Abstract
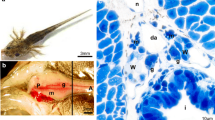
As a flatfish, the turbot (Scophthalmus maximus) is one of the most important farmed fish species with great commercial value, which has a strong sexual dimorphism on growth rate and sexual maturity. In this study, using histology, the basic information on proliferation and migration of germ cells and early gonadal development during sex differentiation in turbot were described in detail. There were six to nine individual primordial germ cells (PGCs) with large nuclei until 15 days post-hatching (dph). The PGCs located under the mesonephric ducts undergo migration along the dorsal mesentery toward the region of the genital ridge from 0 to 15 dph. During migration, the number of PGCs was constant, and the expression of vasa had no significant changes. At 20 dph, the aggregation of somatic cells at genital ridge indicated the formation of primary gonad. Furthermore, the number of PGCs was increased to 60 and the expression of vasa was upregulated for the first time. The undifferentiated gonads with no morphological indications of sex differentiation grew larger with the increase in germ cells and somatic cells number/size from 20 to 35 dph. During 36–52 dph, cytological gonadal differentiation was observed. In presumptive testes of type I gonadal tissue (with a lance shape), the number of germ cells increased steadily and the germ cells had the same characteristics as before. Meanwhile, in presumptive ovaries of type II gonadal tissue (with a club-like shape), the germ cells proliferated and induced in two different populations of germ cells. One type had the morphological characteristics as undifferentiated germ cells, while the other type of germ cells underwent mitosis exhibiting smaller size and mottled nuclei. At 60 dph, ovarian cavity was present in the gonad of type II, which would develop into ovaries. However, spermatogonial cysts were not noticed in the gonad of type I until 90 dph, which indicated the formation of the testes.







Similar content being viewed by others
References
Arezo MJ, D’Alessandro S, Papa N, de Sá R, Berois N (2007) Sex differentiation pattern in the annual fish Austrolebias charrua (Cyprinodontiformes: Rivulidae). Tissue Cell 39:89–98
Chiasson M, Benfey TJ (2007) Gonadal differentiation and hormonal sex reversal in Arctic charr (Salvelinus alpinus). J Exp Zool Part A Ecol Genet Physiol 307:527–534
Dai XY, Jin X, Chen XW, He JY, Yin Z (2015) Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS ONE 10:15. doi:10.1371/journal.pone.0117824
Deniz Koç N, Yüce R (2012) A light- and electron microscopic study of primordial germ cells in the zebra fish (Danio rerio). Biol Res 45:331–336
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Fairchild EA, Rennels N, Howell WH, Wells RE (2007) Gonadal development and differentiation in cultured juvenile winter flounder, Pseudopleuronectes americanus. J World Aquac Soc 38:8
Fatima S, Adams M, Wilkinson R (2011) Histological study of gonadal development and sex differentiation in Salvelinus fontinalis under Tasmanian climate conditions. Aust J Zool 59:321. doi:10.1071/zo11092
FEAP (2015) European aquaculture production report 2005–2014. Federation of European Aquaculture Producers. http://www.feap.info/Default.asp?SHORTCUT=582. Accessed 20 Aug 2015
Foyle TP (1993) A histological description of gonadal development and sex differentiation in the coho salmon (Oncorhynchus kisutch) for both untreated and oestradiol immersed fry. J Fish Biol 42:699–712
Gao Z et al (2009) Gonadal sex differentiation in the bluegill sunfish Lepomis macrochirus and its relation to fish size and age. Aquaculture 294:138–146
Guerrero SM, Moreno-Mendoza N (2009) Gonadal sex differentiation in Chapalichthys encaustus (Teleostei: Goodeidae). Dev Biol 331:443
Haffray P, Lebègue E, Jeu S, Guennoc M, Guiguen Y, Baroiller JF, Fostier A (2009) Genetic determination and temperature effects on turbot Scophthalmus maximus sex differentiation: an investigation using steroid sex-inverted males and females. Aquaculture 294:30–36
Hamaguchi S (1982) A light- and electron-microscopic study on the migration of primordial germ cells in the teleost, Oryzias latipes. Cell Tissue Res 227:139–151. doi:10.1007/BF00206337
Hendry CI, Martin-Robichaud DJ, Benfey TJ (2002) Gonadal sex differentiation in Atlantic halibut. J Fish Biol 60:1431–1442
Kobayashi T, Ishibashi R, Yamamoto S, Otani S, Ueno K, Murata O (2011) Gonadal morphogenesis and sex differentiation in cultured chub mackerel, Scomber japonicus. Aquac Res 42:230–239
Lebrun C, Billard R, Jalabert B (1982) Changes in the number of germ cells in the gonads of the rainbow trout (Salmo gairdneri) during the first 10 post-hatching weeks. Reprod Nutr Dév 22:405–412
Lei J (2003) The development direction of turbot farming industry in China. Sci Fish Farm 7:3–4 (in Chinese)
Lewis ZR, McClellan MC, Postlethwait JH, Cresko WA, Kaplan RH (2008) Female-specific increase in primordial germ cells marks sex differentiation in threespine stickleback (Gasterosteus aculeatus). J Morphol 269:909–921
Lin F et al (2012) Germ line specific expression of a vasa homologue gene in turbot (Scophthalmus maximus): evidence for vasa localization at cleavage furrows in euteleostei. Mol Reprod Dev 79:803–813
Luckenbach JA, Godwin J, Daniels HV, Borski RJ (2003) Gonadal differentiation and effects of temperature on sex determination in southern flounder (Paralichthys lethostigma). Aquaculture 216:13
Mandich A, Massari A, Bottero S, Marino G (2002) Histological and histochemical study of female germ cell development in the dusky grouper Epinephelus marginatus (Lowe, 1834). Eur J Histochem 46:87–100
Nagahama Y (1983) The Functional-morphology of teleost gonads. Fish Physiol 9:223–275
Nagai T, Yamaha E, Arai K (2001) Histological differentiation of primordial germ cells in zebrafish. Zool Sci 18:215–223
Nakamura M, Kobayashi T, Chang X-T, Nagahama Y (1998) Gonadal sex differentiation in teleost fish. J Exp Zool 281:362–372
Nishimura T, Tanaka M (2014) Gonadal development in fish. Sex Dev 8:252–261
Orban L, Sreenivasan R, Olsson PE (2009) Long and winding roads: testis differentiation in zebrafish. Mol Cell Endocrinol 312:35–41
Parmentier HK, Timmermans LPM (1985) The differentiation of germ cells and gonads during development of carp (Cyprinus carpio L.). A study with anti-carp sperm monoclonal antibodies. J Embryol Exp Morphol 90:13–32
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281
Raz E (2003) Primordial germ-cell development: the zebrafish perspective. Nat Rev Genet 4:690–700
Sacobie CFD, Benfey TJ (2005) Sex differentiation and early gonadal development in brook trout. N Am J Aquac 67:181–186
Saito D et al (2007) Proliferation of germ cells during gonadal sex differentiation in medaka: insights from germ cell-depleted mutant zenzai. Dev Biol 310:280–290
Sandra G-E, Norma M-M (2009) Sexual determination and differentiation in teleost fish. Rev Fish Biol Fish 20:101–121
Satoh N, Egami N (1972) Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development. J Embryol Exp Morphol 28:385–395
Takashima F, Patino R, Nomura M (1980) Histological studies on the sex differentiation in rainbow. Bull Jpn Soc Sci Fish 46:1317–1322
Timmermans LPM, Taverne N (1989) Segregation of primordial germ cells: their numbers and fate during early development of Barbus conchonius (Cyprinidae, Teleostei) as indicated by 3H-thymidine incorporation. J Morphol 202:225–237
Uchida D, Yamashita M, Kitano T, Iguchi T (2002) Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol 205:711–718
Uguz C, Iscan M, Togan I (2003) Developmental genetics and physiology of sex differentiation in vertabrates. Environ Toxicol Pharmacol 14:9–16
Yamamoto T-O (1969) 3 Sex differentiation. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 3. Academic Press, New York, pp 117–175
Yamamoto E (1999) Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture 173:235–246. doi:10.1016/S0044-8486(98)00448-7
Yvan F et al (1983) La gonadogenèse des Poissons. Reprod Nutr Dev 23:453–491
Acknowledgments
This research was supported by National Natural Science Foundation of China (Nos. 31372514, 31472264, 31572602), Modern Agro-Industry Technology Research System (nycytx-50), China Postdoctoral Science Foundation (2014M551973), the Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology (Nos. 2015ASKJ02, 2015ASKJ02-03-03), Youth Innovation Promotion Association CAS and Chinese Academy of Science and Technology Service Network Planning (KFJ-EW-STS-060).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, C., Xu, S., Liu, Y. et al. Gonadogenesis analysis and sex differentiation in cultured turbot (Scophthalmus maximus). Fish Physiol Biochem 43, 265–278 (2017). https://doi.org/10.1007/s10695-016-0284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0284-5