Abstract
Water scarcity is a problem that faces many regions. In a developing and arid countries, the need for fresh water is exponentially increasing. One of the most water consuming industries is the construction industry, especially in concrete manufacturing. This research is an evaluation of the Reliability of recycled wastewater to be used in concrete manufacturing. Two different wastewater treatment regimens are implemented: secondary and tertiary treated wastewater. The treated wastewater, either secondary or tertiary, replaces potable water with percentages of 25%, 50%, and 100%. The mixture of the two types of water is used in concrete mixing and curing. Control specimens with pure potable water are cast for the comparison. The experimental program investigated the cement setting, and for fresh concrete slump test is performed on all concrete mixtures. The hardened concrete is tested in compression and flexural strengths at ages of 7 days, 28 days, and 90 days. To investigate concrete durability, permeability test is conducted in addition to sulfate attack resistance. The concrete chemical composition is compared using the X-Ray Defragmentation (XRD) test. The results show that the treated wastewater may delay both the initial and final setting. The phenomenon is more pronounced in secondary treated wastewater. No significant effect on concrete performance in the fresh state is detected. For the compressive strength, both treatment regimens can be safely used. However, the flexural strength is reduced at the late ages. Using treated wastewater affects the permeability of concrete to be more durable and protect the reinforcement steel from rusting. For the XRD test, the results show different intensities and positions of the peaks of the different compounds encountered in the treated wastewater. Under sulfate attach, the treated wastewater specimens give higher expansion compared with potable water. In conclusion secondary and tertiary treated wastewater can be used in concrete mixing and curing. However, the manufactured concrete should be protected from sulfate attack to avoid excessive expansion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recycled water which is also known as reclaimed water is treating wastewater through eliminating all solids and suspended particles. Reclaimed water can be implemented in multiple applications including ground water recharging, irrigation, concrete manufacturing, and landscape applications (Angelakis & Snyder, 2015). A byproduct of water reclamation is a sludge that when mixed with biowaste, results in a compost that is extensively used by farmers (Maroušek et al., 2022). However, some plants can be cultivated directly on wastewater such as spirulina (Maroušek & Maroušková, 2021). In this research focus is on the implementation of recycled water in concrete manufacturing and curing. The suitability of treated wastewater in concrete manufacturing can be of additional economic value. For any investor in the field of treated wastewater, adding another customers’ sector such as the field of construction industry, which is an ever growing economic activity in any society, would encourage investment in this field (Akbari et al., 2021; Pavolová et al., 2021).
According to the American Society for Testing Materials (ASTM C1602-06, 2006), the suitability of non-potable water for concrete production is governed by two factors. Firstly, the difference between the setting time, which is the time required for cement paste to lose its plasticity and withstand some loads, of the cement paste using the non-potable water and the paste made with potable water should be from one hour early to one hour and half later. Secondly, the average compressive strength of concrete made using the unknown quality water should not be less than 90% of the average strength of the control cubes (Vaishali & Prashant, 2023).
Different researchers investigated the effect of using treated wastewater on the properties of cement paste, mortar and concrete (Tonetti et al., 2019).
Al-Joulani (2019) investigated the effect of replacing potable water with tertiary treated wastewater (TTWW) by different percentages. He reported that for water/cement (w/c) ratio of 0.5 to 0.6, an increase in the 14 and 28 days compressive strength with a range of 10–39% takes place. For higher w/c ratio of 0.7, the compressive strength decreased by 16% when the potable water was replaced by TTWW.
Several authors reported that there is no significant impact on concrete strength when secondary treated wastewater (STWW) or TTWW are used; either as partial replacement of potable water or as 100% of mixing water (Al-Jabri et al., 2011).
Others conducted compressive strength investigation while using STWW in mixing either with full replacement of potable water or partial, and reported enhancement in the results among those researchers are; Hegazy and Farouk (2020) and Reddy Babu et al. (2018).
For concrete consistency measured in terms of the slump test results, most authors reported that no significant effect was observed when the results were compared with results of concrete mixed with 100% potable water (Shaikh & Inamdar, 2016).
The impact on setting time attracted the interest of the researcher. This is attributed to the fact that the treated wastewater usually contains organic matter which might retard the cement hydration and affects the formation of Calcium Silicate Hydrate (CSH) (Mohe et al., 2022). The researchers reported an increase in the initial setting with different values. Among them Micheal et al. (2022). The impact is more pronounced for the final setting time (Ghrair & Al-Mashaqbeh, 2016).
Grey water is another source for mixing and curing water for concrete manufacturing. Grey water is defined as the wastewater of kitchens, baths, cleaning activities. Different researchers studied the gray water; either raw or treated, as an alternative to potable water. Among them (Ghrair et al., 2018) and Abushanab and Alnahhal (2021). Moreover, other researchers studied the effect of using industrial waste water in concrete manufacturing (Ahmed & Afifi, 2019). According to (Nasseralshariati et al., 2021), the industrial waste water affects the concrete durability and strength nonlinearly. It is worth mentioning that the implementation of wastewater in concrete manufacturing is not limited to mixing and curing. It can be extended to implementing the phosphorus in the wastewater treatment plants to produce a novel and eco-friendly char cement that can be activated using waste metals (Maroušek & Gavurová, 2022). This novel concrete possesses higher strength and less weight (Maroušek & Maroušková, 2021).
This research is concerned with investigation into the suitability of secondary treated wastewater (STWW) and tertiary treated wastewater (TTWS) for concrete mixing and curing. The investigation covers the effect on cement paste properties, the fresh concrete consistency and the hardened concrete strength and durability. All results will be compared with control case where potable water is used for mixing and curing.
2 Material
The materials used in the experimental work are obtained from the local market. The cement is Ordinary Portland Cement designated as CEM I with strength not less than 42.5 MPa after 28 days designated as grade 42.5. The cement bags are purchased from the local market in 50 kg bags and stored in a dry place for not more than 3 months. The fine aggregate is ordinary desert sand with specific gravity of 2.52 and bulk density of 1345 kg/m3. The coarse aggregate is crushed limestone with maximum nominal size of 10 mm. The mixing water is obtained from a local wastewater treatment plant whose capacity is 60,000 m3/day. Figure 1 shows the treatment procedure adopted in the plant. Eventually, the treatment scheme shown in Fig. 1 is the widely spread scheme for treatment plants of medium scale. However, it is not the only scheme. Other cheaper methods can be found around the world such as the method that incorporate biochar (Maroušek et al., 2019) and extracting water from fermentation residues (Maroušek & Gavurová, 2022).
The chemical composition of the STWW and TTWW are given in Tables 1 and 2 on the days when the treated wastewater is collected.
Where:
-
BOD: Biological oxygen demand
-
COD: Chemical oxygen demand
-
TSS: Total suspended solids in mg/l
-
TDS: Total dissolved salts in mg/l
-
D.O.: Dissolved oxygen in mg/l
-
R-Cl2: Residual Clorine in mg/l
It can be concluded that dissolved salts content in the STWW are higher than the those in the TTWW. This may reflect on the behavior of concrete in its fresh state as will be discussed later. Similarly, the BOD5 content in the STWW is more than the BOD5 in the TTWW. This, in turn, may affect the cement setting time.
To investigate the type of salts encountered in the treated water and to assess its suitability to concrete mixing and curing, chemical analysis is performed on both the STWW and TTWW. The results are shown in Table 3 compared with the ASTM C1602.
Despite that both the STWW and TTWW are conformable with the ASTM C1602 limits for mixing and curing water qualities, the behavior of the concrete made with both waters shows a significant different behavior compared with concrete made with potable water when exposed to sulfates as will be discussed in the results section. According to the codes of practice of different countries, the following aspects should be taken into consideration when using a non-potable water in concrete mixing.
-
The initial setting time for cement samples equipped with this water should not be less than 45 min, provided that the initial setting time does not differ by more than 25% of the initial setting time for samples of the same cement prepared with distilled water.
-
The final setting time for the cement samples equipped with this water does not exceed 12 h, provided that the final setting time does not differ by more than 25% of the final setting time for samples of the same cement prepared with distilled water.
-
The compressive strength of the standard mortar after 7 days in which this water was used is not less than 90% of the average compressive strength of similar samples prepared with distilled water at the same age.
3 Methodology
Different tests are conducted on cement paste and concrete in both fresh and hardened state. For cement paste, the initial and final setting time are determined for pastes that contain 100% potable water and other pastes where 25%, 50%, and 100% of the mixing water is replaced by either STWW or TTWW.
3.1 Concrete mix ingredients
For concrete mix design, the absolute volume method is used to determine the concrete mix proportions. The concrete ingredients ratios for cement: sand: coarse aggregate are 1:2:4. The water/cement ratio is kept at 0.45. For mixing water, four different combinations are used. The control mix contains 100% potable water and other three mixes are used where 25%, 50%, and 100% either STWW or TTWW replaces potable water. It is worth mentioning that the type of water used for mixing is the same water used for curing. The ingredients for all mixes are shown in Table 4. The mixing process is done using a rotary mixer for 2 min.
3.2 Concrete consistency tests
The concrete consistency test adopted in this research is the slump test performed immediately after mixing concert according to ASTM C143 (2020).
3.3 Hardened concrete testing
For hardened concrete, the investigation includes compressive strength and flexural strength at the ages of 7, 28, and 90 days. The hardened concrete durability testing includes permeability and sulfate resisting. Moreover, XRD investigation is conducted for the cement paste including 100% potable water, 100% STWW, and 100% TTWW. Details of all tests are presented in the following sections.
For compressive strength determination cube specimens of 15.8 cm side are used. The specimens are put in compressive machine model ELE 1886B0033 and at a loading rate of 14 MPa/min. For the modulus of rapture determination four points bending test is performed. The test setup is shown in Fig. 2. The beam dimensions are 100 × 100 × 600 mm and the span is 500 mm.
4 Concrete durability investigation
Concrete durability is an important property that signifies a concrete that can withstand the environmental conditions while maintaining same performance in the context of strength and serviceability. Different aspects define the concrete durability such as abrasion, permeability, chemical attacks resistance, and weathering conditions resistance.
4.1 Permeability testing
Permeability is an important property of concrete that largely affects its durability as permeable concrete is prone to deteriorate faster due to chemical attacks. The test is conducted at age of 28 days according to BS EN 12390-8 (2003). The specimens are concrete cubes with dimensions 15.8 cm × 15.8 cm × 15.8 cm with roughened surface placed in the apparatus and subjected to water pressure of about (500 ± 50) kPa for (72 ± 2) h.
The concrete cubes are removed after exposure to the water pressure and split to measure the water penetration depth as shown in Fig. 3.
4.2 Sulfate attack testing
The other aspect that characterizes the concrete durability is the chemical attack resistance. Usually, the chemical attack results in either dissolution of concrete components or chemical reaction resulting in expansion and subsequent deterioration. One of the most harmful attacks on concrete is sulfate. Sulfates usually react with the free Calcium Hydroxide Ca (OH)2 to form Calcium Sulfate (Gypsum) as follows:
The Calcium Sulfate in its turn reacts with Calcium Aluminate to form Calcium Sulfoaluminate according to the equation:
The resulting chemical component occupied a greater volume than that of the compounds it replaces so disruption of hardened concrete takes place.
The test is performed according to ASTM-C452 (ASTM C452-21, 2021). The specimens’ dimensions are 25 × 25 × 285 mm. The percentage of gypsum to cement is determined such that the mixture contains 7.0% mass of SO3, the ratio of cement to gypsum can be determined using the following equation:
where:
-
c = percentage of SO3 content of the Portland cement,
-
g = percentage of SO3 content of the gypsum
To determine the percentage of SO3 in both cement and gypsum, the X-ray fluorescence (XRF) test is performed.
The results of the XRF of both cement and gypsum are shown in Tables 5 and 6 respectively.
Substituting in Eqs. 1 and 2, the percentages of cement and gypsum in the mixture should be 90.17% and 9.83% respectively.
Nine test specimens of cement and gypsum mortar with dimensions 25 × 25 × 285 m are prepared. Three specimens for the percentages 100% Potable Water, three with 100% STTW and three with 100% TTWW.
According to ASTM –C452 (ASTM C452-21, 2021), The specimens are cured in the moist closet at 23°C for 22–23 h. The specimens are then removed from the molds, properly identified, and placed in water at 23 °C for at least 30 min. prior to making the initial length measurement. After initial length measurement, the specimens are stored in water which is replenished with fresh water every 7 days for the first 28 days and every 28 days thereafter. The mold and the specimens are shown in Fig. 4.
4.3 XRD analysis
X-Ray diffraction analysis (XRD) is a nondestructive technique that provides detailed information about the crystallographic structure, chemical composition, and physical properties of a material. XRD works by irradiating a material with incident X-rays and then the intensities and scattering angles of the X-rays that leave the material are measured. This technology is used by many researchers to investigate the chemical compounds encountered in concrete with different additives. Among those researchers are Silva et al (2020), Reddy and Naqash (2019), Kontoleontos et al. (2013), and Singh et al. (2021). For this research, the XRD analysis test is conducted on three specimens: control specimen, 100% STWW, and 100% TTWW.
5 Results
5.1 Initial and final setting results
The initial and setting time result for both STWW and TTWW are shown in Figs. 5 and 6. It can be concluded that all specimens lie in the specification of limit of more than 45 min. However, when compared with the control specimen which records 120 min. for initial setting, it can be concluded that all specimens record an increase in the setting time with about 1%. Nevertheless, this increase is acceptable by all specifications.
For final setting time, the same behavior is encountered. The final setting time for all specimens with STWW and TTWW is more than the final setting time of the control specimen. The increase is around 1%.
This increase can be attributed to the existence of more organic matters in the treated wastewater other than potable water.
5.2 Slump test results
The slump test results are shown in Fig. 7. The control specimen is a dry mix with slump 30 mm. The slump increases with the increase of the replacement percentage of treated wastewater. This can be attributed to the fine suspended solids encountered in the wastewater which work as lubricants. This phenomenon is also reported by other researchers (Asadollahfardi et al., 2016; Hegazy & Farouk, 2020).
5.3 Compressive strength results
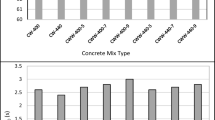
The results of compressive strength at ages of 7, 28 and 90 days are shown in Figs. 8, 9, and 10. It can be concluded that all mixes strength fulfills the specification limit with strength not less than 90% of the strength of the control specimen. The best results are obtained for the mix with 25% STWW. For this mix, the strength is enhanced by around 10% of the control mix. This observation can be found in the work of Ooi et al. (2012).
5.4 Flexural strength result
The flexural strength results for all mixes are shown in Figs. 11, 12 and 13. It can be concluded that the flexural strength at an early age for mixes containing any percentage of treated wastewater is around 83% of the control mix. However, at late ages all mixes prove to be satisfying.
5.5 Permeability test results
The permeability test results are shown in Table 7. The results show that the penetration depth for all specimens containing treated wastewater is less than the penetration of the control specimen which indicates a good concrete quality.
5.6 Sulfate resisting test results
The average linear expansion of the three mixes; control, 100% STWW, and TTWW are shown in Table 8. It can be observed that the linear expansion of specimens containing any percentage of treated wastewater exhibits more expansion than the control specimen. This phenomenon is more pronounced in the TTWW specimens. Comparing the chemical composition of the STWW and TTWW, Table 3, it can be attributed to the sulfate content in the TTWW which is more than the STWW.
5.7 XRD analysis results
The XRD results for the three specimens; control, 100% STWW, and 100% TTWW are shown in Figs. 14, 15 and 16 respectively. The XRD analysis illustrates that there are four matched chemical compounds in the control, secondary and tertiary examined specimens. These chemical compounds are Ca2SiO4H2O, Ca (OH)2, SiO2 and CaCo3.
For control specimens, it is noticed that there are different peaks with different intensities at 2θ = 27°, 35°, 50° and 56° for SiO2, Ca (OH)2, CaCo3 and Ca2 (SiO4) (H2O) respectively, as shown in Fig. 14.
For 100% STWW specimen, Fig. 15, it is noticed that there are obvious differences in the position and the intensity for the four chemical compounds. Firstly, SiO2 peak is in the same position as the control specimens but with smaller intensity. But CaCo3 peak changes its position to be at 2θ = 30° with a little difference in the intensity. While the peak of Ca (OH)2 changes its position to be at 2θ = 40° with a little difference in the intensity. Also, Ca2 (SiO4) (H2O) changes its position to be at 2θ = 70° with a little decrease in the intensity.
Same differences in the intensity and position can be observed for 100% TTWW specimen as shown in Fig. 16. Firstly, SiO2 peaks have more positions with various intensities than the control specimens. But CaCo3 peak changes its position to be at 2θ = 30° and 40° respectively, with variable differences in the intensities. While the peak of Ca (OH)2 changes its position to be at 2θ = 34° and 47° respectively, with variable differences in the intensities. Also, Ca2 (SiO4) (H2O) changes its position to be at 2θ = 27°, 40° and 50° respectively, with variable differences in the intensities.
6 Discussion
Several experiments are conducted on concrete mixed and cured using treated wastewater with different contents. It can be observed that the chemical compounds encountered in all mixes are the same, however, with different intensities. The results show that this change in the intensities has an impact on the produced concrete in terms of compressive and flexural strength yet within specification. It also delays cement setting, yet such an effect can be override using chemical admixture. The effect is more pronounced for the volumetric changes due to sulfate attack. This is attributed to the different chemical components encountered in the treated water. For fresh concrete properties, no significant impact is observed. The results obtained from all the above-mentioned experiments can be used in conjunction with artificial intelligence to predict the behavior of concrete mixed with different water qualities and compounds in terms of strength, consistency, and durability (Zvarikova et al. 2021). From the financial point of view, and for arid countries that depend on desalination of sea water for all purposes, the cost to desalinate 1 m3 of sea water is around 6$ while the cost of treatment of 1 m3 of wastewater is around 3 $ (Alkhudhiri et al., 2019). For ingredients to produce 1 m3 of concrete as shown in Table 4, and taking into account the prices of concrete materials in an arid country like the Saudi Arabia (https://www.ceicdata.com/en/saudi-arabia/average-construction-materials-price), the cost of 1 m3 of concrete will cost 39.05$ for the use of desalinated water. For the use of treated wastewater, the cost will be around 38$. Thus, the reduction on concrete materials cost is around 1.3% not including the cost of curing water. The main advantages to use treated wastewater in concrete manufacturing and curing is to save the energy required to produce desalinated water and to use a byproduct of the essential industry of water reclamation.
7 Conclusion
A study on the reliability of using treated wastewater in concrete mixing and curing is conducted. Focusing on concrete consistency and strength, the results prove that the treated wastewater can be safely used. The setting time increases in the case of using treated wastewater when compared with concrete mixed with potable water. The maximum increase in initial setting time occurs in the specimens mixed with purely secondary treated wastewater. The increase is 40 min. This increase can be taken into consideration when transporting concrete and controlled using chemical admixtures. The same phenomenon can be observed for the final setting time. For compressive strength and flexural strength, both treated water regimens satisfy the specification limits as all specimens do not deviate from the control specimens by not more than 10%. The concrete mixed and cured using wastewater show less permeability. The concrete durability in terms of sulfate attack resistance shows considerable increase in the linear expansion for mixes where treated wastewater is implemented compared with potable water. The XRD analysis shows different chemical compounds’ position and intensities due to the various chemical compounds found in the treated wastewater. It can be concluded that treated wastewater can be safely used for concrete mixing and curing. Special care should be given to concrete in sulfate containing medium. This conclusion can be of benefit for many countries that suffer scarcity in water and need to reuse water from different sources. However, further research is needed for deeper investigation on the durability of concrete mixed with treated wastewater in harsh environments, specially by using sulfate resisting cements. Other durability parameters also need more investigation such as abrasion and freeze and thaw behavior. Moreover, investigation on the crystallization level is suggested to correlate with the concrete properties.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abushanab, A., & Alnahhal, W. (2021). Combined effects of treated domestic wastewater, fly ash, and calcium nitrite toward concrete sustainability. Journal of Building Engineering, 44, 103240. https://doi.org/10.1016/j.jobe.2021.103240
Ahmed, R., & Afifi, M. (2019). Utilizing industrial wastewater in production of concrete: Experimental & feasibility study. In Proceedings, annual conference—Canadian society for civil engineering, 2019-June (pp. 1–9).
Akbari, M., et al. (2021). The dynamic effect of micro-structural shocks on private investment behavior. Acta Montanistica Slovaca, 26(1), 1–17. https://doi.org/10.46544/AMS.v26i1.01
Al-Jabri, K. S., et al. (2011). Effect of using wastewater on the properties of high strength concrete. Procedia Engineering, 14, 370–376. https://doi.org/10.1016/j.proeng.2011.07.046
Al-Joulani, N. M. A. (2019). Effect of using tertiary treated wastewater from nablus wastewater treatment plant (NWWTP), on some properties of concrete. International Journal of Innovative Technology and Exploring Engineering, 8(11), 2460–2466. https://doi.org/10.35940/ijitee.K1709.0981119
Alkhudhiri, A., Darwish, N. Bin., & Hilal, N. (2019). Analytical and forecasting study for wastewater treatment and water resources in Saudi Arabia. Journal of Water Process Engineering, 32, 100915. https://doi.org/10.1016/j.jwpe.2019.100915
Angelakis, A. N., & Snyder, S. A. (2015). Wastewater treatment and reuse: Past, present, and future. Water (Switzerland), 7(9), 4887–4895. https://doi.org/10.3390/w7094887
Asadollahfardi, G., et al. (2016). Use of treated domestic wastewater before chlorination to produce and cure concrete. Construction and Building Materials, 105, 253–261. https://doi.org/10.1016/J.CONBUILDMAT.2015.12.039
ASTM C143-20. (2020). Standard test method for slump of hydraulic-cement concrete.
ASTM C1602-06. (2006). Standard specification for mixing water used in the production of hydraulic cement concrete.
ASTM C452-21. (2021). No standard test method for potential expansion of portland-cement mortars exposed to sulfate itle.
Ghrair, A. M., & Al-Mashaqbeh, O. (2016). Domestic wastewater reuse in concrete using bench-scale testing and full-scale implementation. Water (Switzerland), 8(9), 366. https://doi.org/10.3390/w8090366
Ghrair, A. M., et al. (2018). Influence of grey water on physical and mechanical properties of mortar and concrete mixes. Ain Shams Engineering Journal, 9(4), 1519–1525. https://doi.org/10.1016/j.asej.2016.11.005
Hegazy, M., & Farouk, M. (2020). Effect of using secondary treated wastewater in production and curing of concrete. Journal of Materials and Environmental Science, 11, 1493–1502.
Kontoleontos, F., et al. (2013). Dry-grinded ultrafine cements hydration. Physicochemical and microstructural characterization. Materials Research, 16(2), 404–416. https://doi.org/10.1590/S1516-14392013005000014
Maroušek, J., & Gavurová, B. (2022). Recovering phosphorous from biogas fermentation residues indicates promising economic results. Chemosphere, 291, 133008. https://doi.org/10.1016/j.chemosphere.2021.133008
Maroušek, J., & Maroušková, A. (2021). Economic considerations on nutrient utilization in wastewater management. Energies, 14(12), 3468. https://doi.org/10.3390/en14123468
Maroušek, J., Strunecký, O., & Stehel, V. (2019). Biochar farming: defining economically perspective applications. Clean Technologies and Environmental Policy, 21(7), 1389–1395. https://doi.org/10.1007/s10098-019-01728-7
Maroušek, J., et al. (2022). Economic impacts of soil fertility degradation by traces of iron from drinking water treatment. Environment, Development and Sustainability, 24(4), 4835–4844. https://doi.org/10.1007/s10668-021-01636-1
Micheal, A., Abdel Salam, H., & Ghanem, G. M. (2022). Implementation of secondary and tertiary treated wastewater in concrete manufacturing. In IOP conference series: Earth and environmental science. https://doi.org/10.1088/1755-1315/1056/1/012046
Mohe, N. S., Shewalul, Y. W., & Agon, E. C. (2022). Experimental investigation on mechanical properties of concrete using different sources of water for mixing and curing concrete. Case Studies in Construction Materials, 16, e00959. https://doi.org/10.1016/j.cscm.2022.e00959
Nasseralshariati, E., et al. (2021). The effect of incorporating industrials wastewater on durability and long-term strength of concrete. Materials, 14(15), 4088. https://doi.org/10.3390/ma14154088
Ooi, S. L., et al. (2012). Treated effluent in concrete technology. Jurnal Teknologi [Preprint] (August 2014). https://doi.org/10.11113/jt.v34.648
Pavolová, H., et al. (2021). The analysis of investment into industries based on portfolio managers. Acta Montanistica Slovaca, 26(1), 161–170. https://doi.org/10.46544/AMS.v26i1.14
Reddy Babu, G., Madhusudana Reddy, B., & VenkataRamana, N. (2018). Quality of mixing water in cement concrete "a review. Materials Today: Proceedings, 5, 1313–1320. https://doi.org/10.1016/j.matpr.2017.11.216
Reddy, P. N., & Naqash, J. A. (2019). Experimental study on TGA, XRD and SEM analysis of concrete with ultra-fine slag. International Journal of Engineering, Transactions B: Applications, 32(5), 679–684. https://doi.org/10.5829/ije.2019.32.05b.09
Shaikh, M. A. R., & Inamdar, D. V. M. (2016). Study of Utilization of Waste Water in Concrete. IOSR Journal of Mechanical and Civil Engineering, 13(04), 105–108. https://doi.org/10.9790/1684-130402105108
Silva, L. A., et al. (2020). XRD investigation of cement pastes incorporating concrete floor polishing waste. Ceramica, 66(380), 373–378. https://doi.org/10.1590/0366-69132020663802956
Singh, M., et al. (2021). Recycling of waste bagasse ash in concrete for sustainable construction. Asian Journal of Civil Engineering, 22(5), 831–842. https://doi.org/10.1007/s42107-021-00349-0
Tonetti, A. L., et al. (2019). Environmentally friendly interlocking concrete paver blocks produced with treated wastewater. Water Science and Technology: Water Supply, 19(7), 2028–2035. https://doi.org/10.2166/ws.2019.078
Vaishali, S., & Prashant, K. (2023). Potential use of treated sewage in concrete to meet water crises in megacities. Waste and Resource Management, 176(2), 58–69.
Zvarikova, K, et al. (2021). Sustainable Industry 4.0 wireless networks, smart factory performance, and cognitive automation in cyber-physical system-based manufacturing. Journal of Self-Governance and Management Economics, 9(4), 9–20. Available at: https://www.ceeol.com/search/article-detail?id=1005632
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Micheal, A., Salam, H.A.E. Reliability of using secondary and tertiary treated wastewater in concrete mixing and curing. Environ Dev Sustain (2024). https://doi.org/10.1007/s10668-024-04613-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10668-024-04613-6