Abstract
In recirculating aquaculture systems (RAS), it is important to monitor the water quality to keep the fish healthy. Especially in water treatment with oxidizing agents, for example, ozone (O3) or hydrogen peroxide (H2O2), the monitoring of the quality of dissolved organic matter (DOM) in water is advisable to keep track of the treatment’s effect. Previously in wastewater treatment plant (WWTP) studied, HPLSEC and fluorescence method for monitoring and characterization of organic matter was used here to track the effect of oxidative treatments; 5 duplicated treatments (2 × O3, 1 × O3 + H2O2, 1 × H2O2, 1 × control) were performed for four months with weekly samplings. Systems that contained O3 injection reduced fluorescence on average over 90%, except tyrosine-like fluorescence with removal of 80%. Combined O3 + H2O2 treatment did not bring any advantages over pure O3 treatment, and H2O2 had no significant effect on fluorescence. Humic and fulvic compounds were detected to largely be derived from inlet lake water, while large protein-like structures were mostly created in RAS. A peak of benzoic acid-like molecules was also detected in all RAS waters. Treatments did not change the molecular weight profile of DOM systems and inlet water, having most of their fluorescence coming from medium-sized (108–1322 Da) molecules. DOC was lower in O3 treatments, but the linear connection between DOC and fluorescence was not observed, although this was proposed in earlier studies. Most likely, the oxidizing treatments induce change to DOM in a way that such comparison becomes inaccurate, although this must be studied further in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ozone is a strong organic oxidizer, widely used nowadays in industry and domestic water production due to the ozone’s ability to oxidize most of the organic material. This ability has been noted in the aquacultural industry since ozone can be used to increase fish welfare by disinfecting pathogens and improving overall water quality (Summerfelt et al., 1997; Powell, 2016; Davidson et al., 2021). Ozone also has the potential to remove problematic off-flavor compounds from water that is especially abundant in recirculating aquaculture systems (RAS) (Lindholm-Lehto & Vielma, 2019; Pettersson et al., 2022). Even though ozone rapidly decomposes to oxygen, it is still very toxic to fish, and this raises concern among fish farmers, although the research and usage have steeply risen during the last few decades (Powell, 2016). To mitigate risks and costs, it is important to optimize and monitor the ozone’s performance. One possible way for monitoring is to use high-performance liquid size exclusion chromatography (HPLSEC) with fluorescence and UV detection to track the decomposition of dissolved organic matter (DOM).
Currently, DOM in aquaculture water is studied frequently in multiple different ways. PARAFAC method (one of the most used ones) was used, for example, by Hambly et al. (2015), Yamin et al. (2017), and Kim et al. (2021). These studies have a comprehensive evaluation of fluorescence data, but molecular weights were not studied. Kamjunke et al. (2017) did an extensive evaluation of DOM in aquacultural water with nuclear magnetic resonance spectroscopy, ultrahigh-resolution spectrometry, and fluorescence, especially with tyrosine- and tryptophan-like fluorescence. Aguilar-Alarcon et al. (2020, 2022) used high-resolution and time-of-flight mass spectrometry to evaluate DOM in RAS without fluorescence. The latter study focuses especially on fulvic acids in DOM. Wang et al. (2021) used PARAFAC and cyclotron resonance mass spectrometry to identify DOM from aquacultural ponds.
The usage of HPLSEC and fluorescence in water purification has been previously studied by Ignatev and Tuhkanen (2019). They concluded that it is indeed viable to monitor the change of organic matter during the wastewater purification process with the HPLSEC method. Other studies have also found this method viable for monitoring water treatment processes (Hidayah et al., 2020; Jokubauskaite et al., 2015). Spiliotopoulou et al. (2017, 2018) used fluorescence and HPLSEC in a marine water aquarium system and small RAS to successfully monitor the effect of ozone on the quality of DOM, but other than that, the application of HPLSEC and fluorescence to ozonated RAS to monitor DOM is largely unstudied.
This study is the second part of an earlier study (Pettersson et al., 2022) about the use of oxidizers in removing the off-flavors from RAS and included the off-flavor and fish data (Pettersson et al., 2022). On the other hand, this study contains the characterization of the amount and size distribution of DOM. The aim of this part is to study the viability of the fluorescence and HPLSEC method for RAS water and the change of organic matter due to the ozonation. According to our knowledge, this is the first time when organic matter in continuously ozonated RAS was studied and regularly with different ozone and hydrogen peroxide amounts. It was hypothesized that the more intensive the oxidizing treatment, the lower the fluorescence and UVA-254 would be. With the development of monitoring methods and tools, it is possible to encourage the aquacultural industry to wider use of ozone. Correctly, ozone addition should reduce maintenance costs, keep disease outbreaks under control, and support the growth of the fish, which finally leads to economic savings.
Materials and methods
Experimental setup
This study is the second part of our earlier study (Pettersson et al., 2022), and the data in this article was collected at the same time. A more detailed description of ozonation equipment, experiment conditions, and sampling can be found in the aforementioned article.
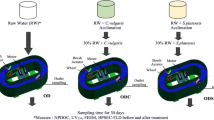
In short, the 10 individual RAS contained a bottom-drained rearing tank, feed collector, solids removal (vortex clarifier and a drum filter with 60-µm mesh size), a moving-bed biofilter, a forced-ventilated cascade aeration column for CO2 removal, oxygen injection, and pH adjustment with NaOH solution. The total volume of the system was 1440 L. At the beginning of the experiment, each system contained approximately 23.8 kg of rainbow trout (Oncorhynchus mykiss). The water renewal rate was 5.4–6.0 L h−1 and was adjusted based on feeding (500 L of water per kg of feed per day). Feeding ratio changed between 0.9 and 1.2% during the experiment. The system pH was maintained at 7. All the systems were maintained identically, excluding the different oxidizing treatments. The experiment lasted for four months (Pettersson et al., 2022).
The experiment included treatments: low O3 (0.4 mg L−1), high O3 (0.8 mg L−1), O3 + H2O2 as advanced oxidation process (0.4 mg L−1 and 0.15 µl L−1), H2O2 (0.15 µl L−1), and control (n = 2, 10 systems in total). The lake water from Lake Peurunka (62.44886, 25.85201, 694 ha, 59,600 m3) was used as new replacement water and was also studied during the experiment (inlet lake water). The flow in the ozonation loop was 0.23 L s−1.
Sampling
The experiment lasted for four months. Samples were collected weekly from the rearing tank below the surface with an empty plastic syringe. Samples were immediately filtered through 0,45-μm prewashed cellulose acetate syringe filters (Sartorius, 16555-Q) to 50-ml sample tubes made of polypropylene and high-density polyethylene (VWR) and stored in a fridge (at 6 °C) for few days before the analysis.
Chromatography and fluorescence method
The HPLSEC method was based on a method described by Ignatev and Tuhkanen (2019). The mobile phase was prepared for the analysis by dissolving 0.8900 g of Na2HPO4 (analytic grade, WVR Chemicals) and 0.7801 g of NaH2PO4 (analytic grade, WVR Chemicals) to 2 L of ultrapure water. The solution was then vacuum filtered through a Whatman 0.2-µm cellulose acetate filter and transferred to an analyzer. A stirred sample (1.5 mL) was transferred to the HPLSEC-glass vial (2 ml Verex vial, Phenomenex). HPLSEC analysis was performed with C196-E061W Prominence (Shimadzu, Japan) measuring tryptophan-, tyrosine-, fulvic-, and humic-like fluorescence and UV absorbance at 254 nm. A separation column (Yarra 3 μm SEC-3000, 300 * 7.8 mm, Phenomenex, USA) was used. Each sample was analyzed twice with two different wavelengths for fluorescence. The used wavelengths were 270 nm excitation and 355 nm emission for tryptophan, 220 nm excitation and 310 nm emission for tyrosine. Humic-like fluorescence was followed at 330 nm excitation and 425 nm for emission, and fulvic had 390 nm excitation and 500 nm for emission. After every three samples, a blank sample (ultrapure water) was run to check that no impurities were present. The fluorescence intensity was integrated into Shimadzu LabSolutions LC/GC (version 5.51.) for each different wavelength.
Fluorescence data was separated into three fractions according to the main bulk of fluorescence intensity. These fractions were as follows (retention time in brackets): large (0.0–9.0 min), medium (9.0–11.8 min), and small (11.8–20 min) (Fig. 1). Fluorescence area integration was performed manually for size fractions. It was calculated that the percentage of one fraction on average contributed to fluorescence and the UVA-254 during the experiment. MS Excel 2016 (v16.0) in Office 2016 (Microsoft Corp. 2015) was used for handling the fraction data.
Standards were run to link the retention times to molecular weights, and a standard curve of R2 = 0.992 was acquired with good logarithmic linearity (Supplementary Fig. 1). The lowest and the highest atomic weights of size fractions are shown in Table 1.
Statistical analyses
Data analyses were performed using Microsoft Excel (2016) and IBM SPSS statistics 24. Kruskal–Wallis’ test (significance level 0.05) was used to test the average fluorescence difference between treatments after week 3. The same Kruskal–Wallis test was also used to compare size fractions between the treatments and inlet lake water. DOC concentrations were compared between treatments with Friedmann’s test (significance level 0.05). The DOC-fluorescence relationship was presented with a general linear model, and the R2 value was determined for models by taking DOC data from the systems and plotting it against fluorescence and UVA-254 separately.
Ozone doses were calculated as mg of injected ozone per mg of DOC in a liter of water. This was calculated for the ozonation loop and its water flow rate. It was assumed that all of the injected ozone were consumed in the process. This was calculated for every week as DOC concentrations changed through the experiment.
Results
Reduction of DOC
The DOC concentrations were lower in ozone-treated systems than in the control and H2O2 systems (n = 28, F = 4, p < 0.05), and the high O3 had the lowest DOC values. High O3 had statistically lower values than O3 + H2O2 (n = 28, F = 4, p < 0.05). Hydrogen peroxide did not seem to have any noticeable effect on DOC concentrations when compared to controls (n = 28, F = 4, p = 0.866) (Supplementary Table 2). The measured DOC concentrations were approximately 3–4 mg L−1 lower in low O3 and O3 + H2O2 treatments than in control and H2O2 (Fig. 2). High O3 systems had over 5 mg L−1 lower DOC concentrations than control and H2O2, closing similar values with inlet water (Fig. 2).
Ozone dose in high O3 systems was about two times higher than in O3 + H2O2 and low O3 systems. The dose in the latter two stayed similar throughout the experiment. Fluctuations and few peaks were recorded in all treatments, especially in weeks 6 and 12–14. The average dose was 0.106 mg (O3) mg−1 (DOC) for high O3, 0.047 mg (O3) mg−1 (DOC) for low O3, and 0.044 mg (O3) mg−1 (DOC) in O3 + H2O2 (Supplementary Fig. 2).
Fluorescence and UVA-254
Humic-like fluorescence was by far the most abundant of all the measured wavelengths, followed by tryptophan-, fulvic-, and tyrosine-like fluorescence. All the treatments, except H2O2, managed to remove the majority of the fluorescence from RAS during the first two weeks, after which the values stayed at similar levels (Fig. 3).
Treatments that included ozone removed 90–96% of fulvic-, humic-, and tryptophan-like fluorescence. High O3 had slightly better fluorescence removal performance than low O3, only by a few percent, but statistically, there was a significant difference in humic (n = 132, F = 5, p < 0.05) and fulvic fluorescence (n = 132, F = 5, p < 0.05), but with tryptophan, no statistical difference was found (n = 132, F = 5, p = 0.086). When compared to O3 + H2O2 treatment, high O3 dose had consistently lower fluorescence (n = 132, F = 5, p < 0.05) in all previously mentioned cases. There was no statistical difference between O3 + H2O2 and low O3 treatments (n = 132, F = 5, p > 0.05), although O3 + H2O2 performance seemed to be 2–5% percent lower overall. All these three ozone treatments had statistically lower fluorescence values than controls and H2O2 treatments (n = 132, F = 5, p < 0.05). No difference was recorded between H2O2 and controls (n = 132, F = 5, p > 0.05) for fluorescence or UVA-254.
Ozonated treatments removed tyrosine-like fluorescence with efficiency of 80–84% (Table 2). There was no statistical difference between them (n = 132, F = 5, p > 0.05), but a clear difference to controls and H2O2 treatment was recorded (n = 132, F = 5, p < 0.05) (Supplementary Table 1).
UVA-254 was removed less efficiently when compared to fluorescence (Table 2). Treatment with pure H2O2 failed to reduce it at all (n = 132, F = 5, p > 0.05), while the removal efficiency in other treatments ranged from 64 to 83%. High O3 had the highest removal efficiency with 83%. There was no statistical difference between O3 + H2O2 and low O3, although the first had UVA-254 mean removal efficiency of 64% and the latter 74% (Table 2).
In many cases, low O3, high O3, and O3 + H2O2 treatments were able to reduce fluorescence and UV-254 lower than in incoming inlet water, excluding tyrosine fluorescence, where the values were in the same range.
The main fluorescence peak was found around 11 min of retention time. Especially humic and fulvic fluorescences were largely defined by this peak, whereas tyrosine and tryptophan fluorescence contained a few other important ones in addition. The peak found around 5 min was exclusive only to these protein-like wavelengths, especially strong in tyrosine-like fluorescence. Tryptophane-like fluorescence also had a peak in 12 min, but this was more strongly present in control and H2O2 treatments during week 4 rather than 2 (Fig. 4). This peak was also missing from inlet lake water chromatograms (Supplementary Fig. 4).
Averaged chromatograms for all treatments (orange = O3 + H2O2, gray = low O3, yellow = high O3, blue = H2O2, light blue = control) and fluorescence including UV-254 (A = humic, B = fulvic, C = tyrosine, D = tryptophan, E = UV-254). The X-axis displays the measured retention time (min), and the Y-axis displays the intensity (I) as absorbance units (AU). Chosen weeks for display were initial week 2 and week 4 when the treatments had stabilized
In general, treatments of high O3, low O3, and O3 + H2O2 were able to reduce the fluorescence substantially when comparing chromatograms from the initial week 2 to week 4. However, H2O2 treatment did not have any effect on chromatograms and resembled closely controlled chromatograms, sometimes demonstrating slightly lower main peaks (for example, tyrosine, 5 min and 11 min). The chromatogram signals seemed to follow the following pattern in week 4 from lowest signal to highest: high O3, low O3, O3 + H2O2, and shared fourth position for H2O2 and control (Fig. 4).
Tyrosine-like fluorescence had the most erratic chromatograms and displayed the effect of treatments the least clearly. However, clear fluorescence reduction can be seen in peaks around 5 min and 9 min with high O3-, low O3-, and O3 + H2O2 treatments (Fig. 4). Tyrosine signal was substantially weaker and clearer in inlet lake water chromatograms when compared to any other RAS chromatograms (Supplementary Fig. 4).
UVA-254 also had initially during week 2 its core intensity found at 9 to 11 min of retention time, but it was spread more evenly. Another slightly weaker peak was found again around 12 min (Fig. 4). Inlet lake water lacked this peak almost completely (Supplementary Fig. 4). The even spread of UVA-254, however, changed in week 4 as between 9 and 11 min; four strong peaks can be easily distinguished in all treatments except high O3. High O3 treatment smoothed the peaks so that possibly only two can be observed. The peak in 12 min also seemed to grow in relation to other peaks, especially in O3-treated systems (for example, peak 12 min is many times higher than peaks around 9–11 min in high O3 treatment). Interestingly, O3 + H2O2 had the strongest fluorescence intensity this time during week 4, even higher than controls or H2O2. Otherwise, UVA reduction happened in a similar way as previously mentioned for fluorescence (Fig. 4).
Molecular size fractions
In terms of molecular size fractions, there was very little statistical difference between any of the treatments. The treatments decreased the fluorescence considerably but did not change the molecular composition in water when compared to the control’s molecular weight profile. However, molecular weight profiles did not stay constant in any of the systems, having minor changes from week to week, but as said earlier, these changes were reflected in all systems at the same time (Fig. 5).
In all cases, the fluorescent DOM was dominantly made of medium-sized molecules (108–1322 Da). There were relatively more small molecules (< 108 Da) than large (> 1322 Da) in DOM, making large molecules the least abundant in water.
There were statistically more medium-sized molecules in high O3 treatment than in inlet lake water (n = 110, F = 5, p = 0.028). Control systems also had more small molecules than inlet lake water (n = 110, F = 5, p = 0.020) (Supplementary Table 2). There were only statistical significances found when size fraction data was compared between treatments.
The average molecular weight profile for fluorescence and UV-254 is presented in Supplementary Fig. 3. It is notable that humic and fulvic fluorescence reflected more medium-sized molecules and tyrosine and tryptophan small ones. Tyrosine also had a significant number of large molecules in its signal (n = 55, F = 4, p < 0.05). UV-254 also had more large molecules when compared to humic (n = 55, F = 4, p < 0.05) and fulvic (n = 55, F = 4, p = 0.009) fluorescence (Supplementary Table 5.).
Fluorescence and UVA-254 – DOC correlation
Figure 6 depicts DOC values plotted against fluorescence responses. Fluorescence gave R2 values between 0.37 and 0.40. Humic- and fulvic-like fluorescence were on the higher side (around 0.40), as protein-like tryptophan and tyrosine were on the lower side (0.36–0.38). UVA-254 had the best correlation with DOC, R2 being 0.49 (Fig. 6). Overall, no strong linear relation between fluorescence/UVA-254 and DOC was found.
Discussion
Initially, all systems started with higher fluorescence and UVA-254 values than inlet lake water. This is to be expected as RAS water is very concentrated with different compounds due to a small water retention rate (Leonard et al., 2002). However, O3 and O3 + H2O2 treatments were able to reduce fluorescence to the same or slightly below inlet water levels. The removal of fluorescence was similar to the earlier observations by Spiliotopoulou et al. (2017), with the removal of intensity being in the same range (over 90%). Protein-like fluorescence being removed with worse efficiency than humic-like fluorescence has also been recorded before by Aguilar-Alarcon et al. (2022). The steep decline in fluorescence was followed by a steady residual fluorescence, which indicates that there are always compounds in water that cannot be oxidized by ozone any further or that the reaction rate is so slow that the system can replace them fast enough. In the case that oxidation is limited by ozone’s selectivity, usage of AOP can be beneficial due to the unselectivity of OH radicals (Von Gunten, 2003). However, in this study, AOP treatment was most likely limited by the production of RAS and did not have any advantage over pure O3 treatment.
The main part of the DOM in RAS was found to be mainly medium-sized molecules (the main part between 108 and 1322 Da), especially the humic and fulvic parts. Wang et al. (2021) recorded average molecular weights of 400–500 Da with mass spectrometry in their study of aquacultural ponds, and Aguilar-Alarcon et al. (2020) reported similar average molecular weights of 390–450 Da for their research with different feeds in RAS. Our results are in the same size class even though there were substantial differences in environment (the first study had aquacultural ponds, not RAS), water exchange rate, and water quality (the latter study used part seawater).
The molecular weight profile is mostly the same between inlet lake water and RAS systems (except for a few exceptions), which is interesting, as RAS water contains excessive amounts of small nitrogen molecules due to biofiltration and slow water retention rate (Paudel et al., 2015). This, in theory, should make RAS water more concentrated with small molecules when compared to natural water. Ozonation should further decrease the molecule size of DOM in natural waters (Świetlik & Sikorska, 2004; Świetlik et al., 2004; Veenstra et al., 1983), but Krumins et al. (2001) reported that ozonation of RAS does not have any effect to molecular weight profile of DOM due to ozone-induced flocculation and removal by clarifiers. Our results strongly support this theory. In addition, the molecular weight profile in RAS is strongly linked to used inlet water despite the oxidizing treatments.
The protein-like fluorescence tyrosine and tryptophan had a peak in 5 min of retention time, which can be considered as large (40,000–50,000 Da) protein-like structures. These are most likely derived from bacteria, fishes, and feed as they accumulate in system water (Yamin et al., 2017). O3 and O3 + H2O2 treatments were able to reduce or keep these compounds stable throughout the experiment, as substantial accumulation can be seen in control and H2O2 treatments. These fluorescences are considered as indicators of biological activity and bioavailability of DOM (Fellman et al., 2010; Hambly et al., 2015), so their increase in control systems is to be expected as time passes. The protein-like fluorescence being much weaker and different in composition in inlet lake water supports the assumption that most of those molecules are produced in RAS (feed, fish feces, microbial parts) rather than imported from inlet water (Wang et al., 2021; Yamin et al., 2017). This is especially relevant with those large protein-like structures that are almost absent from inlet lake water. On the other hand, humic and fulvic fluorescence share many similarities with RAS, which in turn supports the already existing theory that much of those molecules are generally derived from inlet water in RAS systems (Wang et al., 2021).
Humic and fulvic fluorescence were removed with very similar efficiencies, which somewhat contradicts the earlier reports that ozonation usually targets the large humic-like molecules and supports the formation of smaller fulvic-like molecules (Aguilar-Alarcon et al., 2022; Lai et al., 2021; Veenstra et al., 1983). However, the ozone dose in this study was very large, which can create enough oxidization potential so that the fulvic-like molecules can also be oxidized at the same rate as humic-like molecules.
H2O2 treatment had very poor fluorescence removal, not being able to remove fluorescence or UVA-254 at all. A better removal efficiency had been expected as the molecular amount of H2O2 was half of that of low O3. H2O2 is known to be a weaker oxidant than ozone, so the injection amount of oxidizer could have been too low to counter the organic load. There is also a possibility that treatments could boost microbial growth in systems in the same way as ozone transforms large molecules into more bioavailable forms (Wietz et al., 2009). It can also be speculated that Fe3+ ions in water can catalyze H2O2 decomposition if abundant in water (Eisenhauer, 1964). Humic substances can possibly trap this ferric iron to complexes and so reduce their amount in water, which would lead to H2O2 being more stable. This is all just speculation, and no evidence can be presented here, but it could explain the difference among systems as such.
H2O2 underwhelming fluorescence reduction indicates that something might be happening outside of monitoring wavelengths, and so it went unrecorded. In Pettersson et al. (2022), fishes in H2O2 treatment had improved growth, so water quality indeed seemed to improve in some areas. The slight reduction in tryptophan and tyrosine peaks when compared to control could display disinfection happening because of less microbial activity. Turbidity was lower in H2O2 than in control systems but higher than in other treatments (Supplementary Table 3). However, this reduction in turbidity was not noticeable in fluorescence, probably mostly as filtration before HPLSEC analysis removed all the suspended solids, colloids, etc.
The least efficient UVA-254 removal was possibly caused by aromatic rings. These rings are one of the harder things for ozone to oxidize, and UVA-254, being a meter for DOM aromaticity, could explain these results. UVA-254 also represents double and triple bonds, which will be oxidized immediately by ozone, but it is possible that these are in the minority when compared to aromatic rings. The less selective hydroxyl radical from O3 + H2O2 treatment should react with aromatic rings better than pure ozone (Von Gunten, 2003), but this was not observed here. The RAS water is heavily concentrated with different compounds, which simultaneously contribute to the fluorescence and UVA and thus cannot be monitored individually. It is possible that hydroxyl radicals created in the O3 + H2O2 process are more likely to be scavenged by these compounds than ozone, resulting in observed similar efficiency among pure O3 and O3 + H2O2 treatments (Klausen & Gronborg, 2010). Again, it seems that the possible advantages of AOP are limited by DOM concentrations.
The substantial UVA-254 peak of approximately 12 min of retention time is curious, as in that molecular weight range, only benzoic acid and its derivatives can be found. Benzoic acid has been reported to be found in a few different RAS systems (Lindholm-Lehto et al., 2020, 2021), and it is produced by micro-organisms, plants, algae, and animals in their cells as part of their metabolism (Qualley et al., 2012; Joye, 2019). The peak, however, is fairly strong and temporarily growing in relation to other peaks, which indicates that molecules represented by it are relatively abundant. One possibility is that treatments are cutting big aromatic compounds with multiple rings into smaller pieces (possibly attacking the before-mentioned double and triple bonds), which then leads finally to benzoic acid-like compounds. This would explain the relative growth of the peak.
The O3 had a clear reducing effect on DOC concentrations, and that was to be expected from previous studies (Park et al., 2013; Summerfelt et al., 1997). The reduction in DOC was higher in this study than in those referenced ones, but so was the ozone dose, which most probably explains the difference. Interestingly, the O3 + H2O2 treatment had the same DOC concentrations as the low O3 treatment. Both treatments had the same amount of ozone injected into water, which means that O3 + H2O2’s oxidative potential should have been higher and possibly lead to lower DOC concentrations. The high O3 treatment had the lowest concentrations of DOC, which indicates that by increasing the ozone injection to the system, the DOC can be further decreased, but the efficiency will suffer as compounds that are harder to oxidize become more abundant.
There is a significant difference in DOC concentration between O3 + H2O2 and high O3. On the other hand, the fluorescence difference between O3 + H2O2 and high O3 is very small and not notable, so clearly, this missing DOC is something that cannot be seen in the fluorescence measurements. If trusted purely on fluorescence, it could be said that doubling the ozone dose is just a waste of resources as the payoff is nonexistent, but this would then completely ignore the overall DOC reduction if the same comparison is done to O3 + H2O2 and low O3, fluorescence, and DOC line logically.
No strong linear correlation between fluorescence and DOC was observed in this experiment. UVA-254 had the strongest relation, which is not surprising as highly aromatic humic and fulvic acids represent up to 80% of DOM in natural waters (Singh, 2014). UVA-254 is already regularly used as an indicator for organic matter in aquaculture. Ignatev and Tuhkanen (2019) proposed that fluorescence and DOC would have a linear relation that could be used in predicting organic matter in water. It worked well in the case of wastewater purification, but it seems clear that ozonation changes the organic matter structure in RAS water in a way that at least the fluorescence HPLSEC method used here cannot predict the DOC content of water reliably. It could be speculated that in RAS, some fluorescence compounds are formed upon ozonation that emit strong fluorescence signals but are low in concentration. There is no evidence to support this, but it can be one way to explain this phenomenon.
Conclusions
Significant differences in chromatograms were found among the treatments and inlet lake water. Mainly, the intensity was much higher in RAS water when compared to inlet water. O3 and O3 + H2O2 treatments greatly reduced fluorescence, even close to inlet water fluorescence values, while H2O2 had no detectable effect. O3 + H2O2 treatment added no benefits over normal O3 treatment, which indicated that oxidation of DOM was not limited by the selectivity of ozone but rather the organic loading of the systems. Fluorescence demonstrated that most of the humic and fulvic compounds were derived from the inlet water, while protein-like fluorescence, especially the large molecules, is produced in RAS. UV-254 showed that RAS water contained large amounts of small aromatic benzoic acid derivates that were not detected in inlet lake water. The studied fluorescence HPLSEC method proved to be sensitive enough to track the oxidizer-induced change in DOM in the RAS environment.
Most of the fluorescence intensity was produced by medium-sized molecules (108–1322 Da). This was true for both RAS and inlet lake water, despite RAS being usually reported to contain smaller molecules in comparison. Treatments also did not have any effect on the molecular weight profile of the water. This proves that even with strong oxidizing treatments, the molecular weight profile of RAS water is strongly linked to its water source.
The O3 was able to substantially reduce the DOC of RAS water. The deduced relation between fluorescence and DOC is not a valid meter in the RAS environment if water is treated with continuous ozonation. This, however, does not mean that this relation should not be studied further with non-ozonated RAS waters, as the method has worked well in different environments.
Data availability
All data used in this manuscript can be obtained by emailing the corresponding author.
References
Aguilar-Alarcon, P., Gonzales, S. V., Simonsen, M. A., Borrero-Santiago, A. R., Sanchis, J., Meriac, A., Kolarevic, J., Asimakopoulos, A. G., & Mikkelsen, O. (2020). Characterizing changes of dissolved organic matter composition with the use of distinct feeds in recirculating aquaculture systems via high-resolution mass-spectrometry. Science of the Total Environment, 749, 142326. https://doi.org/10.1016/j.scitotenv.2020.142326
Aguilar-Alarcon, P., Zherebker, A., Rubekina, A., Shirshin, E., Simonsen, M. A., Kolarevic, J., Lazado, C. C., Nikolaev, E. N., Asimakopoulos, A. G., & Mikkelsen, O. (2022). Impact of ozone treatment on dissolved organic matter in land-based recirculating aquaculture systems studied by Fourier transform ion cyclotron resonance mass spectrometry. Science of The Total Environment, 843, 157009. https://doi.org/10.1016/j.scitotenv.2022.157009
Davidson, J., Summerfelt, S., Espmark, Å. M. O., Mta, V. C., Marancik, D., Earlye, R. L., Snead, A., & Good, C. (2021). Effects of O3 on post-smolt Atlantic salmon (Salmo salar) performance, health, and maturation in freshwater recirculation aquaculture systems. Aquaculture, 533, 736208. https://doi.org/10.1016/j.aquaculture.2020.736208
Eisenhauer, H. R. (1964). Oxidation of phenolic wastes. Journal of Water Pollution Control Federation, 36, 1116–1128.
Fellman, J. B., Hood, E., & Spencer, G. M. (2010). Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnology and Oceanography, 55, 2452–2462. https://doi.org/10.4319/lo.2010.55.6.2452
Hambly, A. C., Arvin, E., Pedersen, L.-F., Pedersen, P. B., & Seredynska-Sobecka, B. (2015). Characterising organic matter in recirculating aquaculture systems with fluorescence EEM spectroscopy. Water Research, 83, 112–120. https://doi.org/10.1016/j.watres.2015.06.037
Hidayah, E. N., Lai, W. L., Cahyonugroho, O. H., & Rizqa, F. (2020). Organic matter from biofilter nitrification by high performance size exclusion chromatography and fluorescence excitation-emission matrix. Global Journal of Environmental Science and Management, 6, 133–144. https://doi.org/10.22034/gjesm.2020.02.01
Ignatev, A., & Tuhkanen, T. (2019). Monitoring WWTP performance using size-exclusion chromatography with simultaneous UV and fluorescence detection to track recalcitrant wastewater fractions. Chemosphere, 214, 587–597. https://doi.org/10.1016/j.chemosphere.2018.09.099
Jokubauskaite, I., Amaleviciute, K., Lepane, V., Sleptiene, A., Slepetys, J., Liaudanskiene, I., Karcauskiene, D., & Booth, C. A. (2015). High-performance liquid chromatography (HPLC)-size exclusion chromatography (SEC) for qualitative detection of humic substances and dissolved organic matter in mineral soils and peats in Lithuania. International Journal of Environmental Analytical Chemistry, 95, 508–519. https://doi.org/10.1080/03067319.2015.1048435
Joye, I. J. (2019). Acids and bases in food. Encyclopedia of food chemistry, Academic press. 1–9. https://doi.org/10.1016/B978-0-08-100596-5.21582-5 5Get rights and content
Kamjunke, N., Nimptsch, J., Harir, M., Herzsprung, P., Schmitt-Kopplin, P., Neu, T. R., Graeber, D., Osorio, S., Valenzuela, J., Reyes, J. C., Woelfi, S., & Hertkorn, N. (2017). Land-based salmon aquacultures change the quality and bacterial degradation of riverine dissolved organic matter. Science and Reports, 7, 43739. https://doi.org/10.1038/srep43739
Kim, J., Kim, Y., Park, S. E., Kim, T.-H., Kim, B.-G., Kang, D.-J., & Rho, T. (2021). Impact of aquaculture on distribution of dissolved organic matter in coastal Jeju Island, Korea, based on absorption and fluorescence spectroscopy. Environmental Science and Pollution Research, 29, 553–563. https://doi.org/10.1007/s11356-021-15553-3
Klausen, M. M., & Gronborg, O. (2010). Pilot scale testing of advanced oxidation processes for degradation of geosmin and MIB in recirculated aquaculture. Water Supply, 10, 217–225. https://doi.org/10.2166/ws.2010.246
Krumins, V., Ebeling, J., & Wheaton, F. (2001). Ozone’s effects on power-law particle size distribution in recirculating aquaculture systems. Aquacultural Engineering, 25, 13–24. https://doi.org/10.1016/S0144-8609(01)00064-4
Lai, C., He, C., Han, F., Xu, H., Huang, B., Dionysiou, D. D., & Pam, X. (2021). Simultaneous changes of exogenous dissolved organic matter treated by ozonation in properties and interaction behavior with sulfonamides. Environmental Pollution, 275, 116546. https://doi.org/10.1016/j.envpol.2021.116546
Leonard, N., Guiraud, J. P., Gasset, E., Cailleres, J. P., & Blancheton, J. P. (2002). Bacteria and nutrients—nitrogen and carbon—in a recirculating system for sea bass production. Aquacultural Engineering, 26, 111–127. https://doi.org/10.1016/S0144-8609(02)00008-0
Lindholm-Lehto, P. C., & Vielma, J. (2019). Controlling of geosmin and 2-methylisoborneol induced off-flavours in recirculating aquaculture system farmed fish—a review. Aquaculture Research, 50, 9–28. https://doi.org/10.1111/are.13881
Lindholm-Lehto, P. C., Pulkkinen, J., Kiuru, T., Koskela, J., & Vielma, J. (2020). Water quality in recirculating aquaculture system using woodchip denitrification and slow sand filtration. Environmental Science and Pollution Research, 27, 17314–17328. https://doi.org/10.1007/s11356-020-08196-3
Lindholm-Lehto, P. C., Pulkkinen, J. T., Koskela, J., & Vielma, J. (2021). Efficient water treatment achieved in recirculating aquaculture system using woodchip denitrification and slow sand filtration. Environmental Science and Pollution Research, 28, 65333–65348. https://doi.org/10.1007/s11356-021-15162-0
Park, J., Kim, P.-K., Lim, T., & Daniels, H. V. (2013). Ozonation in seawater recirculating systems for black seabream Acanthopagrus schlegelii (Bleeker): effects on solids, bacteria, water clarity, and color. Aquacultural Engineering, 55, 1–8. https://doi.org/10.1016/j.aquaeng.2013.01.002
Paudel, S. R., Choi, O., Khanal, K. S., Chandran, K., Kim, S., & Lee, J. W. (2015). Effects of temperature on nitrous oxide (N2O) emission from intensive aquaculture system. Science of the Total Environment, 518–519, 16–23. https://doi.org/10.1016/j.scitotenv.2015.02.076
Pettersson, S. P., Lindholm-Lehto, P. C., Pulkkinen, J. T., Kiuru, T., & Vielma, J. (2022). Effect of ozone and hydrogen peroxide on off-flavor compounds and water quality in a recirculating aquaculture system. Aquacultural Engineering, 98, 102277. https://doi.org/10.1016/j.aquaeng.2022.102277
Powell, A. (2016). Direct application of ozone in aquaculture systems. Aquaculture, 10, 424–438. https://doi.org/10.1111/raq.12169
Qualley, A. V., Widhalm, J. R., Adebesin, F., Kish, C. M., & Dudareva, N. (2012). Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proceedings of the National Academy of Sciences, 109, 16383–16388. https://doi.org/10.1073/pnas.1211001109
Singh, R. (2014). Membrane technology and engineering for water purification (2nd ed.), Water and Membrane Treatment. Butterworth-Heinemann, Oxford, pp. 81–178.
Spiliotopoulou, A., Martin, R., Pedersen, L.-F., & Andersen, H. R. (2017). Use of fluorescence spectroscopy to control ozone dosage in recirculating aquaculture systems. Water Research, 111, 357–365. https://doi.org/10.1016/j.watres.2016.12.036
Spiliotopoulou, A., Rojas-Tirado, P., Chetri, R. K., Kaarsholm, K. M. S., Martin, R., Pedersen, P. B., Pedersen, L. F., & Andersen, H. R. (2018). Ozonation control and effects of ozone on water quality in recirculating aquaculture systems. Water Research, 133, 289–298. https://doi.org/10.1016/j.watres.2018.01.032
Summerfelt, S. T., Hankins, J. A., Weber, A. L., & Durant, M. D. (1997). Ozonation of a recirculating rainbow trout culture system II. Effects on microscreen filtration and water quality. Aquaculture, 158, 57–67. https://doi.org/10.1016/S0044-8486(97)00064-1
Świetlik, J., & Sikorska, E. (2004). Application of fluorescence spectroscopy in the studies of natural organic matter fractions reactivity with chlorine dioxide and ozone. Water Research, 38, 3791–3799. https://doi.org/10.1016/j.watres.2004.06.010
Świetlik, J., Dąbrowska, A., Raczyk-Stanisławiak, U., & Nawrocki, J. (2004). Reactivity of natural organic matter fractions with chlorine dioxide and ozone. Water Research, 38, 547–558. https://doi.org/10.1016/j.watres.2003.10.034
Veenstra, J. N., Barber, J. B., & Khan, P. A. (1983). Ozonation: Its effect on the apparent molecular weight of naturally occurring organics and trihalomethane production. Ozone Science and Engineering, 5, 225–244. https://doi.org/10.1080/01919518308550990
Von Gunten, U. (2003). Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Research, 37, 1443–1467. https://doi.org/10.1016/S0043-1354(02)00457-8
Wang, D., Song, C., Zhang, B., Chen, J., Luo, A., Wang, X., Wu, S., & Ye, Y. (2021). Deciphering dissolved organic matter from freshwater aquaculture ponds in eastern China based on optical and molecular signatures. Process Safety and Environment Protection, 155, 122–130. https://doi.org/10.1016/j.psep.2021.09.025
Wietz, M., Hall, M. R., & Hoj, L. (2009). Effects of seawater ozonation on biofilm development in aquaculture tanks. Systematic and Applied Microbiology, 32, 266–277. https://doi.org/10.1016/j.syapm.2009.04.001
Yamin, G., Borisover, M., Cohen, E., & Van Rijn, J. (2017). Accumulation of humic-like and proteinceous dissolved organic matter in zero-discharge aquaculture systems as revealed by fluorescence EEM spectroscopy. Water Research, 108, 412–421. https://doi.org/10.1016/j.watres.2016.11.028
Acknowledgements
We greatly appreciate the work of the staff at the Laukaa fish farm for their fish husbandry and system management. The assistance of the laboratory technicians in the Faculty of Biology and Environmental Sciences was greatly appreciated.
Funding
Open Access funding provided by University of Jyväskylä (JYU). Funding for the study came from the European Maritime and Fisheries Fund.
Author information
Authors and Affiliations
Contributions
The experiment was planned by P.S, T.T, L-L.P and I.A. Execution of experiment and measurements was carried out by P.S. Preparation of manuscript was done by P.S under supervision of T.T. I.A and L-L.P critically evaluated the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The authors declare no competing interests. Great emphasis was put on the well-being of the fishes used in the experiment. The study did not include any human participants.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pettersson, S., Ignatev, A., Lindholm-Lehto, P. et al. Monitoring of water quality with HPLSEC and fluorescence method in the ozonated recirculating aquaculture system. Environ Monit Assess 195, 1497 (2023). https://doi.org/10.1007/s10661-023-12117-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-12117-5