Abstract
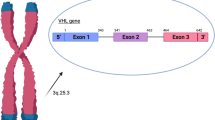
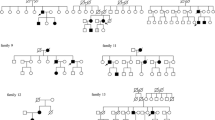
The general prevalence of the familial multi-organ tumor disorder, von Hippel–Lindau syndrome (VHL), was estimated to be 1 in 25–40,000 in western studies two decades back. Few studies were done in Indian sub-continent, amidst a surge in clinical reports on VHL specific manifestations. The syndrome is correlated with mutations of the gene VHL (located in Chr 3p25.3). We aimed to conduct a prospective case series describing phenotypic and genotypic characteristics in Indian population. The VHL-specific clinical and radiological features were collected from patients and family members. Genotypic changes such as deletion/duplication or point mutation in the VHL locus were identified using sequencing and MLPA. Thirty-one subjects, from fifteen families with diagnosed VHL, were included in the study. Multicystic pancreas was found in 71 % (22/31), CNS hemangioblastoma in 68 % (21/31), renal cell carcinoma and retinal angiomas in 23 % (7/31) each, pheochromocytoma in 9.7 % (3/31) of the population and endolymphatic sac tumor in one subject. Four families (9 subjects) had full length deletion of VHL, three families (4 subjects) had a deletion of exon 3, eight families (18 subjects) had different exonic, splice-site and intronic point mutations and one subject had a de novo in-frame indel in exon 1. Multicystic pancreas and CNS hemangioblastomas were the most common manifestations in our population. The phenotypic expression patterns in terms of tumorigenesis, tissue tropism and penetrance in comparison to the genotypic features were found to be different from previous correlative studies.


Similar content being viewed by others
References
von Hippel E (1904) On a very rare malady of the retina. Von Graefe Arch Ophthalmol 59:83–106 in German
Lindau A (1926) Studies on the small cysts of the brain. Pathogenesis and relationship to angiomatosis retinae. Acta Pathol Microbiol Scand (Supp. 1): Pl
Melmon KL, Rosen SW (1964) Lindau’s disease: review of the literature and study of a large kindred. Am J Med 36:595–617
Maher ER, Bentley E, Yates JR et al (1991) Mapping of the von Hippel–Lindau disease locus to a small region of chromosome 3p by genetic linkage analysis. Genomics 10(4):957–960
Seizinger BR, Smith DI, Filling-Katz MR et al (1991) Genetic flanking markers refine diagnostic criteria and provide insights into the genetics of Von Hippel Lindau disease. Proc Natl Acad Sci USA 88(7):2864–2868
Richards FM, Maher ER, Latif F et al (1993) Detailed genetic mapping of the von Hippel–Lindau disease tumour suppressor gene. J Med Genet 30(2):104–107
Latif F, Tory K, Gnarra J et al (1993) Identification of the von Hippel–Lindau disease tumor suppressor gene. Science 260(5112):1317–1320
Maddock IR, Moran A, Maher ER et al (1996) A genetic register for von Hippel–Lindau disease. J Med Genet 33(2):120–127
Maher ER, Iselius L, Yates JR et al (1991) Von Hippel–Lindau disease: a genetic study. J Med Genet 28(7):443–447
Dwarakanath S, Suri A, Sharma BS, Mehta VS (2006) Intracranial hemangioblastomas: an institutional experience. Neurol India 54(3):276–278
Padhi S, Sarangi R, Challa S, Bussary P, Panigrahi MK, Purohit AK (2011) A 10-year retrospective study of hemangioblastomas of the central nervous system with reference to von Hippel–Lindau (VHL) disease. J Clin Neurosci 18(7):939–944. doi:10.1016/j.jocn.2010.12.050
Dogra P, Kumar A, Singh A (2007) An unusual case of Von Hipple Lindau (VHL) syndrome with bilateral multicentric renal cell carcinoma with synchronous solitary urinary bladder metastasis. Int Urolo Nephrol 39(1):11–14. doi:10.1007/s11255-006-6656-5
Agarwal N, Kumar S, Dass J, Arora VK, Rathi V (2009) Diffuse pancreatic serous cystadenoma associated with neuroendocrine carcinoma: a case report and review of literature. J Pancreas 1:55–58
Jain V, Bishnoi A, Meena K et al (2011) Metachronous occurrence of multifocal phaeochromocytoma. Indian J Pediatr 78(5):620–622. doi:10.1007/s12098-010-0292-x
Rao PV, Lu X, Pattee P et al (2005) Peripheral genotype–phenotype correlations in Asian Indians with type 2 diabetes mellitus. J Assoc Physicians India 53:521–526
Wu P, Zhang N, Wang X et al (2012) Family history of von Hippel–Lindau disease was uncommon in Chinese patients: suggesting the higher frequency of de novo mutations in VHL gene in these patients. J Hum Genet 57(4):238–243. doi:10.1038/jhg.2012.10
Wang X, Zhang N, Ning X et al (2014) Higher prevalence of novel mutations in VHL gene in Chinese Von Hippel–Lindau disease patients. Urology 83(3):6751–6755. doi:10.1016/j.urology.2013.09.069
Bailes SM, Devers JJ, Kirby JD, Rhoads DD (2007) An inexpensive, simple protocol for DNA isolation from blood for high-throughput genotyping by polymerase chain reaction or restriction endonuclease digestion. Poult Sci 86(1):102–106
Crossey PA, Foster K, Richards FM et al (1994) Molecular genetic investigations of the mechanism of tumourigenesis in von Hippel–Lindau disease: analysis of allele loss in VHL tumours. Hum Genet 93(1):53–58
Beroud C, Collod-Beroud G, Boileau C, Soussi T, Junien C (2000) UMD (Universal mutation database): a generic software to build and analyze locus-specific databases. Hum Mutat 15(1):86–94. doi:10.1002/(SICI)1098-1004(200001)15:1<86:AID-HUMU16>3.0.CO;2-4
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G (2002) Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 30(12):e57
Cascon A, Escobar B, Montero-Conde C et al (2007) Loss of the actin regulator HSPC300 results in clear cell renal cell carcinoma protection in Von Hippel–Lindau patients. Hum Mutat 28(6):613–621. doi:10.1002/humu.20496
Wong WT, Agron E, Coleman HR et al (2007) Genotype–phenotype correlation in von Hippel–Lindau disease with retinal angiomatosis. Arch Ophthalmol 125(2):239–245
McNeill A, Rattenberry E, Barber R, Killick P, MacDonald F, Maher ER (2009) Genotype–phenotype correlations in VHL exon deletions. Am J Med Genet A 149A(10):2147–2151. doi:10.1002/ajmg.a.33023
Friedrich CA (2001) Genotype–phenotype correlation in von Hippel–Lindau syndrome. Hum Mol Genet 10(7):763–767
Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG Jr (2001) von Hippel–Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet 10(10):1019–1027
Simpson JL, Carson SA, Cisneros P (2005) Preimplantation genetic diagnosis (PGD) for heritable neoplasia. J Natl Cancer Inst Monogr 34:87–90. doi:10.1093/jncimonographs/lgi027
Stebbins CE, Kaelin WG Jr, Pavletich NP (1999) Structure of the VHL–ElonginC–ElonginB complex: implications for VHL tumor suppressor function. Science 284(5413):455–461
Kaelin WG Jr (2002) Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2(9):673–682
Kaelin WG, Iliopoulos O, Lonergan KM, Ohh M (1998) Functions of the von Hippel–Lindau tumour suppressor protein. J Intern Med 243(6):535–539
Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG Jr (1995) Binding of the von Hippel–Lindau tumor suppressor protein to Elongin B and C. Science 269(5229):1444–1446
Miller F, Kentsis A, Osman R, Pan ZQ (2005) Inactivation of VHL by tumorigenic mutations that disrupt dynamic coupling of the pVHL.hypoxia-inducible transcription factor-1alpha complex. J Biol Chem 280(9):7985–7996. doi:10.1074/jbc.M413160200
Ohh M, Park CW, Ivan M et al (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel–Lindau protein. Nat Cell Biol 2(7):423–427. doi:10.1038/35017054
Min JH, Yang H, Ivan M, Gertler F, Kaelin WG Jr, Pavletich NP (2002) Structure of an HIF-1alpha–pVHL complex: hydroxyproline recognition in signaling. Science 296(5574):1886–1889. doi:10.1126/science.1073440
Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W (2003) Regulation of microtubule stability by the von Hippel–Lindau tumour suppressor protein pVHL. Nat Cell Biol 5(1):64–70. doi:10.1038/ncb899
Domene C, Illingworth CJ (2012) Effects of point mutations in pVHL on the binding of HIF-1alpha. Proteins 80(3):733–746. doi:10.1002/prot.23230
Varela I, Tarpey P, Raine K et al (2011) Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469(7331):539–542. doi:10.1038/nature09639
Hammel PR, Vilgrain V, Terris B et al (2000) Pancreatic involvement in von Hippel–Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel–Lindau. Gastroenterology 119(4):1087–1095
van Asselt SJ, de Vries EG, van Dullemen HM et al (2013) Pancreatic cyst development: insights from von Hippel–Lindau disease. Cilia 2(1):3. doi:10.1186/2046-2530-2-3
Wu J, Jiao Y, Dal Molin M et al (2011) Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA 108(52):21188–21193. doi:10.1073/pnas.1118046108
Gomy I, Molfetta GA, de Andrade Barreto E et al (2010) Clinical and molecular characterization of Brazilian families with von Hippel–Lindau disease: a need for delineating genotype–phenotype correlation. Fam Cancer 9(4):635–642. doi:10.1007/s10689-010-9357-2
Franke G, Bausch B, Hoffmann MM et al (2009) Alu–Alu recombination underlies the vast majority of large VHL germline deletions: molecular characterization and genotype–phenotype correlations in VHL patients. Hum Mutat 30(5):776–786. doi:10.1002/humu.20948
Gautreau A, Ho HY, Li J, Steen H, Gygi SP, Kirschner MW (2004) Purification and architecture of the ubiquitous Wave complex. Proc Natl Acad Sci USA 101(13):4379–4383. doi:10.1073/pnas.0400628101
Bompard G, Caron E (2004) Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol 166(7):957–962. doi:10.1083/jcb.200403127
Kim H, D’Andrea AD (2012) Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev 26(13):1393–1408. doi:10.1101/gad.195248.112
Garner E, Smogorzewska A (2011) Ubiquitylation and the Fanconi anemia pathway. FEBS Lett 585(18):2853–2860. doi:10.1016/j.febslet.2011.04.078
Stickle NH, Chung J, Klco JM, Hill RP, Kaelin WG Jr, Ohh M (2004) pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development. Mol Cell Biol 24(8):3251–3261
Ohh M, Yauch RL, Lonergan KM et al (1998) The von Hippel–Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell 1(7):959–968
Acknowledgments
The authors wish to acknowledge Dr. Catherine Stolle of Children’s Hospital of Philadelphia for her contributions on the design and analyses of VHL genotyping. The authors also wish to acknowledge Mrs. Krishna Chandra and Mrs. Gisha Girish for their contributions in the VHL clinic coordination and SciGenome Laboratories, Kakkanad Kochi India for their contributions in sequencing. The study was supported by Kerala State Council for Science, Technology and Environment (222/SRSLS/2004/CSTE) and Indian Council of Medical Research (54/18/2011-HUM/BMS), Internal seed grant from Amrita Institute of Medical Sciences and VHL Family Alliance to ABP. Research fellowship to NV was funded by Council of Scientific and Industrial Research, India (09/963(0009)/2011-EMR-I).
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Vikkath, N., Valiyaveedan, S., Nampoothiri, S. et al. Genotype–phenotype analysis of von Hippel–Lindau syndrome in fifteen Indian families. Familial Cancer 14, 585–594 (2015). https://doi.org/10.1007/s10689-015-9806-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-015-9806-z