Abstract
Malaria has a significant impact on the lives of many in Ghana. It is one of the key causes of mortality and morbidity, resulting in 32.5% of outpatient visits and 48.8% of under 5-year-old hospital admissions. Future climate change may impact on this risk. This study aims at estimating the impact of climate variables and health facilities on malaria prevalence in Ghana using regional data from January 2012 to May 2017. This study links data at a regional level on malaria cases with weather data to evaluate the impact that changes in weather may have on malaria prevalence in Ghana. The results of fixed-effect modelling show that the maximum temperature has a statistically significant negative impact on malaria in the context of Ghana, and rainfall with a lag of two months has a positive statistically significant impact. Adapting to climate change in Ghana requires a better understanding of the climate-malaria relationship and this paper attempts to bridge this gap.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Malaria is caused by parasitic protozoans of the genus Plasmodium and is mainly transmitted by the female Anopheles mosquito. The World Health Organization (WHO) reports that nearly 50% of the global population was at risk of malaria in 2015 [1]. In the same year, about 212 million malaria cases and 429,000 malaria deaths were reported [1]. These deaths comprised mostly children in Sub-Saharan Africa. Despite these figures, it was reported that malaria prevalence and deaths across Africa had drastically reduced over the previous 15 years mainly due to malaria control interventions such as insecticide-treated bed nets, indoor residual spraying, and rapid treatment of clinical cases with the more effective artemisinin-based combination therapy [2]. However, malaria remains one of the primary causes of mortality and morbidity in Ghana, and accounts for 38% of outpatient visits, 36% of hospital admissions, and estimated 30% of all deaths in children under 5 years of age [3]. Ghana reported only 599 malaria deaths in 2017, but the WHO estimated about 10,900 total malaria deaths in that same year [4]. In comparison to the first quarter of 2015 when approximately 17,820 suspected cases per day were recorded, in the first quarter of 2016 there were 18,443 suspected cases per day [5]. The number of malaria cases may be underestimated as some cases are unreported. The Ghana Statistical Service estimates that about 37% of malaria cases in Ghana go unreported [6]. Malaria is also a major cause of poverty and low productivity in Ghana, with the total economic cost of malaria in Ghana being estimated at US$6.68 million in 2014, with 3,913 workdays lost from 2012 to 2014 [7].
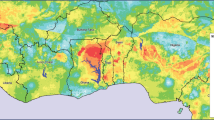
Ghana comprises three agro-ecological zones: savannah, forest, and coastal. Historically there were 10 regions, but this changed in December 2018 and there are now 16 regions in Ghana [8]. Each zone has unique characteristics, due to variations in temperature, rainfall, and humidity, that influence the malaria vector and parasite, and subsequent transmission [9]. A wide range of factors, including climate and environmental changes, influence malaria prevalence [10, 11]. Climate change is expected to increase malaria exposure by up to 28% in person-months [12] by 2100 especially in high-altitude areas in Africa [13]. Temperature and rainfall are the key drivers of mosquito population dynamics and subsequent malaria transmission [14, 15]. These factors affect the seasonal and inter-annual variability of malaria [16] and can increase or decrease malaria transmission [17].
Temperatures above certain thresholds are known to be unfavourable to mosquitoes and parasites, leading to a decline in malaria prevalence. Temperatures between 20 and 30 °C have been found to be ideal for adult mosquito survival [15, 18, 19] while the parasite has optimum temperatures of 32–33 °C [14]. Any temperature outside this range can substantially alter the extrinsic incubation period of the parasite development in the mosquito. Malaria transmission is also dependent on rainfall, given the need for available aquatic habitats for the mosquito vector. Previous studies have shown that rainfall and temperature are significantly correlated with malaria prevalence with a lag time of 1 to 2 months [16, 20], and 2 to 5 months respectively [16].
Other factors such as knowledge and awareness of malaria, housing, income, health facilities, per capita gross domestic product (GDP), humidity, and education level have been found to have significant effects on malaria prevalence [21,22,23,24].
Unlike other malaria-endemic countries, such as Uganda and Kenya, where the relationship between malaria and climate has been studied extensively, fewer studies have been conducted in Ghana [9, 23, 25]. The main challenge with understanding how climate variables affect malaria prevalence in Ghana is the scarcity of continuous data covering a sizeable proportion of Ghana and spanning several years. Our study estimates the influence of the key climate drivers (temperature and rainfall) on malaria prevalence in Ghana using matching malaria prevalence and climate data at monthly levels over the period January 2012 to May 2017. Better understanding of how climate influences malaria incidence rates can help inform strategies for managing malaria and inform future adaptation to climate change.
2 Methods
2.1 Study Design
Longitudinal data, collected monthly from January 2012 to May 2017, are used to estimate the effect of climate variables (temperature, rainfall) and non-climate variables (health facilities) on estimated malaria prevalence across the ten regions of Ghana (Fig. 1): Ashanti, Brong Ahafo, Central, Eastern, Greater Accra, Northern, Upper East, Upper West, Western, and Volta. Malaria prevalence is estimated based on laboratory confirmed hospital cases, as described in Sec. 2.2. At the time of our study, there were only ten administrative regions in Ghana but in 2018, the Government of Ghana created six new regions by splitting four of the pre-existing regions: Northern, Volta, Western and Brong Ahafo. The geographical boundaries of Ghana did not change. Although this study mainly aims at estimating the impact of climate variables on malaria, it is important to include health facilities as a non-climatic factor. This is because all malaria cases were based on clinical reports, hence the number of health facilities per region is likely to influence the estimated malaria prevalence.
2.2 Malaria and Health Facilities Data
Clinically confirmed malaria case data at regional levels were obtained from the Centre for Health Information Management (CHIM): the focal unit for collating, analysing, reporting and presenting health service information in the Ministry of Health, Ghana. The data were anonymized. Malaria prevalence was expressed as malaria cases per 100,000 population based on the regional 2010 Ghana population and housing census [26]. Malaria prevalence per region was calculated as:
This was done to account for increasing population per year. Annual growth rate is the rate at which regional population increases for a given year. The annual growth rate for each region was derived from the regional decadal growth rate from the 2010 Ghana census as specified by the Ghana Statistical Service.
Clinical reports of malaria cases are determined in part by the extent of health care provision in a region. Therefore, data for the number of health facilities across regions were obtained from CHIM and Policy Planning Monitoring and Evaluation Division of the Ghana Health Service [27]. Health facilities comprise government, Christian Health Association of Ghana (the second largest healthcare provider in Ghana), quasi government and private health institutions. These include hospitals, health centres, clinics, Community-based Health and Planning Services (CHPS) and maternity homes. Similar to malaria prevalence, health facilities for each region was expressed per 100,000 population.
2.3 Climate Data
Monthly rainfall and monthly temperature data were obtained from two sources: Ghana Meteorological Agency (GMet) office in Accra, Ghana and the National Climate Data Centre (NCDC), now National Centres for Environmental Information (NCEI), available online [https://www.ncdc.noaa.gov/cdo-web/datasets/GSOM/locations/FIPS:GH/detail]. Climate data (minimum temperature, maximum temperature and rainfall) was extracted for all stations within each region from both data sets and merged. The average across the sites within a region for a specific year and month was calculated and used for data analysis.
2.4 Statistical Methods
First, the data was explored by computing summary regional statistics of malaria prevalence, and climate variables. Plots were then made describing the relationships between variables. These include scatterplots fitted with linear regression lines and local regression curves. Additional descriptive plots were made to show heterogeneity of malaria prevalence, and climate variables across the study period.
To estimate the impact of temperature and rainfall on malaria prevalence, we applied panel data analysis, using procedures outlined by Torres-Reyna [28] and Croissant and Millo [29]. Two modelling approaches were used: fixed-effects models (FEM) and random effects models (REM). All variables, both dependent and independent, were standardized by converting to z-scores and used in fitting these models.
A major assumption made of the FEM is that both the outcome and the predictor variables may be affected by time-invariant unobservable characteristics [30]. Not taking this into account would lead to inconsistent estimates of the model’s parameters. The general equation for FEM is
where yit is the outcome variable (malaria prevalence) for individual i (region) at time t (year, month), xit is a vector of predictor variables which vary with time, αi represents individual and time-invariant observable variables as well as time-invariant unobservable factors and uit is the error term associated with the model.
Through the fixed-effect transformation of the model, the variable αi is cancelled out. This allows to consistently estimate the model parameters.
Unlike the FEM, REM assumes that the explanatory variables are uncorrelated with (unobservable) time invariant factors [31]. This model allows time invariant variables to be included in the model rather than to have them cancelled out as in FEM. The general equation for REM is
where yit is the outcome variable (malaria prevalence) for individual ‘i’ (region) at time ‘t’ (year, month), xit is a vector of predictor variables which vary with time and vit is the composite error term associated with the model, with vit = αi + uit.
Fixed-effect models (FEM) and random effect models (REM) were estimated. After fitting the FEM and REM models, the Hausman test [31, 32] was used to select a suitable model. The Hausman test of FEM against REM for only climate models indicated that FEM was the preferred model (chisq = 13.106, df = 2, p-value = 0.001). Similarly, the Hausman test between FEM and REM for all variables (including number of health facilities per 100,000) indicated that FEM is the preferred model (chisq = 37.053, df = 3, p-value = 4.485e − 08). This means that the null hypothesis that the individual effects of the explanatory variables are random and uncorrelated was rejected therefore FEM was selected.
The cross correlation function (CCF) was then used to determine the lags to be used for maximum temperature and rainfall that were significantly correlated with malaria prevalence. For CCF of maximum temperature and malaria prevalence, the dominant cross correlations were observed between lag = − 4 and lag = − 10, while the dominant cross correlations were observed for CCF for rainfall and prevalence between lag = − 2 and lag = − 3. Multiple ordinary least square regressions were run with the (past) lags 4, 5, 6, 7, 8, 9 and 10 of maximum temperature, and (past) lags 2 and 3 of rainfall. Only the lags with statistically significant coefficients were included in the final models to assess their effect on prevalence. These were lags 2 and 5 for rainfall and maximum temperature respectively. The pattern of the peak in CCF is important for determining whether the outcome variable may be considered as a predictor [33]. A tapering pattern following a peak in the CCF plots suggests that the lag 1 value of the outcome variable is a potential predictor. Therefore, lag 1 of prevalence was included in the final model. However, inclusion of the dependent variable in the model is likely to create a bias. To control for the bias associated with adding the lagged prevalence as a predictor, the ‘Arellano-Bond’ method was applied [33]. This controls for both heteroskedasticity and autocorrelation. The final FEM model equation is
where uit is the error term associated with the model.
The R statistical package version 3.4.1 (R Core Development Team, Vienna) was used for all statistical analyses — which involved using the packages ggplot2 for visualizations, and plm for panel linear models.
3 Results
3.1 Descriptive Statistics and Correlation Analysis
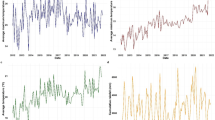
Monthly climate variables (temperature and rainfall) and corresponding monthly malaria prevalence were analysed across the ten regions of Ghana. A total of 20,165,123 laboratory-confirmed clinical malaria cases were recorded over the 5-year study period (from January 2012 to May 2017). Table 1 presents an overview of the prevalence data for malaria in the different regions. The Upper East region recorded the highest prevalence while Greater Accra region recorded the lowest. Malaria prevalence gradually declined from 2012 to 2017 (Fig. 2). A major peak in prevalence was observed in each year (Fig. 3) from August to October, and the lowest prevalence from January to April. These periods of high and low malaria prevalence coincide with the wet and dry seasons respectively.
Ashanti region recorded the highest monthly rainfall (528.32 mm), over the study period. However, some months were completely dry with no rainfall recorded (0 mm). All regions had dry months. Using the Köppen-Geiger climate classification [34], where months with rainfall below 60 mm in tropical areas are considered as dry, the Central region had the highest number of dry months and the Volta region had the lowest number of dry months. The mean rainfall across the country was 65.92 mm. The region with the highest average rainfall was Brong Ahafo (90.53 mm) while the Central region had the lowest average rainfall (31.21 mm) (Table 4 in Appendix). Two major rainfall peaks were observed for each year with the first peak generally higher than the second peak except in 2016 (Figs. 4a and 7 in Appendix). The highest rainfall was recorded from April to June, and from August to September, with the lowest rainfall being recorded from October to January (Figs. 4a and 7 in Appendix ).
The lowest temperature (16.1 °C) was recorded in the Northern region, while the highest minimum temperature was in the Western region (29.3 °C)) (Table 5 in Appendix). The Western region had the lowest maximum temperature (23.3 °C), and Upper East had the highest maximum temperature (42.1 °C) (Table 6 in Appendix). Minimum temperature was highest from March to April and lowest from December to January (Fig. 4b and Fig. 8 in Appendix). The highest maximum temperature was recorded from February to March, and the lowest maximum temperature was observed in August (Fig. 4b and Fig. 9 in Appendix).
Overall, there was a weak positive association between malaria prevalence and rainfall (ρ = 0.06, p-value > 0.05) (Fig 5a). The three northern regions (Northern, Upper East and Upper West) showed the strongest positive correlation between malaria prevalence and rainfall: Northern (ρ = 0.45, p-value < 0.05), Upper East (ρ = 0.31, p-value < 0.05) and Upper West (ρ = 0.44, p-value < 0.05) (Fig. 6a). Malaria prevalence showed a significant negative correlation with mean temperature over the whole country (ρ = − 0.17, p-value < 0.05) (Fig 5b).
Rainfall precedes malaria prevalence peaks by 1 to 3 months (Fig. 4). The lowest rainfall records were followed by the lowest prevalence. For example, a decline in rainfall to nearly 0 mm in January 2013 was followed by a decline in malaria prevalence of about 1500 (per 100,000 inhabitants) in March 2013.
3.2 Effects of Rainfall and Maximum Temperature on Malaria Prevalence
Our FEM model explains approximately 61% of the variation in malaria prevalence using rainfall, maximum temperature, malaria prevalence at lag 1 month, maximum temperature at lag 5 months, rainfall at lag 2 months and health facilities as the independent variables. These lags were included because they were found to have the strongest and significant correlation with the dependent variable, malaria prevalence, using the cross correlation function (CCF) (Figs. 10 and 11 in Appendix).
Maximum temperature, maximum temperature at a lag of 5 months and rainfall at a lag of 2 months emerged as statistically significant predictors of malaria prevalence (Table 2). An increase in maximum temperature is associated with a decrease in malaria prevalence. For one standard deviation increase in maximum temperature, malaria prevalence decreases by approximately 0.22 standard deviation on average. In contrast, maximum temperature at a lag of 5 months is associated with an increase in malaria prevalence by approximately 0.11 standard deviation. Although small, maximum temperature and maximum temperature at a lag of 5 months have a greater effect on malaria prevalence compared to rainfall (|beta estimate|= 0.04, p-value > 0.05) and rainfall at a lag of two months (|beta estimate|= 0.04, p-value < 0.05). As expected, an increase in the number of health facilities is associated with an increase in the number of reported malaria cases (|beta estimate|= 0.23, p-value < 0.05). Overall, the strongest predictor is malaria prevalence at lag 1 (|beta estimate|= 0.68, p-value < 0.05). From our model, the effect of maximum temperature, rainfall and health facilities on malaria prevalence is not uniform across the regions. Regional fixed-effect estimates (Table 3) are highest for the Upper East (|estimate|= 0.392, p-value < 0.05) and lowest for Greater Accra (|estimate|= 0.162, p-value < 0.05).
The blue line represents the ‘line of best fit’ using local polynomial regression fitting, and the grey shading represents the 95% confidence interval around the line of best fit.
The blue line represents the ‘line of best fit’ using linear regression and the grey shading represents the 95% confidence interval around the line of best fit.
4 Discussion
Malaria remains an important public health concern in Sub-Saharan Africa despite substantial gains in malaria treatment and prevention in recent decades. We investigated the relationship between climate variables (temperature, rainfall) and malaria prevalence across 10 regions of Ghana over five years from 2012 to 2017. At the national level, mean temperature (ρ = − 0.17, p-value < 0.05) and rainfall (ρ = 0.06, p-value > 0.05) are weakly correlated with malaria prevalence. There are statistically significant moderate correlations between rainfall and prevalence for the three northern regions (Northern: ρ = 0.45, Upper East: ρ = 0.31, Upper West: ρ = 0.44). While rainfall is positively correlated with malaria prevalence in a few regions, the mean temperature is negatively correlated with prevalence in all 10 regions (ρ range: − 0.68 to − 0.28, p-value < 0.05). The highest maximum temperature (42.1 °C) for Upper East in this study is comparable to maximum temperature (41.1 °C) reported for Upper East by Issahaku et al. [35] from 1954 to 2012. Rainfall at a lag of 2 months was found to be positively correlated with malaria prevalence (Fig. 11 in Appendix), consistent with other studies in East African Highlands [16], Kenya [36, 37] and Ghana [9, 25]. Non-climate variables may contribute to explaining the relationship between climate variables and malaria prevalence. For example, the positive correlation between rainfall and malaria prevalence in Upper East (Table 3; Fig. 6a) may be linked to increased malaria vector breeding sites due to relatively low urbanization and poor housing in this region. The northern regions, especially Upper East and Upper West, are particularly characterized by poor quality housing, with a high percentage of buildings being from mud and mud bricks and having roofs made from thatch of grass and wood. These collect water from rainfall and serve as potential breeding sites for mosquitoes. Given that these breeding sites are close to the dwelling, the Anopheles mosquito has easy access to humans thereby transmitting malaria. The female Anopheles gambiae generally favours small open, and temporal pools such as footprints, roadside ditches and man-made holes in buildings for oviposition [38, 39] likely due to lower prevalence of larval predation compared to large permanent habitats [40, 41]. Rainfall promotes malaria transmission by creating more breeding sites for mosquitoes; however, intense rainfall may decrease malaria transmission by destroying breeding sites, leading to a decline in prevalence. Rainfall also increases relative humidity to optimum levels (above 10%), supporting mosquito survival [42, 43]. Increase in temperature on the other hand has a negative effect on malaria prevalence. Temperatures above 30 °C are detrimental to mosquitoes and parasites [15, 18, 19].
We estimated the effect of temperature and rainfall on malaria prevalence using a fixed-effect model with lags of the dependent and independent variables (Table 2). Maximum temperature, maximum temperature at lag 5, rainfall at lag 2, health facilities and malaria prevalence at a lag time of 1 month had significant effects on malaria prevalence. Maximum temperature was associated with a decrease in malaria prevalence. Over the study period, maximum temperature ranged from 23.3 to 42.1 °C with 98.6% of these values outside the reported optimum temperature (17–30 °C) for mosquito survival [19], hence the association with the decline in malaria prevalence. Among the climate variables, maximum temperature had the largest effect on malaria prevalence. Our model also showed that malaria prevalence at a lag time of 1 month has a significant effect on malaria prevalence, similar to a previous study in the East African Highlands [16]. Zhou et al. [16] suggested that after a period of high prevalence, the number of Plasmodium falcipurum gametocyte carriers will increase in a period of 1 to 2 months. This facilitates malaria transmission and potentially leads to a rise in malaria prevalence at least 1 month later. Therefore, malaria prevalence at a lag time of 1 month is an important predictor of malaria incidence. The number of health facilities per 100,000 population for each region also showed a statistically significant positive effect on malaria prevalence. Since our study focused on clinically reported malaria cases this association was expected.
Our multivariate fixed-effect model showed that the average effect of predictor variables on malaria prevalence (Table 3) is significantly greater for Upper East than Greater Accra which recorded the highest and lowest mean prevalence respectively (Table 1). Variations in prevalence across the regions may also be attributed to additional regional differences in socio-economic — such as urban–rural distribution [9], income [23] — and environmental factors including vector and parasite distribution [44, 45], and land use and agriculture [46]. Additionally, Upper East and Upper West regions have the highest poverty rates in Ghana while Greater Accra has the lowest poverty rate in the country [47]. Increased urbanisation, defined by eight components (population, economic activity, healthcare, transportation, housing, education, sanitation and service-infrastructure), has also been found to be associated with decreasing malaria transmission rates in Ghana [48]. Upper East is ranked the least urbanized while Greater Accra, the most urbanized. The association between malaria and poverty [24, 49,50,51,52,53] and urbanization [54,55,56] has also been observed in other studies.
The general decline in malaria prevalence supports reports by WHO that malaria has reduced in Ghana between 2000 and 2015 as a result of increased malaria control measures [57]. On a yearly scale, malaria prevalence decreases over the study period (Fig. 2). However, on a monthly scale, the decline is less pronounced although decreased malaria prevalence over the peak prevalence months (August to October) can be observed (Fig. 3). While peak prevalence occurs between August and October, following the main rainy season (April–July), the lowest prevalence occurs between January and April, following the dry season (December–March). These observations overlap previous findings (low: February–April, high: July–November) [9].
Despite these findings, our study has some limitations. The study period of 5 years and 5 months is not an extended period, although it is sufficient to observe climatic variation. There is a need to consider other non-climatic factors such as education, income, land use and income levels in estimating the impact of climate variables on malaria prevalence. Another limitation is that the malaria data is limited to only cases reported at health facilities and hence does not account for the vast number of people who engage in self-medication or unorthodox medications to treat malaria. As reported by the Ghana Statistical Service, about 37% of malaria cases in Ghana go unreported [6].
Another limitation of the modelling conducted in this study is the risk of ecological fallacy as there may be differences between individual- and regional-level associations between outcome and predictor variables. However, as discussed in previous studies including Chirombo et al. [58], the analyses may still be useful in giving better understanding of the key impacts. Identification of a climate impact on malaria is arguably a topic which lends itself to ecological studies of this type — given the spatiotemporal nature of the link between the vector and the environment (including temperature and precipitation) which is a function of geography and the difficulties in accurately assessing individual behaviours (e.g. insecticide treated net usage, wearing long sleeve clothing or use of mosquito repellent). Savitz [59] highlights that there is a place for such ecological studies in understanding underexplored relationships between the environment and health — and though it is noted above that there are a number of studies linking malaria and climate change, few exist in Ghana.
Regions with the highest prevalence are in the northern parts of Ghana. These regions have received several interventions including insecticide treated nets (ITNs), and intermittent preventive treatment (IPTs) [60]. However, malaria prevalence remains high in these regions, suggesting that addressing the problems of systemic poverty including poorly resourced health facilities could provide long term gains.
5 Conclusions
Malaria is a major cause of morbidity and mortality in Ghana. In this study, we have shown that there is a statistically significant association between malaria prevalence and climatic variation using fixed-effect modelling. A greater understanding of the influence of climate on malaria would aid in developing awareness campaigns and preparing the health system to respond to climate-induced malaria.
The relationships found in the data suggest that the lag structure is complex. Current temperature has a negative impact on malaria incidence, with rainfall having a positive influence.
Further work may include analysis of malaria data at a more granular level (for example, hospital level) and linking this to climate projections for Ghana to estimate future risks. As more data becomes available this may be possible — the current analyses focus on a 5-year period and so extrapolation would be difficult. Socioeconomic and demographic change may also impact on malaria risk — and this would need to be accounted for in any work to estimate future climate-based malaria risks. We provide these results to inform stakeholders to utilise climate-based warnings to prepare the health sector for major outbreaks while taking regional-level actions focusing on regions more prone to malaria prevalence. It is important to highlight the importance of lagged prevalence to help understand and possibly forecast malaria prevalence.
Availability of Data and Materials
The data used and analysed in the current study are available from the corresponding author on reasonable request.
Code Availability
Software codes are available from the corresponding author on reasonable request.
References
World Health Organization. (2017). WHO | Malaria. WHO.
World Health Organization. (2014). World Malaria Report.
Usaid, & CDC. (2009). The President’s Malaria Initiative (PMI) - Working with Communities to Save Lives in Africa - Third Annual Report, March 2009.
WHO, W. H. O. (2018). World Malaria Report 2018. Geneva.
Ghana Health Service. (2016). Malaria 1st Quarter Bulletin-2016.
Ghana Statistical Service (GSS), Ghana Health Service (GHS), & I. M. (2009). Ghana Demographic and Health Survey 2008. Ghana Statistical Service (GSS) Ghana Demographic and Health Survey. Accra, Ghana: GSS, GHS, and ICF Macro.
Nonvignon, J., Aryeetey, G. C., Malm, K. L., Agyemang, S. A., Aubyn, V. N. A., Peprah, N. Y., … & Aikins, M. (2016). Economic burden of malaria on businesses in Ghana: a case for private sector investment in malaria control. Malaria Journal, 15. https://doi.org/10.1186/s12936-016-1506-0
Zurek, K. (2018). CONFIRMED: Results of the 2018 Referendum on new regions - Graphic Online. Accessed from, https://www.graphic.com.gh/news/politics/confirmed-results-of-the-2018-referendum-on-new-regions.html
Asare, E., & Amekudzi, L. (2017). Assessing climate driven malaria variability in Ghana using a regional scale dynamical model. Climate, 5(1), 20. https://doi.org/10.3390/cli5010020
Nájera, J. A., Kouznetsov, R., Delacollette, C., & World Health Organisation. (1998). Malaria epidemics: detection and control, forecasting and prevention: Citeseer.
Centre for Disease Control. (2016). CDC - Malaria - Malaria Worldwide - Impact of Malaria.
Tanser, F., Sharp, B., & le Sueur, D. (2003). Potential effect of climate change on malaria transmission in Africa. The Lancet, 362, 1792–1798. https://doi.org/10.1016/S0140-6736(03)14898-2
Patz, J., & Olson, S. (2006). Climate change and health: Global to local influences on disease risk. Annals of Tropical Medicine and Parasitology, 100, 535–549. https://doi.org/10.1179/136485906X97426
Parham, P. E., & Michael, E. (2010). Modeling the effects of weather and climate change on malaria transmission. Environmental Health Perspectives, 118(5), 620–626. https://doi.org/10.1289/ehp.0901256
Craig, M., Snow, R., & le Sueur, D. (1999). A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitology Today, 15, 105–111. https://doi.org/10.1016/s0169-4758(99)01396-4
Zhou, G., Minakawa, N., Githeko, A. K., & Yan, G. (2004). Association between climate variability and malaria epidemics in the East African highlands. Proceedings of the National Academy of Sciences, 101(8), 2375–2380. https://doi.org/10.1073/pnas.0308714100
Leedale, J., Tompkins, A. M., Caminade, C., Jones, A. E., Nikulin, G., & Morse, A. P. (2016). Projecting malaria hazard from climate change in eastern Africa using large ensembles to estimate uncertainty. Geospatial Health, 11(1S). https://doi.org/10.4081/gh.2016.393
Bi, P., Tong, S., Donald, K., Parton, K. A., & Ni, J. (2003). Climatic variables and transmission of malaria: A 12-year data analysis in Shuchen County, China. Public Health Reports, 118, 65–71. https://doi.org/10.1093/phr/118.1.65
Beck-Johnson, L. M., Nelson, W. A., Paaijmans, K. P., Read, A. F., Thomas, M. B., Bjørnstad, O. N., & Costa, F. T. M. (2013). The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS One, 8(11). https://doi.org/10.1371/journal.pone.0079276
Ferrão, J. L., Mendes, J. M., Painho, M., & Zacarias, S. (2017). Malaria mortality characterization and the relationship between malaria mortality and climate in Chimoio, Mozambique. Malaria Journal, 16. https://doi.org/10.1186/s12936-017-1866-0
Yadav, K., Dhiman, S., Rabha, B., Saikia, P., & Veer, V. (2014). Socio-economic determinants for malaria transmission risk in an endemic primary health centre in Assam, India. Infectious Diseases of Poverty, 3, 19. https://doi.org/10.1186/2049-9957-3-19
Agomo, C. O., & Oyibo, W. A. (2013). Factors associated with risk of malaria infection among pregnant women in Lagos Nigeria. Infectious Diseases of Poverty, 2, 19. https://doi.org/10.1186/2049-9957-2-19
Akpalu, W., & Codjoe, S. N. A. (2013). Economic analysis of climate variability impact on malaria prevalence: The case of Ghana. Sustainability (Switzerland), 5(10), 4362–4378. https://doi.org/10.3390/su5104362
Tusting, L. S., Ippolito, M. M., Willey, B. A., Kleinschmidt, I., Dorsey, G., Gosling, R. D., & Lindsay, S. W. (2011). The evidence for improving housing to reduce malaria: A systematic review and meta-analysis. https://doi.org/10.1186/s12936-015-0724-1
Klutse, N. A. B., Abooagye-Antwi, F., Owusu, K., & Ntiamoa-Baidu, Y. (2001). Assessment of patterns of climate variables and malaria cases in two ecological zones of Ghana. Open Journal of Ecology, 4(4), 764–764. https://doi.org/10.4236/oje.2014.412065
Ghana Statistical Service. (2012). Population and Housing Census.
Netherlands Enterprise Agency. (2015). Sector Report: Health and Life Sciences Ghana.
Torres-reyna, O. (2010). Getting started in fixed / random effects models using R.
Croissant, Y., & Millo, G. (2008). Panel data econometrics in R: the plm package. Journal of Statistical Software, 27(2), 1–43. https://doi.org/10.18637/jss.v027.i02
Frees, E. W. (2003). Longitudinal and panel data: analysis and applications for the social sciences.
Hausman, J., & Taylor, W. (1981). Panel data and unobservable individual effects. Journal of Econometrics, 16(1), 155. https://doi.org/10.1016/0304-4076(81)90085-3
Hausman, J. A. (1978). Specification tests in econometrics. Econometrica, 46(6), 1251–1271.
Arellano, M., & Bond, S. (1991). Some tests of specification for panel data: Monte Carlo evidence and an application to employment equations. Review of Economic Studies, 58, 277–297. Retrieved from https://academic.oup.com/restud/article/58/2/277/1563354
Peel, M. C., Finlayson, B. L., & Mcmahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644.
Issahaku, A.-R., Campion, B. B., & Edziyie, R. (2016). Rainfall and temperature changes and variability in the Upper East Region of Ghana. Earth and Space Science, 3(8), 284–294. https://doi.org/10.1002/2016EA000161
Kipruto, E. K., Ochieng, A. O., Anyona, D. N., Mbalanya, M., Mutua, E. N., Onguru, D., … & Estambale, B. B. A. (2017). Effect of climatic variability on malaria trends in Baringo County, Kenya. Malaria Journal. https://doi.org/10.1186/s12936-017-1848-2
Karuri, S. W., & Snow, R. W. (2016). Forecasting paediatric malaria admissions on the Kenya Coast using rainfall. Global Health Action, 9. https://doi.org/10.3402/gha.v9.29876
Opoku, A., & Ansa-Asare, O. (2009). The occurrences and habitat characteristics of mosquitoes in Accra, Ghana. West African Journal of Applied Ecology, 11(1). https://doi.org/10.4314/wajae.v11i1.45730
Bentley, M. D., & Day, J. F. (1989). Chemical, ecological and behavioural aspect of mosquito oviposition. Ann. Rev. Ent., 34, 401–421.
Sunahara, T., Ishizuka, K., & Mogi, M. (2002). Habitat size: A factor deterormining the opportunity f encounters between mosquito larvae and aquatic predators. J. Vect. Ecol., 27, 820.
Washburn, J. Q. (1995). Regulation of factors affecting larval mosquito population in container and pool habitats: Implication for biological control. Journal of the American Mosquito Control Association, 11, 279–283.
Wu, Y., Qiao, Z., Wang, N., Yu, H., Feng, Z., Li, X., & Zhao, X. (2017). Describing interaction effect between lagged rainfalls on malaria: an epidemiological study in south–west China. Malaria Journal, 16. https://doi.org/10.1186/s12936-017-1706-2
Yamana, T. K., & Eltahir, E. A. B. (2013). Incorporating the effects of humidity in a mechanistic model of Anopheles gambiae mosquito population dynamics in the Sahel region of Africa. Parasites & Vectors, 6, 1. https://doi.org/10.1186/1756-3305-6-235
De Souza, D., Kelly-Hope, L., Lawson, B., Wilson, M., & Boakye, D. (2010). Environmental factors associated with the distribution of Anopheles gambiae ss in Ghana; an important vector of lymphatic filariasis and malaria. PLoS One, 5, e9927.
Dinko, B., Oguike, M. C., Larbi, J. A., Bousema, T., & Sutherland, C. J. (2013). Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. International Journal for Parasitology: Drugs and Drug Resistance, 3, 45–50.
Klinkenberg, E., McCall, P., Wilson, M., Amerasinghe, F., & Donnelly, M. (2008). Impact of urban agriculture on malaria vectors in Accra, Ghana. Malaria Journal, 7, 151.
National Malaria Control Programme. (2013). An Epidemiological Profile of Malaria and its Control in Ghana.
Frank, C., Krumkamp, R., Sarpong, N., Sothmann, P., Fobil, J. N., Foli, G., … & Kreuels, B. (2016). Spatial heterogeneity of malaria in Ghana: a cross-sectional study on the association between urbanicity and the acquisition of immunity. Malar J, 15. https://doi.org/10.1186/s12936-016-1138-4
Teklehaimanot, A., & Mejia, P. (2008). Malaria and poverty. Annals of the New York Academy of Sciences, 1136(1), 32–37. https://doi.org/10.1196/annals.1425.037
Caulfield, L. E., Richard, S. A., & Black, R. E. (2004). Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. The American journal of tropical medicine and hygiene, 71(2_suppl), 55–63.
Crookston, B. T., Alder, S. C., Boakye, I., Merrill, R. M., Amuasi, J. H., Porucznik, C. A., & Ansong, D. (2010). Exploring the relationship between chronic undernutrition and asymptomatic malaria in Ghanaian children. Malaria Journal, 9, 39.
Friedman, J. F., Kwena, A. M., Mirel, L. B., Kariuki, S. K., Terlouw, D. J., Phillips-Howard, P. A., Hawley, W. A., ... & ter Kuile, F. O. (2005). Malaria and nutritional status among pre-school children: Results from cross-sectional surveys in western Kenya. American Journal of Tropical Medicine and Hygiene, 73, 698–704.
Almeida, M., Alexandre, A., Benzecry, S. G., Siqueira, A. M., Vitor-Silva, S., Melo, G. C., & Alecrim, G. C. (2015). The association between nutritional status and malaria in children from a rural community in the Amazonian Region: A longitudinal study. PLoS Neglected Tropical Diseases, 9(4), 1–15. https://doi.org/10.1371/journal.pntd.0003743.
Ghana Health Service and National Malaria Control Programme. (2013). Ghana Malaria Programme Review Final Report.
Hay, S. I., Guerra, C. A., Tatem, A. J., Atkinson, P. M., & Snow, R. W. (2005). Urbanization, malaria transmission and disease burden in Africa. Nature Reviews Microbiology, 3, 81–90.
Tatem, A. J., Gething, P. W., Smith, D. L., & Hay, S. I. (2013). Urbanization and the global malaria recession. Malaria Journal, 12.
World Health Organization. (2016). WHO | Fact Sheet: World Malaria Report 2015. WHO.
Chirombo, J., Ceccato, P., Lowe, R., Terlouw, D. J., Thomson, M. C., Gumbo, A., ... & Read, J. M. (2020). Childhood malaria case incidence in Malawi between 2004 and 2017: Spatio-temporal modelling of climate and non-climate factors. Malaria Journal, 19(1), 1–13. https://doi.org/10.1186/s12936-019-3097-z
Savitz, D. A. (2012). Commentary: A niche for ecologic studies in environmental epidemiology. Epidemiology, 23(1), 53–54. https://doi.org/10.1097/EDE.0B013E31823B5633
United Nations Children‘s Fund (UNICEF). (2007). UNICEF Ghana Fact Sheet Malaria 2007. Accra, Ghana.
Acknowledgements
The authors wish to thank the Centre of Health Information Management Division of the Ghana Health Service and the Christian Health Association of Ghana for providing access to the data used in this study. They are grateful to Dr. Kwasi Amuzu and Mr. Ivan Tetteh Essegbey for helping them with the health data from the Christian Health Association of Ghana. They also thank Jolyon Medlock from Public Health England for his valuable comments and suggestions. The study was undertaken while TVO was a Master’s student supported by funding of the University of Exeter and the Commonwealth Shared Scholarship Commission.
Funding
The study was undertaken while TVO was a Master’s student supported by funding of the University of Exeter and the Commonwealth Shared Scholarship Commission.
Author information
Authors and Affiliations
Contributions
TVO developed the research proposal and carried out the data collection, literature review, preliminary descriptive data analysis, interpretation of data, and drafted the initial manuscript. SA was involved in descriptive and statistical data analyses and data visualization. SA also provided inputs to the first draft of the manuscript. AM, with the support of TT, gave directions on the data analysis and helped review and edit the manuscript. All authors made significant contributions to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oheneba-Dornyo, T.V., Amuzu, S., Maccagnan, A. et al. Estimating the Impact of Temperature and Rainfall on Malaria Incidence in Ghana from 2012 to 2017. Environ Model Assess 27, 473–489 (2022). https://doi.org/10.1007/s10666-022-09817-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10666-022-09817-6