Abstract
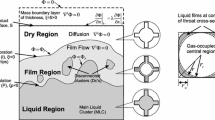
Asymptotic methods are employed to analyse a commonly used one-dimensional transient model for coupled heat and mass transfer in the primary drying stage of freeze-drying (lyophilization) in a vial. Mathematically, the problem constitutes a two-phase moving boundary problem, in which one of the phases is a frozen porous matrix that undergoes sublimation, and the other is a low-pressure binary gaseous mixture. Nondimensionalization yields a model with 19 dimensionless parameters, but a systematic separation of timescales leads to a reduced model consisting of just a second-order differential equation with two initial conditions for the location of a sublimation front; the temperature and gas partial pressures can be found a posteriori. The results of this asymptotic model are compared with those of earlier experimental and theoretical work. Most significantly, the current model would be a computationally efficient tool for predicting the onset of secondary drying.








Similar content being viewed by others
References
Muzzio CR, Dini NG (2011) Simulation of freezing step in vial lyophilization using finite element method. Comput Chem Eng 35:2274–2283
Sheehan P, Liapis AI (1998) Modelling of the primary and secondary drying stages of the freeze-drying of pharmaceutical product in vials: numerical results obtained from the solution of a dynamic and spatially multidimensional lyophilisation model for different operational policies. Biotechnol Bioeng 60:712–728
Pikal MJ, Cardon S, Bhugra C, Jameel F, Rambhatla S, Mascarenhas WJ, Akay HU (2005) The nonsteady state modeling of freeze drying: in-process product temperature and moisture content mapping and pharmaceutical product quality applications. Pharm Dev Tech 1:17–32
Pikal MJ (1985) Use of laboratory data in freeze-drying process design: heat and mass transfer coefficients and the computer simulation of freeze-drying. J Parenter Sci Tech 39:115–139
Millman MJ, Liapis AI, Marchello JM (1985) An analysis of the lyophilisation process using a sorption sublimation model and various operational policies. AIChE J 31:1594–1604
Mascarenhas WJ, Akay HU, Pikal MJ (1997) A computational model for finite element analysis of the freeze-drying process. Comput Meth Appl Mech Eng 148:105–124
Song CS, Nam JH, Kim CJ, Ro ST (2002) A finite volume analysis of vacuum freeze drying processes of skim milk solution in trays and vials. Dry Tech 20:283–305
Song CS, Nam JH, Kim CJ, Ro ST (2005) Temperature distribution in a vial during freeze-drying of skim milk. J Food Eng 67:467–475
Brülls M, Rasmuson A (2009) Ice sublimation in vial lyophilization. Dry Tech 27:695–706
Zhai S, Su H, Taylor R, Slater KH (2005) Pure ice sublimation within vials in a laboratory lyophiliser; comparison of theory with experiments. Chem Eng Sci 60:1167–1176
Hottot A, Peczalski R, Vessot S, Andrieu J (2006) Freeze-drying of pharmaceutical proteins in vials: modeling of freezing and sublimation steps. Dry Tech 24(5):561–570
Velardi SA, Barresi AA (2008) Development of simplified models for the freeze-drying process and investigation of the optimal operating conditions. Chem Eng Res Design 86:9–22
Lopez-Quiroga E, Antelo LT, Alonso AA (2012) Time-scale modeling and optimal control of freeze-drying. J Food Eng 111(4):655–666
Jackson R (1977) Transport in porous catalysts. Elsevier, Amsterdam
Mason EA, Malinauskas AP (1983) Gas transport in porous media: the dusty-gas model. Elsevier, New York
Gloor PJ, Crosser OK, Liapis AI (1987) Dusty-gas parameters of activated carbon adsorbent particles. Chem Eng Commun 59:95–105
Kast W, Hohenthanner CR (2000) Mass transfer within the gas-phase of porous media. Int J Heat Mass Transfer 43:807–823
Liapis AI, Bruttini R (1995) Freeze-drying of pharmaceutical crystalline and amorphous solutes in vials: dynamic multi-dimensional models of the primary and secondary drying stages and qualitative features of the moving interface. Dry Tech 13:43–72
Sheehan P (1998) Internal report No. 10. Tech. Rep., Department of Chemical Engineering, University of Missouri-Rolla, Rolla, MO
Mitchell SL, Vynnycky M (2009) Finite-difference methods with increased accuracy and correct initialization for one-dimensional Stefan problems. Appl Math Comput 215:1609–1621
Mitchell SL, Vynnycky M (2012) An accurate finite-difference method for ablation-type Stefan problems. J Comput Appl Math 236:4181–4192
Mitchell SL, Vynnycky M, Gusev IG, Sazhin SS (2011) An accurate numerical solution for the transient heating of an evaporating droplet. Appl Math Comput 217:9219–9233
Vynnycky M (2009) An asymptotic model for the formation and evolution of air gaps in vertical continuous casting. Proc R Soc A 465:1617–1644
Wagner W, Saul A, Pruss A (1994) International equations for the pressure along the melting and along the sublimation curve of ordinary water substance. J Phys Chem Ref Data 23:515–527
Boss EA, Filho RM, de Toledo ECV (2004) Freeze drying process: real time model and optimization. Chem Eng Process 43:1475–1485
Sadikoglu H, Liapis AI (1997) Mathematical modelling of the primary and secondary drying stages of bulk solution freeze-drying in trays: parameter estimation and model discrimination by comparison of theoretical results with experimental data. Dry Tech 15:791–810
Bruttini R, Rovero G, Baldi G (1991) Experimentation and modelling of pharmaceutical lyophilization using a pilot plant. Chem Eng J 45:B67–B77
Nail SL (1980) The effect of pressure on heat transfer in the freeze-drying of parenteral solutions. J Parenter Drug Assoc 34:358–368
Demirel Y, Saxena SC (1996) Heat transfer through a low-pressure gas enclosure as a thermal insulator: design considerations. Int J Energy Res 20:327–338
Gan KH, Bruttini R, Crosser OK, Liapis AI (2004) Heating policies during the primary and secondary drying stages of the lyophilization process in vials: effects of the arrangements of vials in clusters of square and hexagonal arrays on trays. Dry Tech 22:1539–1575
Acknowledgments
The author acknowledges the support of Enterprise Ireland, within the framework of the Pharmaceutical Manufacturing Technology Centre Initial Research Programme, Grant TC/2012/5105. The author would also like to thank Dr W. T. Lee for useful discussion and the constructive comments of the anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Further considerations relating to boundary condition (2.24)
Note that if \(p_{\mathrm{w}}^{H}>p_{\mathrm{w}}^\mathrm{sat}\left( T_{\mathrm{init} }\right) ,\) where \(p_{\mathrm{w}}^\mathrm{sat}\) is the temperature-dependent water vapour saturation pressure, then sublimation cannot begin instantaneously at \(t=0.\) As \(T\) at \(z=H\) increases, eventually sublimation can begin when
On the other hand, if \(p_{\mathrm{w}}^{H}<p_{\mathrm{w}}^\mathrm{sat}\left( T_{\mathrm{init}}\right) ,\) sublimation would begin instantaneously at \(t=0.\) However, this would seem to be an unrealistic scenario, given that the upper surface of the frozen matrix is continuously in contact with the water vapour above it during freezing. Thus, most realistic and expedient seems to be
as this avoids a post-freezing pre-sublimation stage\(;\) in turn, this means that we must have
In summary, it seems that setting \(p_{\mathrm{c}}\) and \(T_{\mathrm{init}}\) would suffice in order to determine \(p_{\mathrm{a}}^{H}\) and \(p_{\mathrm{w} }^{H}.\)
However, there are two further points of confusion. First of all, different authors use different expressions for \(p_{\mathrm{w}}^\mathrm{sat}\left( T\right) .\) Velardi and Barresi [12] use
whereas
is used in [2, 7, 25, 26]; these are plotted in Fig. 9, along with tabulated values [24], and suggest that the units for (A.4) are bars, rather than Pa as given in [12]. Moreover, it is far from clear that all earlier models are thermodynamically consistent: Velardi and Barresi [12] took \(p_{\mathrm{c}}=26\) Pa, \(T_{\mathrm{init} }=228\) K, yet \(p_{\mathrm{w}}^\mathrm{sat}\approx 28.7\) Pa; others [2, 7, 25, 26] took \(p_{\mathrm{c}}\approx 5\hbox { Pa, }T_{\mathrm{init}}=233\) K, yet \(p_{\mathrm{w}}^\mathrm{sat}\approx 48.9\hbox { Pa};\) Mascarenhas et al. [6] took \(p_{\mathrm{w}}^{H}=5.3\hbox { Pa, }T_{\mathrm{init}}=242\) K, but \(p_{\mathrm{c}}\) was not specified at all. For the computations, we therefore use the data for \(p_{\mathrm{w}}^\mathrm{sat}\) given in [24] and the value for \(p_{\mathrm{c} }\) used in [12] and as given in Table 1.
Appendix B: Forms for \(Q_{\mathrm{v}}\)
For \(Q_{\mathrm{v}},\) some authors [13, 26, 27] simply use
whereas others [2, 7, 12] take the expression given in (2.26); this expression takes into heat conduction through the glass of the vial and heat transfer in the air gap between the vial and the heating surface, which may be expected to consist of both conduction and radiation. For \({\mathfrak {h}}_{\mathrm{v}}\) in (2.26), some [2, 7] take \(30\hbox { Wm}^{-2} \hbox {K}^{-1},\) whereas others [12] take the linear sum
where \(k_{\mathrm{a}}\) is the thermal conductivity of the gas in the air gap, \(\Lambda _{0}\) is the free molecular conductivity of the gas at 273.15 K, \(T_{\mathrm{g}}\) is equal to the mean temperature value between the bottom surface of the vial and the shelf surface, \(a^{*}\) is an accommodation coefficient which takes into account the efficiency of energy transfer between gas molecules striking the surface of the glass and of the shelf, \(\left[ h_{\mathrm{a}}\right] \) is the characteristic air-gap width, which can typically vary between 0.05 and 0.6 mm [9], \(T_{\mathrm{gl}}\) is the temperature of the bottom surface of the vial, and \(\varepsilon _{\mathrm{sh}}\) and \(\varepsilon _{\mathrm{gl}}\) denote the radiative emissivities of shelf and the glass vial, respectively. Further, we have [4, 28]
with
where \(T_{\mathrm{sg}}=(T_{\mathrm{sh}}+T_{\mathrm{gl}})/2\) [9], and
where \(a_{\mathrm{gl}}^{*}\) is the accommodation coefficient for the gas and the glass and \(a_{\mathrm{sh}}^{*}\) is the accommodation coefficient for the gas and the shelf [29]. In turn,
Heat transfer coefficient \({\mathfrak {h}}_{\mathrm{v}}\) versus temperature \(T\) as given by Eq. (B.2) for two values of vial–shelf gap width \(\left[ h_{a}\right] \)
where \(M_{\mathrm{g}}\) is the molecular weight of the gas, \({\mathfrak {M}}\) is the ratio of molecular weights of the gas and the surface, i.e. the bottom of the glass vial and the shelf, and \(\varphi \) is the fractional coverage of the adsorption layer, which depends on the nature of the surface; one form for this is [30]
where \(T_{\mathrm{r}}\) is the temperature of the gas leaving the surface, which can be taken to be the mean of \(T_{\mathrm{sh}}\) and \(T_{\mathrm{gl}},\) i.e. \(T_{\mathrm{sg}}\) as defined earlier\(,\) and \(\Phi \) is a parameter related to the nature of the surface, which was taken to be 2 in [9]. Although Eqs. (B.2)–(B.7) were not implemented in the final model, it is interesting to note that they lead to \({\mathfrak {h}}_{\mathrm{v}}\sim \) 15–25 \(\hbox {Wm}^{-2}~\hbox {K}^{-1},\) as shown in Fig. 10, which is of the same order of magnitude as that given by [2, 7]; furthermore, for \(p_{\mathrm{c} }\approx 20\) Pa, all approaches lead to \(Q_{\mathrm{v}}\sim 30\hbox { Wm}^{-2}\,\hbox { K}^{-1}.\) Consequently, as a balance between oversimplicity and overcomplexity, and so as to be able to allow for the explicit effect of the properties of the vial, i.e. \(w\) and \(k_{\mathrm{gl}},\) (2.26) has been used, in tandem with \({\mathfrak {h}}_{\mathrm{v}}=30\hbox { Wm}^{-2}\,\hbox {K}^{-1} .\)
Rights and permissions
About this article
Cite this article
Vynnycky, M. An asymptotic model for the primary drying stage of vial lyophilization. J Eng Math 96, 175–200 (2016). https://doi.org/10.1007/s10665-015-9789-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10665-015-9789-7