soybean proteins. Vet Immunol Immunopathol 72:277–288
Carroll KK, Hamilton RMG (1975) Effects of dietary protein and carbohydrate on plasma cholesterol levels in relation to atherosclerosis. J Food Sci 40:18–23
Carter CG, Sajjadi M (2011) Low fish meal diets for Atlantic salmon, Salmo salar L., using soy protein concentrate treated with graded levels of phytase. Aquacult Int 19:431–444
Carver JD, Walker WA (1995) The role of nucleotides in human nutrition. Nutr Biochem 6:58–72
Celi P, Sullivan M, Evans D (2010) The stability of the reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) tests on stored horse blood. Vet J 183:217–218
Chen W, Ai QH, Mai KS, Xu W, Liufu ZG, Zhang WB, Cai YH (2011) Effects of dietary soybean saponins on feed intake, growth performance, digestibility and intestinal structure in juvenile Japanese flounder (Paralichthys olivaceus). Aquaculture 318:95–100
Cheng ZJ, Hardy RW (2004) Protein and lipid sources affect cholesterol concentrations of juvenile Pacific white shrimp, Litopenaeus vannamei (Boone). J Anim Sci 82:1136–1145
Cheng ZJ, Hardy RW, Verlhac V, Gabaudan J (2004) Effects of microbial phytase supplementation and dosage on apparent digestibility coefficients of nutrients and dry matter in soybean product-based diets for rainbow trout Oncorhynchus mykiss. J World Aquac Soc 35:1–15
Chou RL, Her BY, Su MS, Hwang G, Wu YH, Chen HY (2004) Substituting fishmeal with soybean meal in diets of juvenile cobia (Rachycentron canadum). Aquaculture 229:325–333
Colburn HR, Walker AB, Breton T, Stilwell JM, Sidor IF, Gannam AL, Berlinsky DL (2012) Partial replacement of fishmeal with soybean meal and soy protein concentrate in diets of Atlantic Cod. N Am J Aquac 74:330–337
Davis DA, Jirsa D, Arnold CR (1995) Evaluation of soybean proteins as replacements for menhaden fish meal in practical diets for red drum (Sciaenops ocellatus). J World Aquac Soc 26:48–58
Day OJ, Plascencia-Gonzalez HG (2000) Soybean protein concentrate as a protein source for turbot (Scophthalmus maximus L.) Aquac Nut 6:221–228
Deng JM, Mai KS, Ai QH, Zhang WB, Wang XJ, Xu W, Liufu ZG (2006) Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 258:503–513
Dias J (1999) Lipid deposition in rainbow trout, Oncorhynchus mykiss and European seabass, Dicentrarchus labrax L.: nutritional regulation of hepatic lipogenesis. PhD thesis, Univ. Porto (Portugal) and Univ. Bordeaux I (France), pp. 190
Drew MD, Borgeson TL, Thiessen DL (2007) A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim Feed Sci Tech 138:118–136
Fletcher TC (1997) Dietary effects on stress and health. In: Iwama GK, Pickering AD, Schreck CB (eds) Fish stress and health in aquaculture. Cambridge University Press, Cambridge, pp 223–244
Francis G, Makkar HPS, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227
Freitas LEL, Nunes AJP, Sa MVC (2011) Growth and feeding responses of the mutton snapper, Lutjanus analis (Cuvier 1828), fed on diets with soy protein concentrate in replacement of Anchovy fish meal. Aquac Res 42:866–877
Furukawa A, Tsukahara H (1966) On the acid digestion method for the determination of chromic oxides as an index substance in the study of digestion of fish feed. Nippon Suisan Gakkaishi 32:502–506
Gabrielsen BO, Austreng E (1998) Growth, product quality and immune status of Atlantic salmon, Salmo salar L., fed wet feed with alginate. Aquac Res 29:397–401
Gatlin DM III, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–152
Hagemeister H, Scholz-Ahrens KE, Schulte-Coerne H, Barth CA (1990) Plasma amino acids and cholesterol following consumption of dietary casein or soy protein in minipigs. J Nutr 120(11):1305–1311
Hernández PV, Olvera-Novoa MA, Rouse DB (2004) Effect of dietary cholesterol on growth and survival of juvenile redclaw crayfish Cherax quadricarinatus under laboratory conditions. Aquaculture 236:405–411
Hernández MD, Martínez FJ, Jover M, García GB (2007) Effects of partial replacement of fish meal by soybean meal in sharpsnout seabream (Diplodus puntazzo) diet. Aquaculture 263:159–167
Holme MH, Zeng C, Southgate PC (2006) The effects of supplemental dietary cholesterol on growth, development and survival of mud crab, Scylla serrata, megalopa fed semi-purified diets. Aquaculture 261:1328–1334
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM (2016a) Effects of dietary administration of guanosine monophosphate on the growth, digestibility, innate immune responses and stress resistance of juvenile red sea bream, Pagrus major. Fish and shellfish Immunol 57:96–106
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM (2016b) Dietary nucleotide administration influences growth, immune responses and oxidative stress resistance of juvenile red sea bream (Pagrus major). Aquaculture 455:41–49
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM, Ono S, Fujieda T (2016c) Comparison of the effects of inosine and inosine monophosphate on growth, immune response, stress resistance and gut morphology of juvenile red sea bream, Pagrus major. Aquaculture 458:64–74
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM (2016d) Dietary effects of adenosine monophosphate to enhance growth, digestibility, innate immune responses and stress resistance of juvenile red sea bream, Pagrus major. Fish and shellfish Immunol 56:523–533
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Maekawa M, Fujieda T (2017a) Effects of dietary administration of inosine on growth, immune response, oxidative stress and gut morphology of juvenile amberjack Seriola dumerili. Aquaculture 468:534–544
Hossain MS, Kader MA, Dey T, Sony NM, Bulbul M, Koshio S (2017b) Effect of high inclusion of rendered animal by-product ingredients on growth, digestibility and economic performances in climbing perch Anabas testudineus. Aquac Res 48:931–940
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM, Fujieda T (2017c) Nucleoside by-product dietary supplementation influences blood chemistry, immune response, oxidative stress resistance and intestinal morphology of juvenile amberjack, Seriola dumerili. Aquac Nutr: 1–11. doi:10.1111/anu.12514
Huang SSY, Oo AN, Higgs DA, Brauner CJ, Satoh S (2007) Effect of dietary canola oil level on the growth performance and fatty acid composition of juvenile red sea bream, Pagrus major. Aquaculture 271:420–431
Ikeda I, Hosokawa H, Shimeno S, Takeda M (1991) Feeding stimulant activity of nucleotides, tryptophan, and their related compounds of jack mackerel. Nippon Suisan Gakkaishi 57(42):1539–1515
Kader MA, Koshio S, Ishikawa M, Yokoyama S, Bulbul M (2010) Supplemental effects of some crude ingredients in improving nutritive values of low fishmeal diets for red sea bream, Pagrus major. Aquaculture 308:136–144
Kader MA, Bulbul M, Koshio S, Ishikawa M, Yokoyama S, Nguyen BT, Komilus CF (2012) Effect of complete replacement of fishmeal by dehulled soybean meal with crude attractants supplementation in diets for red sea bream, Pagrus major. Aquaculture 350–353:109–116
Kaushik SJ, Cravedi JP, Lalles JP, Sumpter J, Fauconneau B, Laroche M (1995) Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 133:257–274
Kaushik SJ, Covès D, Dutto G, Blanc D (2004) Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 230:391–404
Kim JD, Kaushik SJ, Breque J (1998) Nitrogen and phosphorus utilization in rainbow trout, Oncorhynchus mykiss fed diets with or without fish meal. Aquacult Living Res 11:261–264
Kiron V, Puangkaew J, Ishizaka K, Satoh S, Watanabe T (2004) Antioxidant status and nonspecific immune responses in rainbow trout (Oncorhynchus mykiss) fed two levels of vitamin E along with three lipid sources. Aquaculture 234:361–379
Kissil GW, Lupatsch I, Higgs DA, Hardy RW (2000) Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquac Res 31:595–601
Kofuji PYM, Hosokawa H, Masumoto T (2006) Effects of dietary supplementation with feeding stimulants on yellowtail Seriola quinqueradiata (Temminck & Schlegel; Carangidae) protein digestion at low water temperatures. Aquac Res 37:366–373
Krogdahl A, Bakke-Mckellep AM, Baeverfjord G (2003) Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.) Aquac Nutr 9:361–371
Kubilay A, Ulukoy G (2002) The effects of acute stress on rainbow trout (Oncorhynchus mykiss). Turk J Zool 26(2):249–254
Lanari D, Agaro E, Turri C (1998) Use of nonlinear regression to evaluate the effects of phytase enzyme treatment of plant protein diets for rainbow trout, Oncorhynchus mykiss. Aquaculture 161:345–356
Lemaire P, Drai P, Mathieu A, Lemaire S, Carriere S, Giudicelli J, Lafaurie M (1991) Changes with different diets in plasma enzymes (GOT, GPT, LDH, ALP) and plasma lipids (cholesterol, triglycerides) of sea bass (Dicentrarchus labrax). Aquaculture 93:63–75
Li P, Gatlin DM (2006) Nucleotide nutrition in fish: current knowledge and future applications. Aquaculture 251:141–152
Li Y, Ai QH, Mai KS, Cheng ZY (2011) Effects of the partial substitution of dietary fishmeal by two types of soybean meals on the growth performance of juvenile Japanese seabass, Lateolabrax japonicus (Cuvier 1828). Aquac Res 1–9. doi:10.1111/j.1365-2109.2011.02849.x
Lin YH, Wang H, Shiau SY (2009) Dietary nucleotide supplementation enhances growth and immune responses of grouper (Epinephelus malabaricus). Aquac Nutr 15:117–122
Lygren B, Sveier H, Hjeltnes B, Waagbo R (1999) Examination of the immunomodulatory properties and the effect on disease resistance of dietary bovine lactoferrin and vitamin C fed to Atlantic salmon (Salmo salar) for a short-term period. Fish Shellfish Immunol 9:95–107
Mambrini M, Roem AJ, Cravedi JP, Lalles JP, Kaushik SJ (1999) Effects of replacing fish meal with soy protein concentrate and of DL-methionine supplementation in high energy, extruded diets on the growth and nutrient utilization of rainbow trout, Oncorhynchus mykiss. J Anim Sci 77:2990–2999
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15(1–2):75–88
Meilahn CW, Davis DA, Arnold CR (1996) Effects of commercial fish meal analog and menhaden fish meal on growth of red drum fed isonitrogenous diets. Progres Fish-Cult 58:111–116
Morganti P, Bruno C, Guarneri F, Cardillo A, Del Ciotto P, Valenzano F (2002) Role of topical and nutritional supplement to modify the oxidative stress. Int J Cosmet Sci 24(6):331–339
Ngandzali BO, Zhou F, Xiong W, Shao QJ, Xu JZ (2011) Effect of dietary replacement of fish meal by soybean protein concentrate on growth performance and phosphorus discharging of juvenile black sea bream, Acanthopagrus schlegelii. Aquac Nutr 17:526–535
Papatryphon E, Soares JH Jr (2000) The effect of dietary feeding stimulants on growth performance of striped bass, Morone saxatilis, fed-a-plant feedstuff-based diet. Aquaculture 185:329–338
Peng M, Xu W, Ai Q, Mai K, Liufu Z, Zhang K (2013) Effects of nucleotide supplementation on growth, immune responses and intestinal morphology in juvenile turbot fed diets with graded levels of soybean meal (Scophthalmus maximus L.) Aquaculture 392–395:51–58
Reigh RC, Ellis SC (1992) Effects of dietary soybean and fish-protein ratios on growth and body composition of red drum (Sciaenops ocellatus) fed isonitrogenous diets. Aquaculture 104:279–292
Rumsey GL, Siwicki AK, Anderson DP, Bowser PR (1994) Effect of soybean protein on serological response, nonspecific defense-mechanisms, growth, and protein-utilization in rainbow-trout. Vet Immunol Immunopathol 41:323–339
Sagstad A, Sanden M, Krogdahl Å, Bakke-Mckellep AM, Frøystad M, Hemre GI (2008) Organs development, gene expression and health of Atlantic salmon (Salmo salar L.) fed genetically modified soybeans compared to the near-isogenic nonmodified parental line. Aquac Nutr 14:556–572
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MA (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol 25:114–123
Salze G, McLean E, Battle PR, Schwarz MH, Craig SR (2010) Use of soy protein concentrate and novel ingredients in the total elimination of fish meal and fish oil in diets for juvenile cobia, Rachycentron canadum. Aquaculture 298:294–299
Sato N, Murakami Y, Nakano T, Sugawara M, Kawakami H, Idota T, Nakajima I (1995) Effects of dietary nucleotides on lipid metabolism and learning ability of rats. Biosci Biotechnol Biochem 59(7):1267–1271
Storebakken T, Shearer KD, Roem AJ (1998) Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein-concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture 161:365–379
Storebakken T, Refstie S, Ruyter B (2000) Soy products as fat and protein sources in fish feeds for intensive aquaculture. In: Drackley JK (ed) Soy in animal nutrition. Federation of Animal Science Societies, Savoy IL, USA, pp 127–170
Swain P, Dash S, Sahoo P, Routray P, Sahoo S, Gupta SD, Meher PK, Sarangi N (2007) Non-specific immune parameters of brood Indian major carp Labeo rohita and their seasonal variations. Fish Shellfish Immunol 22:38–43
Tacon AGJ, Metian M (2008) Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture 285:146–158
Takagi S, Hosokawa H, Shimeno S, Maita M, Ukawa M, Ueno S (1999) Utilization of soy protein concentrate in a diet for red sea bream, Pagrus major. Suisanzoshoku 47:77–87
Takagi S, Shimeno S, Hosokawa H, Ukawa M (2001) Effect of lysine and methionine supplementation to a soy protein concentrate diet for red sea bream, Pagrus major. Fish Sci 67:1088–1096
Takii K, Shimeno S, Takeda M, Kamekawa S (1986) The effects of feeding stimulants in diet on digestive enzyme activities of eel. Bull Jpn Soc Sci Fish 52:1449–1454
Takii K, Shimeno S, Akutsu M, Takeda M (1990) Dietary supplement of feeding stimulants on performance and digestive function of yellowtail, Seriola quinqueradiata. Bull Fish Lab, Kinki Univ 4:127–137
Vielma J, Lall SP, Koskela J, Schoner FJ, Mattila P (1998) Effects of dietary phytase and cholecalciferol on phosphorus bioavailability in rainbow trout (Oncorhynchus mykiss). Aquaculture 163:309–323
Vielma J, Makinen T, Ekholm P, Koskela J (2000) Influence of dietary soy and phytase levels on performance and body composition of large rainbow trout (Oncorhynchus mykiss) and algal availability of phosphorus load. Aquaculture 183:349–362
Waagbø R (1994) The impact of nutritional factors on the immune system in Atlantic salmon, Salmo salar L.: a review. Aquacult Fish Manag 25:175–197
Walker AB, Sidor IF, O’Keefe T, Cremer M, Berlinsky DL (2010) Partial replacement of fish meal with soy protein concentrate in diets of Atlantic cod. N Am J Aquac 72:343–353
Wu Y, Han H, Qin J, Wang Y (2015) Replacement of fishmeal by soya protein concentrate with taurine supplementation in diets for golden pompano (Trachinotus ovatus). Aquacult Nutri 21:214–222
Yokoyama S, Koshio S, Takakura N, Oshida K, Ishikawa M, Gallardo-Cigarroa FJ, Teshima S (2005) Dietary bovine lactoferrin enhances tolerance to high temperature stress in Japanese flounder Paralichthys olivaceus. Aquaculture 249:367–373
Acknowledgments
The first author would like to thank the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MONBUKAGAKUSHO) for supporting this research work. The research was partially funded by the Management Expenses Grants of the United Graduate School of Agricultural Sciences, Kagoshima University, provided to Dr. Shunsuke Koshio.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hossain, M.S., Koshio, S. Dietary substitution of fishmeal by alternative protein with guanosine monophosphate supplementation influences growth, digestibility, blood chemistry profile, immunity, and stress resistance of red sea bream, Pagrus major . Fish Physiol Biochem 43, 1629–1644 (2017). https://doi.org/10.1007/s10695-017-0398-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0398-4