Abstract
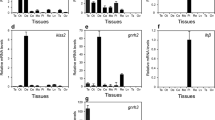
Arginine vasotocin is a hormone produced in the hypothalamus of teleost fish that has been shown to regulate gonad development and sexual behavior. To study the role of arginine vasotocin in the gonadal cycle of the hermaphrodite gilthead seabream, Sparus aurata, we cloned the seabream arginine vasotocin (avt) complementary DNA (cDNA). We investigated the expression of brain avt throughout the gonad cycle using real-time quantitative PCR and compared its expression levels to the expression levels of two key gonadal steroidogenic enzymes, cyp19a1a and cyp11b2. In July, when the process of sex reversal is thought to begin, avt expression was elevated over the previous 2 months. Avt in the brain remained at or above the level of July until November then peaked again in December. There was no difference between males and females in the expression levels of brain avt throughout the year. However, only in ambisexual fish was the expression of the cyp19a1a gonadal aromatase correlated to the expression of avt in the brain. Cyp11b2 did not show any correlation to brain avt expression. We also found that females had more intense body coloration than males and that this intensity peaked prior to spawning. Avt expression and female coloration were positively correlated. The fact that brain avt expression was lowest during gonad quiescence, together with the observation of a correlation between brain avt with gonadal cyp19a1a and body coloration during that time suggests that avt may play a role during the process of sex reversal and spawning of the gilthead seabream.





Similar content being viewed by others
References
Albers HE (2012) The regulation of social recognition, social communication and aggression: vasopressin in the social behavior network. Horm Behav 61:283–292
Balment RJ, Lu W, Weybourne E, Warne JM (2006) Arginine vasotocin a key hormone in fish physiology and behavior: a review with insights from mammalian models. Gen Comp Endocr 147:9–16
D'Ancona U (1941) Ulteriori osservazioni sulľ ermafroditismo e il differenziamento sessuale delľ orata (Sparus aurata L.). Pubblicazioni della Stazione Zoologica di Napoli 18:313–336
Desjardins JK, Fernald RD (2009) Fish sex: why so diverse? Curr Opin Neurobiol 19:648
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM (1998) Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med 4:1329–1333
Foran CM, Bass AH (1999) Preoptic GnRH and AVT: axes for sexual plasticity in teleost fish. Gen Comp Endocr 116:141–152
Godwin J (2010) Neuroendocrinology of sexual plasticity in teleost fishes. Front Neuroendocrin 31:203–216
Godwin J, Thompson R (2012) Nonapeptides and social behavior in fishes. Horm Behav 61:230–238
Guiguen Y, Fostier A, Piferrer F, Chang CF (2010) Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol 165(3):352–366
Happe A, Zohar Y (1988) Self-fertilization in the protandrous hermaphrodite Sparus aurata: development of the technology. In: Zohar, Brenton (eds) Reproduction in fish: basic and applied aspects in endocrinology and genetics. INRA Press, Paris, pp 177–180
Higa M, Ogasawara K, Sakaguchi A, Nagahama Y, Nakamura M (2003) Role of steroid hormones in sex change of protogynous wrasse. Fish Physiol Biochem 28(1–4):149–150
Huffman LS, Hinz FI, Wojcik S, Aubin-Horth N, Hofmann HA (2014) Arginine vasotocin regulates social ascent in the African cichlid fish Astatotilapia burtoni. Gen Comp Endocrinol 212:106–113
Insel TR, Young L (2000) Neuropeptides and the evolution of social behavior. Curr Opin Neurobio 10:784–789
Iwata E, Nagai Y, Sasaki H (2010) Social rank modulates brain arginine vasotocin immunoreactivity in false clown anemonefish (Amphiprion ocellaris). Fish Physiol Biochem 36:337–345
Kleszczynska A, Kulczykowska E (2013) Stocking density influences brain arginine vasotocin (AVT) and isotocin (IT) levels in males and females of three-spined stickleback (Gasterosteus aculeatus). Gen Comp Endocr 183:14–16
Kleszczynska A, Sokolowska E, Kulczykowska E (2012) Variation in brain arginine vasotocin (AVT) and isotocin (IT) levels with reproductive stage and social status in males of three-spined stickleback (Gasterosteus aculeatus). Gen Comp Endocr 175:290–296
Kulczykowska E, Cardoso SC, Gozdowska M, Andre GI, Paula JR, Slebioda M, Oliveira RF, Soares MC (2015) Brain levels of nonapeptides in four labrid fish species with different levels of mutualistic behavior. Gen Comp Endocr 222:99–105
Kline RJ, Holt GJ, Khan IA (2016) Arginine vasotocin V1a2 receptor and GnRH-I co-localize in preoptic neurons of the sex changing grouper, Epinephelus adscensionis. Gen Comp Endocr 225:33–44
Kline RJ, Khan IA, Holt GJ (2011) Behavior, color change and time for sexual inversion in the protogynous grouper (Epinephelus adscensionis). PLoS One 6:e19576
Kobayashi Y, Nagahama Y, Nakamura M (2013) Diversity and plasticity of sex determination and differentiation in fishes. Sex Dev 7:115–125
Kuwamura T, Suzuki S, Kadota T (2011) Reversed sex change by widowed males in polygynous and protogynous fishes: female removal experiments in the field. Naturwissenschaften 98:1041–1048
Lema SC, Slane MA, Salvesen KE, Godwin J (2012) Variation in gene transcript profiles of two V1a-type arginine vasotocin receptors among sexual phases of bluehead wrasse (Thalassoma bifasciatum). Gen Comp Endocr 179(3):451–464
Lema SC, Sanders KE, Walti KA (2015) Arginine vasotocin, isotocin and nonapeptide receptor gene expression link to social status and aggression in sex-dependent patterns. J Neuroendocrinol 27(2):142–157
Liu M, Sadovy Y (2004) The influence of social factors on adult sex change and juvenile sexual differentiation in a diandric, protogynous epinepheline, Cephalopholis boenak (Pisces, Serranidae). J Zool 264:239–248
Lorenzi V, Earley RL, Grober MS (2006) Preventing behavioural interactions with a male facilitates sex change in female bluebanded gobies, Lythrypnus dalli. Behav Ecol Sociobiol 59:715–722
Mank JE, Promislow DEL, Avise JC (2006) Evolution of alternative sex-determining mechanisms in teleost fishes. Biol J Linn Soc 87:83–93
Maruska KP (2009) Sex and temporal variations of the vasotocin neuronal system in the damselfish brain. Gen Comp Endocr 160:194–204
Mototiashi E, Hamabata T, Hironorl A (2008) Structure of neurohypophysial hormone genes and changes in the levels of expression dring spawning season in grass puffer (Takifugu niphobles). Gen Comp Endocr 155:456–463
Nagarajan G, Aruna A, Chang CF (2015) Neuropeptide arginine vasotocin positively affects neurosteroidogenesis in the early brain of grouper, Epinephelus coioides. J Neuroendocrinology 27(9):718–736
Nozu R, Kojima Y, Nakamura M (2009) Short term treatment with aromatase inhibitor induces sex change in the protogynous wrasse, Halichoeres trimaculatus. Gen Comp Endocr 161:360–364
Perry AN, Grober MS (2003) A model for social control of sex change: interactions of behavior, neuropeptides, glucocorticoids, and sex steroids. Horm Behav 43:31–38
Quinitio GF, Caberoy NB, Reyes DM (1997) Induction of sex change in female Epinephelus coioides by social control. Bamidgeh 49:77–83
Reyes-Tomassini J (2009) Behavioral and neuroendocrine correlates of sex change in the gilthead seabream Sparus aurata. Thesis. University of Maryland
Reyes-Tomassini J, Wong T, Zohar Y (2013) GnRH isoforms expression in relation to the gonadal cycle and to dominance rank in the gilthead seabream, Sparus aurata. Fish Physiol Biochem 39:1681
Ripley JL, Foran CM (2010a) Quantification of whole brain arginine vasotocin for two Syngnathus pipefishes: elevated concentrations correlated with paternal brooding. Fish Physiol Biochem 36:867–874
Ripley JL, Foran CM (2010b) Elevated whole brain arginine vasotocin with Aroclor 1254 exposure in two Syngnathus pipefishes. Fish Physiol Biochem 36:917–921
Semsar K, Godwin J (2003) Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. J Neuroscience 23:4386–4393
Shapiro DY (1980) Serial female sex change after simultaneous removal of males from social groups of a coral reef fish. Science 209:1136–1137
Sokolowska E, Kleszczynska A, Nietrzeba M, Kulczykowska E (2015) Annual changes in brain concentration of arginine vasotocin and isotocin correspond with phases of reproductive cycle in round goby, Neogobius melanostomus. Chronobiol Int 32(7):917–924
Uno T, Ishizuka M, Itakura T (2012) Cytochrome P450 (CYP) in fish. Environ Toxicol Phar 34(1):1–13
Wong TT, Gothilf Y, Zmora N, Kight KE, Meiri I, Elizur A, Zohar Y (2004) Developmental expression of three forms of gonadotropin-releasing hormone and ontogeny of the hypothalamic-pituitary-gonadal axis in gilthead seabream (Sparus aurata). Biol Reprod 71:1026–1035
Wong TT, Ijiri S, Zohar Y (2006) Molecular biology of ovarian aromatase in sex reversal: complementary DNA and 5 '-flanking region isolation and differential expression of ovarian aromatase in the gilthead seabream (Sparus aurata). Biol Reprod 74:857–864
Zohar Y, Abraham M, Gordin H (1978) The gonadal cycle of the captivity reared hermaphroditic teleost, Sparus aurata (L.) during the first two years of life. Ann Biol Anim Biochem Biophys 18:877–882
Zohar Y, Billard R, Weil C (1984) La reproduction de la daurade et du bar: Le cycle sexuel et l’induction de la ponte. In: Barnabe G (ed) Billard R. INRA Press Paris, Aquaculture de Bar et des Sparides. edn, pp 3–24
Acknowledgements
The present work was supported by the NOAA-Educational Partnership Program (EPP)-funded Living Marine Resources Cooperative Science Center and the NOA-EPP funded Graduate Science Program. We are also thankful to Steven Rodgers, Eric Evans, Chris Tollini, and John Stubblefield for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reyes-Tomassini, J.J., Wong, TT. & Zohar, Y. Seasonal expression of arginine vasotocin mRNA and its correlations to gonadal steroidogenic enzymes and sexually dimorphic coloration during sex reversal in the gilthead seabream (Sparus aurata). Fish Physiol Biochem 43, 823–832 (2017). https://doi.org/10.1007/s10695-017-0338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0338-3