Abstract
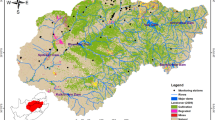
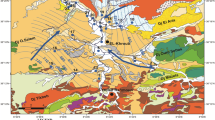
This study compiles commonly available groundwater chemistry data from the Pearl Harbor Sole Source Aquifer (SSA), Hawai‘i—O‘ahu’s primary drinking water source—and applies hierarchical clustering analysis (HCA), principal component analyses (PCA), piper diagrams, and box plots with geospatial analysis to better define groundwater regions and correlate groundwater chemistry in those regions with land use. Groundwater in this aquifer recharges and flows through chemically similar soil and rocks, such that anthropogenic activities are a primary influence on the chemical variability of the aquifer’s differing regions. Our analyses link specific chemical species in groundwater to land use/cover categories: urban, agriculture, and natural and anthropogenically-induced saline water intrusion. To create distinct statistical groupings with different groundwater chemistry compositions, it was important that the suite of parameters used in the statistical analysis do not covary. In our case, Cl− covaried with several major ions; however, by including F−, alkalinity, and SiOx that do not covary with Cl− in the covariance matrix, we produced improved spatial grouping of HCA clusters and stronger affinities to land use designations. Results show that dominant groundwater chemistry changes with land use along flow paths. These results pertain to areas where groundwater flows from conservation land in high recharge areas of O‘ahu’s mountain ranges to urban and agricultural land use regions: groundwater retains its source characteristics until about 3–6 km into agricultural and urban zoned lands. Ultimately, this study outlines a simple method for water quality regulators to use groundwater chemistry to identify risks of target contaminants based on land use.











Similar content being viewed by others
Data availability
The data used in this study are publicly available from a variety of sources, cited in the text with full details in the Reference List.
References
Anthony, S. S., Hunt, C. D., Brasher, A. M. D., Miller, L. D., & Tomlinson, M. S. (2004). Water quality on the island of O‘ahu, Hawaii, 1999–2001, U.S. Geological Survey Circular 1239.
Babcock, R., Amantaid, E., Ishikawa, C., & Uehara, M. (1999). Bench study of chlordane and dieldrin adsorption – WRRC report 99–06. Water Resources Research Center, University of Hawaii. https://scholarspace.manoa.hawaii.edu/server/api/core/bitstreams/08499d67-4b24-48bd-bcf5-257b75fecbb0/content
Commission on Water Resources Management. (2021). Groundwater information website. https://dlnr.hawaii.gov/cwrm/groundwater/
Department of the Navy. (2016–2020). Red Hill Bulk fuel storage quarterly groundwater monitoring reports. Available at: Solid & Hazardous Waste Branch | U.S. Navy Red Hill Bulk Fuel Storage Facility UST project. https://health.hawaii.gov/ust/ust-home-test/ust-red-hill-project-main/#technical-docs
Dores, D. E. (2018). Stable isotope and geochemical source-tracking of groundwater and surface water pollution to Kāne‘ohe Bay, Hawai‘i, M.S. thesis, Honolulu, USA, University of Hawai‘i at Mānoa, 144 p.
Engott, J. A., Johnson, A. G., Bassiouni, M., Izuka, S. K., & Rotzoll, K. (2017). Spatially distributed groundwater recharge for 2010 land cover estimated using a water-budget model for the Island of O‘ahu, Hawai‘i (ver. 2.0, December 2017): U.S. Geological Survey Scientific Investigations Report 2015–5010, 49 p. https://doi.org/10.3133/sir20155010
Guler, G., Thyne, G. D., McCray, J. E., & Turner, A. K. (2002). Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeology Journal, 10, 255–474. https://doi.org/10.1007/s10040-002-0196-6
Hawaiʻi Department of Health. (2019). Safe drinking water branch, drinking water compliance database data extracted November 7, 2019.
Hawaiʻi Department of Health. (2021). Groundwater contamination viewer. https://health.hawaii.gove/sdwb/groundwater-contamination-viewer/
Hunt, C. D. (1996). Geohydrology of the island of Oʻahu, Hawaii, regional aquifer-system analysis, U.S. geological survey professional paper 1412-B, 63 pp.
Hunt, C. D. (2004). Ground-water quality and its relation to land use on O‘ahu, Hawaii, 2000–01. U.S. Geological Survey Water-Resources Investigations Report 03–4305. https://pubs.usgs.gov/wri/wri034305/
Izuka, S. K., Engott, J. A., Rotzoll, K., Bassiouni, M., Johnson, A. G., Miller, L. D., & Mair, A. (2018). Volcanic aquifers of Hawai‘i—hydrogeology, water budgets, and conceptual models (ver. 2.0, March 2018): U.S. Geological Survey Scientific Investigations Report 2015–5164, 158 p. https://doi.org/10.3133/sir20155164
Izuka, S. K., Rotzoll, K., & Nishikawa, T. (2021). Volcanic Aquifers of Hawai‘i—construction and calibration of numerical models for assessing groundwater availability on Kaua‘i, O‘ahu, and Maui: U.S. Geological Survey Scientific Investigations Report 2020–5126, 63 p. https://doi.org/10.3133/sir20205126
Kaunde, M. C., & Mandal, B. (2008). Agricultural activities influence nitrate and fluoride contamination in drinking groundwater of an intensively cultivated district in India. Water, Air, Soil Pollution, 198, 243–252.
Lau, S. (1987). Organic chemical contamination of Oʻahu groundwater – technical report no. 181. Water Resources Research Center, University of Hawaiʻi. 171 p. https://scholarspace.manoa.hawaii.edu/items/92a2a319-a549-404d-b530-6416cc0a55ff/full
Lautze, N., Thomas, D., Hinz, N., Ito, G., Frazer, N., & Waller, D. (2017). Play fairway analysis of geothermal resources across the state of Hawaii: 1. Geological, geophysical, and geochemical datasets. Geothermics, 70, 376–392. https://doi.org/10.1016/j.geothermics.2017.02.001
Lautze, N. C., Ito, G., Thomas, D. M., Frazer, N., Martel, S., Hinz, N., Tachera, D. K., Hill, G., Pierce, H. A., Wannamaker, P. E., & Martin, T. (2020). Play Fairway analysis of geothermal resources across the State of Hawai‘i: 4. Updates with new groundwater chemistry subsurface stress analysis and focused geophysical surveys. Geothermics. https://doi.org/10.1016/j.geothermics.2019.101798
Mair, A., & El-Kadi, A. I. (2013). Logistic regression modeling to assess groundwater vulnerability to contamination in Hawaii, USA. Journal of Contaminant Hydrology., 153, 1–23. https://doi.org/10.1016/j.jconhyd.2013.07.004
Melrose, J., Perroy, R., & Cares, S. (2016), Statewide agricultural land use baseline 2015. Hawaiʻi State Department of Agriculture. https://hdoa.hawaii.gov/wp-content/uploads/2016/02/StateAgLandUseBaseline2015.pdf. GIS data available at https://planning.hawaii.gov/gis/download-gis-data-expanded/
Mink, J. F. (1962). Irrigation and the soils and Groundwater of Oahu Hawaii. Science., 135(3504), 672–673. https://doi.org/10.1126/science.135.3504.672.b
Mink, J. F., & Lau, L. S. (1987). Aquifer identification and classification of Oahu: groundwater protection strategy for Hawaii - WRRC technical report no. 179. Water Resources Research Center, University of Hawaiʻi at Mānoa, Honolulu, HI. 28 pg.
Naval Facilities Engineering Command. (2020). Final-Third Quarter 2020 – Quarterly Groundwater Monitoring Report – Red Hill Bulk Fuel Storage Facility Joint Base Pearl Harbor-Hickam, Oahu, Hawaii. Prepared by AECOM Technical Services, Inc. Honolulu, HI. November 2020.
Naval Facilities Engineering Command, NAVFAC Hawaii. (2019). Conceptual site model, investigation and remediation of releases and groundwater protection and evaluation, – Red Hill Bulk fuel storage facility joint base pearl Harbor-Hickam, Oahu, Hawaii. Prepared by AECOM Technical Services, Inc. Honolulu, HI. June 30, 2019. https://www.epa.gov/sites/default/files/2019-07/documents/red_hill_conceptual_site_model_20190630-redacted.pdf
Nelson, S. T., Tingey, D. G., & Selck, B. (2013). The denudation of ocean islands by ground and surface waters: The effects of climate, soil thickness, and water contact times on Oahu. Hawaii. Geochimica et Cosmochimica Acta, 103, 276–294. https://doi.org/10.1016/j.gca.2012.09.046
Nichols, W. D., Shade, P. J., & Hunt, C. D. (1997). Summary of the Oahu, Hawaii regional aquifer-system analysis. U.S. Geological Survey Professional Paper 1412-A, 61 pp.
Raj, D., & Shaji, E. (2017). Fluoride contamination in groundwater resources of Alleppy, southern India. Geoscience Frontiers., 8, 117–124.
Ramteke, L. P., Sahayam, A. C., Ghosh, A., Rambabu, U., Reddy, M. R. P., Popat, K. M., Rebaryu, R., Kubavat, D., Marathe, K. V. M., & Ghosh, P. K. (2018). Study of fluoride content in some commercial phosphate fertilizers. Journal of Fluorine Chemistry., 210, 149–155. https://doi.org/10.1016/j.jfluchem.2018.03.018
Rotzoll, K. (2012), Numerical simulation of flow in deep open boreholes in a coastal freshwater lens, Pearl Harbor Aquifer, O‘ahu, Hawai‘i: U.S. Geological Survey Scientific Investigations Report 2012–5009, 39 p.
Sherrod, D. R., Sinton, J. M., Watkins, S. E., & Brunt, K. M. (2021). Geologic map of the State of Hawaiʻi: U.S. Geological Survey Scientific Investigations Map 3143, pamphlet 72 p., 5 sheets, scales 1:100,000 and 1:250,000. https://doi.org/10.3133/sim3143
State of Hawaiʻi. (Various dates). Commission on water resources management quarterly conductivity-temperature-depth profiles for the Halawa deep monitoring well, state well number 3–2253–003. Available upon email request to dlnr.cwrm@hawaii.gov
State of Hawaiʻi. (2019). Hawaii water plan - water resource protection plan, 2019 update. Prepared by Townscape, Inc. Honolulu, Hawaiʻi, for Commission on Water Resource Management, 746 pages.
State of Hawaiʻi. (2020). Latest population estimate data. https://census.hawaii.gov/home/population-estimate/
Stearns, H. T., & Vaksvik, K. N. (1935). Geology and ground-water resources of the Island of Oahu, Hawaii, U.S. Geological Survey Bulletin I, 536 p.
Takasaki, K., & Mink, J. F. (1985). Evaluation of major dike-impounded ground-water reservoirs, Island of Oahu: water supply paper 2217, 77 pp. https://doi.org/10.3133/wsp2217
U.S. Environmental Protection Agency. (2022) Overview of the drinking water sole source aquifer program. https://www.epa.gov/dwssa/overview-drinking-water-sole-source-aquifer-program#What_Is_SSA
U.S. Environmental Protection Agency and Hawai’i Department of Health. (2022). Disapproval of the groundwater flow model report. Letter to Capt. Gordie Meyer, U.S. Navy. Dated March 17, 2022. https://www.epa.gov/system/files/documents/2022-03/epa-hdoh-groundwater-flow-model-report-disapproval-with-attachments-2022-03-17.pdf
U.S. Geological Survey (USGS). (2016). Source-tracking approach for detecting and identifying sources of wastewater in waters of Hawaii,” U.S. Geological Survey National Water Information System website, accessed July 17, 2020, at https://doi.org/10.5066/F7P55KJN
USGS. (2014a). North-central Oahu pharmaceutical sampling results for the Waihawa I P2 Well. https://waterdata.usgs.gov/nwis/inventory?agency_code=USGS&site_no=212945158014302
USGS. (2014b). North-Central Oahu Pharmaceutical Sampling Results for the Wahiawa I P1 Well. https://waterdata.usgs.gov/nwis/inventory?agency_code=USGS&site_no=212946158014301
USGS. (2014c). North-Central Oahu Pharmaceutical Sampling Results for the Wahiawa II P2 Well. https://waterdata.usgs.gov/nwis/inventory?agency_code=USGS&site_no=212936158020601
USGS. (2014d). North-Central Oahu Pharmaceutical Sampling Results for the Waialua P2 Well. https://waterdata.usgs.gov/nwis/inventory?agency_code=USGS&site_no=213427158055501
USGS. (2014e). North-Central Oahu Pharmaceutical Sampling Results for the Haleiwa P2 Well. https://waterdata.usgs.gov/nwis/inventory?agency_code=USGS&site_no=213448158054301
Visher, F. N., & Mink, J. F. (1964). Ground-water resources in Southern Oahu, Hawaii: Geological Survey Water-Supply Paper 1778, 142 p.
Warner, T. B. (1971). Normal fluoride content of seawater. Deep-Sea Research, 18, 1255–1263.
Wentworth, C. K. (1942). Geology and ground-water resources of the Moanalua-Halawa district: Board of Water Supply, 222 p.
Wilcox, C. (1996). Sugar Water Hawaii’s Plantation Ditches. University of Hawaiʻi Press.
Acknowledgements
The research was a partnership among HDOH Safe Drinking Water Branch and the University of Hawai‘i (UH) at Mānoa, Hawai‘i Institute of Geophysics and Planetology. This project is part of a broad effort to test the feasibility of using general groundwater chemistry to assess the anthropogenic contamination risk to public drinking water systems and to strengthen the research partnership between UH and HDOH staff to use science to investigate critical groundwater quality and quantity issues.
Funding
Funding was provided by the Hawai‘i Department of Health (HDOH) under a Memorandum of Agreement SDWB-18–001-RW, NSF Hawaiʻi EPSCoR Program through the National Science Foundation’s Research Infrastructure Improvement award (RII) Track-1: ‘Ike Wai: Securing Hawaiʻi’s Water Future Award #OIA-1557349, and the U.S. Department of Energy’s Geothermal Technologies Program Award #DE-EE6729DOE-GTO.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s design and execution. Daniel Dores and Robert Whittier wrote the original manuscript text, with significant revisions by Nicole Lautze and major contributions from Donald Thomas. Figures were prepared by Daniel Dores and Robert Whittier, with editing from Nicole Lautze and Donald Thomas. All authors reviewed the manuscript, an effort led by Nicole Lautze.
Corresponding author
Ethics declarations
Ethical approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1. Discussion of the method
A.1 Multiple samples from the same well
Samples used in this study were collected at different times and by different organizations and were analyzed by different laboratories; several sampling locations have multiple samples over time. We recognize that this diversity may affect the robustness of the results and the conclusions drawn. It is a key part of our study to demonstrate the utility of existing data for regional evaluations, thus removing the need for expensive field sampling for each new hydrologic investigation. Additionally, it is important to note that, for this study, our priority was to capture substantial differences in groundwater composition across broad gradients. Therefore, we completed this analysis with all available data that met our vetting process. It was necessary to include datasets from multiple studies, as no single past project had a broad enough reach to meet our needs. Having duplicate samples from different time intervals allowed for greater insight into the results of the coupled HCA-PCA approach as it showed how local groundwater composition can vary through time due to changes in pumping, climate variations, anthropogenic contamination, and other external stressors. We then investigated how those processes affect groundwater chemistry and resulting relationships to other regional groundwater compositions.
A.2 Statistical approach
Considering the two data management methods to prepare the HCA-PCA pipelines, the approach that incorporated F− and SiOx into the log-normalized ion data to produce HCA2 and PCA2 appears to be more robust (Fig. 13). HCA1, which utilized the % meq/L data input for the Piper diagram showed six clusters at a Euclidean distance of 100 (Fig. 2). In contrast, HCA2 using the log-normalized ion data and included SiOx and F− showed six clusters at a Euclidean distance of 9 (Fig. 4). The shorter Euclidean distance suggests a greater general similarity between the log-normalized data points. The difference of essentially an order of magnitude between the Euclidean distances could be due in part to data management, as HCA1 data was normalized on a percent scale, where HCA2 data was normalized on a log scale. Additionally, there could be some improved clustering results from the separation of the Na+-K+ parameter and inclusion of the SiOx and F− variables in HCA2.
Both PCA1 and PCA2 demonstrate a general geochemistry for O‘ahu groundwater. In PCA1, PCA1-1 accounts for the majority of explained variance (0.471) with Na+-K+, Ca2+, and Mg2+ being the most important features to PCA1-1 (0.536, 0.501, and 0.453, respectively). SO42−, alkalinity, and Cl−, are secondary features in PCA1-1 (0.332. 0.290, and 0.249, respectively). PCA1-2 of PCA1 accounts for only 0.347 of explained variance, but Alkalinity (0.597) and Cl− (0.596) are the key features. The variance in PCA1-1 implies that there is a general variance among the chemical composition of groundwater across the study area, attributable to a variety of common ion concentrations. The variance in PCA1-2 attributable to alkalinity and Cl−, key components of seawater, show that a somewhat secondary driver to the differences seen in groundwater compositions across O‘ahu is the presence of saltwater intrusion. It could be argued that PCA1-1 of PCA1 is a measurement of general groundwater chemistry variability, whereas PCA1-2 is a metric of seawater contamination.
PCA2 continues the narrative of a general chemistry for local groundwater, as Ca2+, Mg2+, Na+, K+, and Cl− all carried similar weight (between 0.380 and 0.402) for PCA2-1, which accounted for 0.662 of the data variance. PCA2 also showed the importance of incorporating SiOx, alkalinity, and F− into the analysis, as they were by far the most important variables of PCA2-2 (0.685, 0.565, and 0.387). The implication from PCA2-2 of PCA2 is that SiOx alkalinity, and F− could be important measurements for differentiating local regions of groundwater on O‘ahu as the other ion concentration variables often co-vary. The differentiations offered by these additional parameters indicates a robustness for the HCA2-PCA2 dataset and approach.
When small-batch PCAs were run for each cluster of HCA2 with the log-normalized ions including SiOx and F−, the majority of variance was explained for each by their PC1s, with the majority of parameters covarying together. One exception was cluster 2a, in which 0.733 of variance was explained by PCA2-1; within that PCA2-1, SiOx had a weight of 0.998. It seems that for cluster 2a, SiOx concentrations were the defining variable.
HCA2 clusters 2b and 2a appear to be the most unique. Cluster 2a has higher alkalinity, while cluster 2b has higher F− concentrations, although there is a large range of F− concentrations for all clusters. Cluster 2b and 2a both have higher Ca2+, Mg2+, Cl−, Na+, K+, and SO42−, although cluster 2b is always higher than cluster 2a. Again, the PCA partnered with its HCA, in this case, PCA2, shows agreement with the box plot chemistry: clusters 2b and 2a are plotted in distinct and separate regions from the rest of the clusters, which otherwise plot adjacent to one another (Fig. 5). Interestingly, the upper and lower quartile boundaries for each cluster defined by HCA2 are generally closer together than in those clusters defined by HCA1. This could imply that the grouping is more robust for HCA2 and more accurately groups samples that are similar to one another, as discussed earlier in reference to the Euclidean distances and inclusion of additional variables.
Appendix 2. Piper diagrams
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dores, D., Whittier, R., Lautze, N. et al. Application of statistics to correlate groundwater chemistry with land use on O‘ahu, Hawai‘i. Environ Monit Assess 195, 551 (2023). https://doi.org/10.1007/s10661-023-11030-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11030-1