Abstract
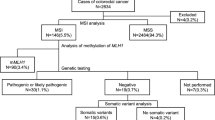
Current algorithms for diagnosing Lynch syndrome (LS) include multistep molecular tumor tests to distinguish LS-associated from sporadic colorectal cancer (CRC), which add cost and complexity to the evaluation. We hypothesized that PREMM5, a clinical LS prediction tool, could be an alternative approach to screen for LS, thereby lessening the need for specialized molecular diagnostics. We reviewed a consecutively ascertained institutional cohort of 1058 CRC patients on whom pathologic and clinical data were available, including prior LS germline testing. Data from MMR-D/MSI-H CRC patients were reviewed and PREMM5 scores were calculated for each individual. Using a PREMM5 score cutoff ≥ 2.5% to characterize the need for germline testing, we determined the rate of pathogenic/likely pathogenic germline variants (PGVs) in LS genes in patients with PREMM5 scores ≥ 2.5% versus < 2.5%. Sensitivity and negative predictive values (NPV) of PREMM5 were calculated for all MMR-D/MSI-H CRC patients, and those with MLH1-deficient CRC. MMR IHC and/or MSI results were available on 572/1058 cases. We identified 74/572 (12.9%) cases as MMR-D/MSI-H, of which 28/74 (37.8%) harbored a LS PGV. 11/49 (22.4%) patients with MLH1-deficient CRC harbored a LS PGV. PREMM5 had 100% sensitivity (95% CI: 87.7–100 for any MMR-D/MSI-H; 95% CI: 71.5–100 for MLH1-deficient CRC) and 100% NPV (95% CI: 83.2–100 for any MMR-D/MSI-H; 95% CI: 82.4–100 for MLH1-deficient CRC) for identifying LS PGVs in these cohorts. PREMM5 accurately distinguishes LS- from non-LS-associated MMR-D/MSI-H CRC without additional somatic molecular testing. These findings are particularly relevant for limited-resource settings where advanced molecular diagnostics may be unavailable.
Similar content being viewed by others
References
Hampel H, Frankel WL, Martin E et al (2008) Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 26(35):5783–5788. https://doi.org/10.1200/JCO.2008.17.5950
Yurgelun MB, Kulke MH, Fuchs CS et al (2017) Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol 35(10):1086–1095. https://doi.org/10.1200/JCO.2016.71.0012
Abu-Ghazaleh N, Kaushik V, Gorelik A et al (2022) Worldwide prevalence of Lynch syndrome in patients with colorectal cancer: systematic review and meta-analysis. Genet Med 24(5):971–985. https://doi.org/10.1016/j.gim.2022.01.014
Win AK, Jenkins MA, Dowty JG et al (2017) Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 26(3):404–412. https://doi.org/10.1158/1055-9965.EPI-16-0693
Yurgelun MB, Hampel H (2018) Recent advances in Lynch syndrome: diagnosis, treatment, and cancer prevention. Am Soc Clin Oncol Educ Book 38:101–109. https://doi.org/10.1200/EDBK_208341
Drogan C, Kupfer SS (2022) Colorectal cancer screening recommendations and outcomes in Lynch syndrome. Gastrointest Endosc Clin N Am 32(1):59–74. https://doi.org/10.1016/j.giec.2021.08.001
Latham A, Srinivasan P, Kemel Y et al (2019) Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol 37(4):286–295. https://doi.org/10.1200/JCO.18.00283
Monahan KJ, Bradshaw N, Dolwani S et al (2020) Hereditary CRC guidelines eDelphi consensus group. Guidelines for the management of hereditary colorectal cancer from the british society of gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 69(3):411–444. https://doi.org/10.1136/gutjnl-2019-319915
Seppälä TT, Latchford A, Negoi I et al (2021) European Hereditary Tumour Group (EHTG) and european Society of Coloproctology (ESCP). European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br J Surg 108(5):484–498. https://doi.org/10.1002/bjs.11902
National Comprehensive Cancer Network, Inc (2022) Genetic/Familial High Risk Assessment: Colorectal v.2.2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf
Leclerc J, Vermaut C, Buisine MP (2021) Diagnosis of Lynch syndrome and strategies to distinguish Lynch-related tumors from sporadic MSI/dMMR tumors. Cancers (Basel) 13(3):467. https://doi.org/10.3390/cancers13030467
Pearlman R, Frankel WL, Swanson BJ et al (2021) Prospective statewide study of universal screening for hereditary colorectal cancer: the Ohio Colorectal Cancer Prevention Initiative. JCO Precis Oncol 5. https://doi.org/10.1200/PO.20.00525. PO.20.00525
Kastrinos F, Uno H, Ukaegbu C et al (2017) Development and validation of the PREMM5 model for Comprehensive Risk Assessment of Lynch Syndrome. J Clin Oncol 35(19):2165–2172. https://doi.org/10.1200/JCO.2016.69.6120
Kastrinos F, Samadder NJ, Burt RW (2020) Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology 158(2):389–403. https://doi.org/10.1053/j.gastro.2019.11.029
Sina M, Ghorbanoghli Z, Abedrabbo A et al (2021) Middle East Network on Hereditary Colorectal Cancer (HCCN-ME). Identification and management of Lynch syndrome in the Middle East and North African countries: outcome of a survey in 12 countries. Fam Cancer 20(3):215–221. https://doi.org/10.1007/s10689-020-00211-3
Karlitz JJ, Hsieh MC, Liu Y et al (2015) Population-based Lynch syndrome screening by microsatellite instability in patients ≤ 50: prevalence, testing determinants, and result availability prior to colon surgery. Am J Gastroenterol 110(7):948–955. https://doi.org/10.1038/ajg.2014.417
Cruz-Correa M, Pérez-Mayoral J, Dutil J et al (2017) Puerto Rico Clinical Cancer Genetics Consortia. Clinical Cancer Genetics Disparities among Latinos. J Genet Couns 26(3):379–386. https://doi.org/10.1007/s10897-016-0051-x
Pan JY, Haile RW, Templeton A et al (2018) Worldwide practice patterns in Lynch syndrome diagnosis and management, based on data from the International Mismatch Repair Consortium. Clin Gastroenterol Hepatol 16(12):1901–1910e11. https://doi.org/10.1016/j.cgh.2018.04.025
Della Valle A, Rossi BM, Palmero EI et al (2019) Collaboration with LA-GETH. A snapshot of current genetic testing practice in Lynch syndrome: the results of a representative survey of 33 latin american existing centres/registries. Eur J Cancer 119:112–121. https://doi.org/10.1016/j.ejca.2019.07.017
Unim B, Pitini E, Lagerberg T et al (2019) Current genetic service delivery models for the provision of genetic testing in Europe: a systematic review of the literature. Front Genet 10:552. https://doi.org/10.3389/fgene.2019.00552
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Chen W, Swanson BJ, Frankel WL (2017) Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol 12(1):24. https://doi.org/10.1186/s13000-017-0613-8
Eikenboom EL, van der Werf-‘t Lam AS, Rodríguez-Girondo M et al (2022) Universal immunohistochemistry for Lynch syndrome: a systematic review and meta-analysis of 58,580 colorectal carcinomas. Clin Gastroenterol Hepatol 20(3):e496–e507. https://doi.org/10.1186/s13000-017-0613-8
Herman JG, Umar A, Polyak K et al (1998) Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A 95(12):6870–6875. https://doi.org/10.1073/pnas.95.12.6870
Adar T, Rodgers LH, Shannon KM et al (2017) A tailored approach to BRAF and MLH1 methylation testing in a universal screening program for Lynch syndrome. Mod Pathol 30(3):440–447. https://doi.org/10.1038/modpathol.2016.211
Cabreira V, Pinto C, Pinheiro M et al (2017) Performance of Lynch syndrome predictive models in quantifying the likelihood of germline mutations in patients with abnormal MLH1 immunoexpression. Fam Cancer 16(1):73–81. https://doi.org/10.1007/s10689-016-9926-0
Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009) Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 11(1):35–41. https://doi.org/10.1097/GIM.0b013e31818fa2ff
Singh V, Mezzacappa C, Gershkovich P et al (2022) Systems approach to enhance Lynch syndrome diagnosis through tumour testing. J Med Genet 17. https://doi.org/10.1136/jmg-2022-108770. :jmg-2022-108770
Geurts-Giele WR, Leenen CH, Dubbink HJ et al (2014) Somatic aberrations of mismatch repair genes as a cause of microsatellite-unstable cancers. J Pathol 234(4):548–559. https://doi.org/10.1002/path.4419
Haraldsdottir S, Hampel H, Tomsic J et al (2014) Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology 147(6):1308–1316e1. https://doi.org/10.1053/j.gastro.2014.08.041
Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA et al (2014) Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology 146(3):643–646e8. https://doi.org/10.1053/j.gastro.2013.12.002
Mannucci A, Furniss CS, Ukaegbu C et al (2020) Comparison of colorectal and endometrial microsatellite instability tumor analysis and Premm5 risk assessment for predicting pathogenic germline variants on multigene panel testing. J Clin Oncol 38(34):4086–4094. https://doi.org/10.1200/JCO.20.01470
Funding
Supported by the National Institutes of Health (National Cancer Institute) R01CA132829 (S.S.), The Helen Gurley Brown Presidential Initiative (C.U.), The Lesswitz Fund for Lynch Syndrome (M.B.Y.), The Sweet Family Fund (S.S.), and The Terry T. Sweet Fund for Lynch Syndrome (M.B.Y.), The Rasmussen Family Fund for Colorectal Cancer Research (M.B.Y.), The Scragg Family Fund (M.B.Y.), and The Stephani Batchelor and Andrew Robert Whittaker Family Fund for Lynch Syndrome Research (S.S.).
Author information
Authors and Affiliations
Contributions
Study conception and design (RLS, CU, CSF, SS, MBY)Acquisition and assembly of data (RLS, MH, CU, HU, MBY)Data analysis and interpretation (RLS, MH, CU, HU, SS, MBY)Statistical analysis (MH, HU)Obtained funding (CU, SS, MBY)Administrative support (SS, MBY)Study supervision (HU, SS, MBY)Drafting of the manuscript (RLS, MH, MBY)Critical revision of the manuscript (all authors)Approval of the final manuscript (all authors).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board. This study was performed in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
Dr. Uno reports prior consulting/advisory payments from Roche. Dr. Syngal reports prior consulting fees from Myriad Genetics Laboratories, Inc., and inventor/royalties from PREMM model. Dr. Yurgelun reports consulting/scientific advisory board fees and research funding from Janssen Pharmaceuticals as well as payments for peer review services from UpToDate. Drs. Sandoval, Horiguchi, Ukaegbu and Furniss have no financial or non-financial conflicts of interest to declare.
Prior publications
Preliminary data from this manuscript were presented as an abstract at the Annual Meeting of the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer (Nashville, TN) in November 11–13, 2022.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10689_2023_345_MOESM1_ESM.pdf
Supplementary Material 1: Histogram of PREMM5 scores among individuals with MMR-D/MSI-H colorectal cancer (n = 74), MLH1-deficient colorectal cancer (n = 49), and other patterns of MMR-D/MSI-H colorectal cancer, both with (LS+) and without (LS-) Lynch syndrome.
10689_2023_345_MOESM2_ESM.pdf
Supplementary Material 2: Performance characteristics of PREMM5 at different score thresholds for identifying Lynch syndrome carriers from noncarriers among individuals with MMR-D/MSI-H colorectal cancer. NPV, negative predictive value; PPV, positive predictive value.
10689_2023_345_MOESM3_ESM.pdf
Supplementary Material 3: Receiver operating characteristic (ROC) curve to discriminate Lynch syndrome carriers from noncarriers among individuals with MMR-D/MSI-H colorectal cancer. AUC, area under the ROC curve.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sandoval, R.L., Horiguchi, M., Ukaegbu, C. et al. PREMM5 distinguishes sporadic from Lynch syndrome-associated MMR-deficient/MSI-high colorectal cancer. Familial Cancer 22, 459–465 (2023). https://doi.org/10.1007/s10689-023-00345-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-023-00345-0